Abstract
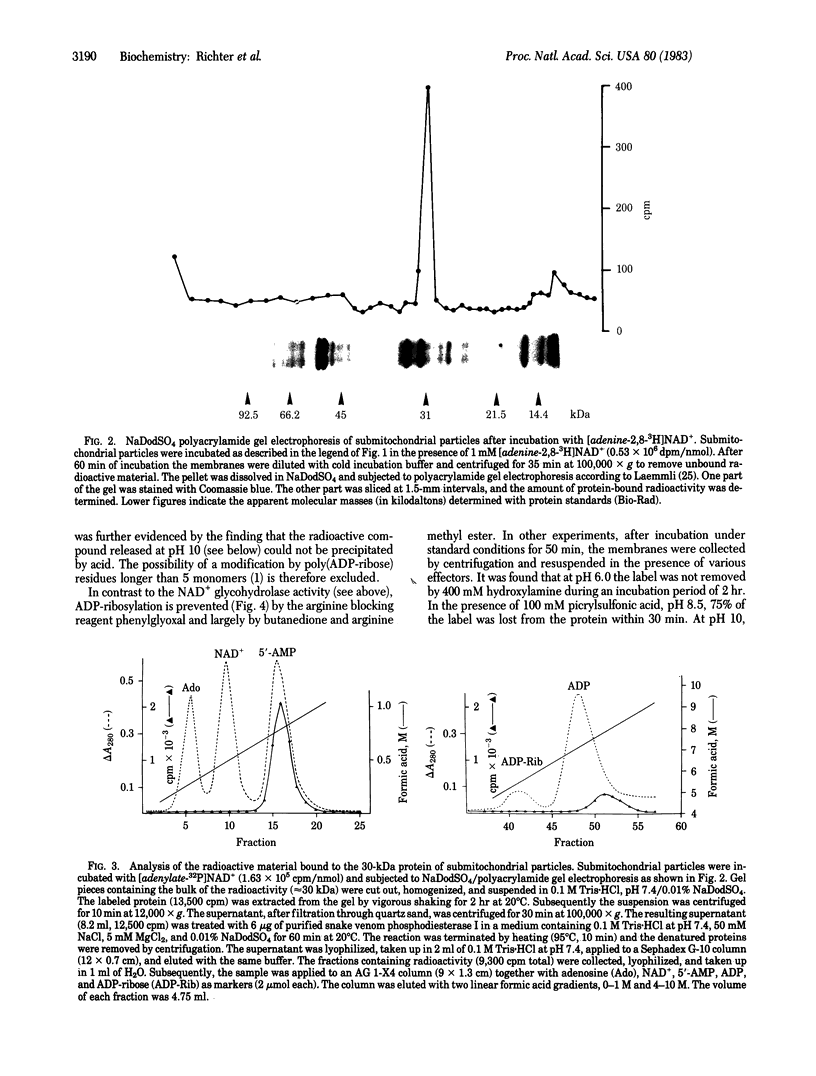
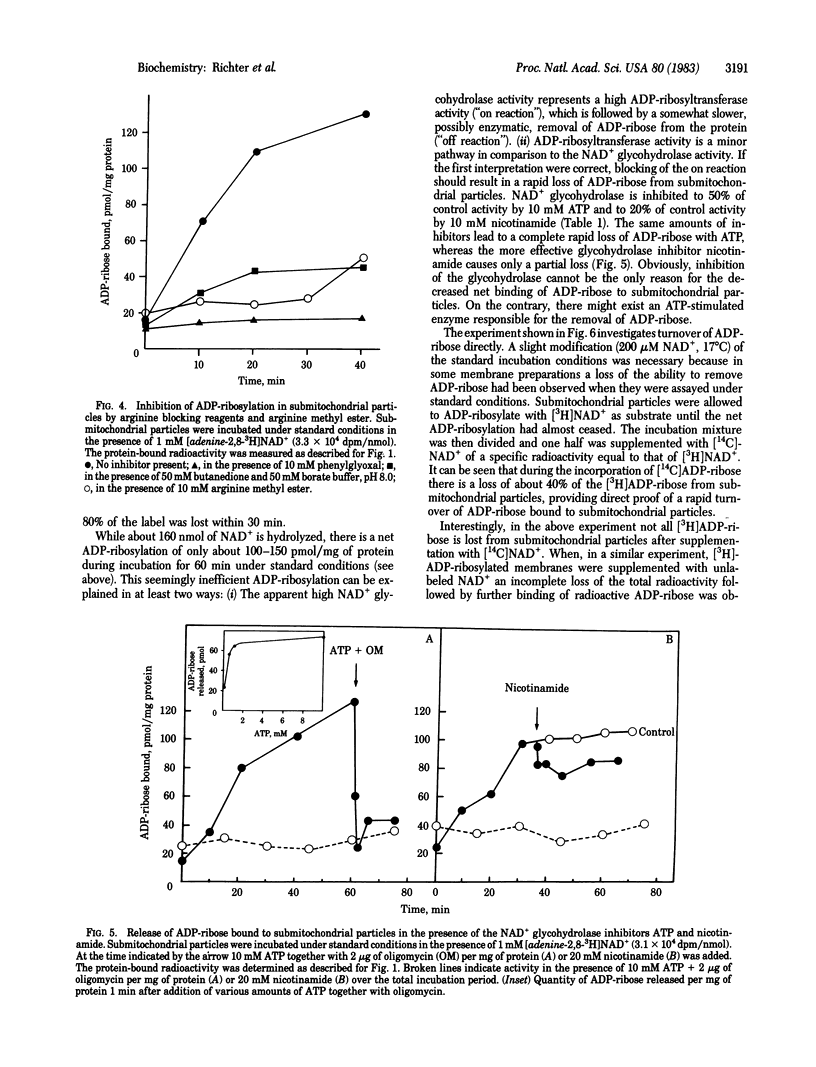
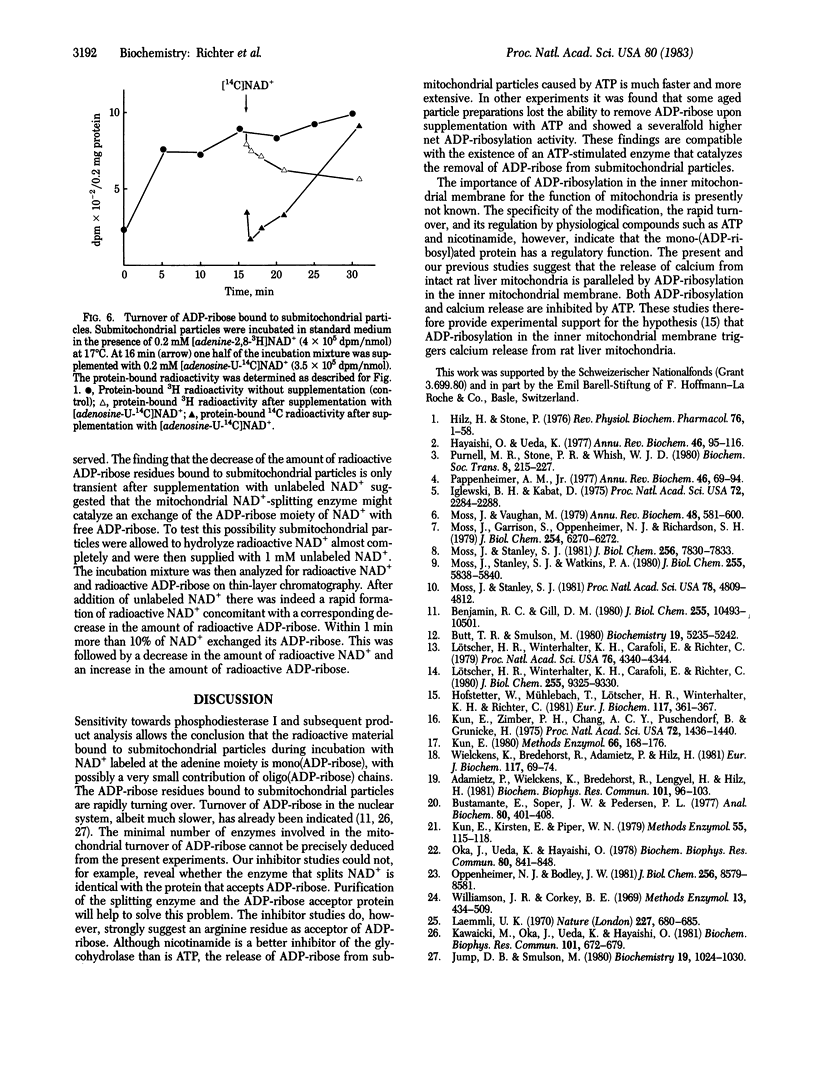
NAD+ glycohydrolase activity is found at high levels in submitochondrial particles. It leads to the reaction products ADP-ribose, nicotinamide, and small amounts of 5'-AMP. Furthermore, submitochondrial particles catalyze the exchange reaction: [adenosine-14C]ADP-ribose + NAD+ in equilibrium [adenosine-14C]-NAD+ + ADP-ribose. When submitochondrial particles are incubated with NAD+, mono(ADP-ribosyl)ation of protein molecules migrating with an apparent molecular weight of 30,000 in sodium dodecyl sulfate/polyacrylamide gel electrophoresis is demonstrable. Inhibitor studies suggest attachment of ADP-ribose to arginine residues. ADP-ribose bound to submitochondrial particles is rapidly turning over. The release of ADP-ribose from the protein is probably enzyme catalyzed. The rapid turnover, the specificity of the modification, and the inhibition of ADP-ribosylation by ATP and nicotinamide suggest a regulatory role of mono(ADP-ribosyl)ation of a protein in the inner mitochondrial membrane.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamietz P., Wielckens K., Bredehorst R., Lengyel H., Hilz H. Subcellular distribution of mono(ADP-ribose) protein conjugates in rat liver. Biochem Biophys Res Commun. 1981 Jul 16;101(1):96–103. doi: 10.1016/s0006-291x(81)80015-0. [DOI] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. ADP-ribosylation in mammalian cell ghosts. Dependence of poly(ADP-ribose) synthesis on strand breakage in DNA. J Biol Chem. 1980 Nov 10;255(21):10493–10501. [PubMed] [Google Scholar]
- Bustamante E., Soper J. W., Pedersen P. L. A high-yield preparative method for isolation of rat liver mitochondria. Anal Biochem. 1977 Jun;80(2):401–408. doi: 10.1016/0003-2697(77)90661-3. [DOI] [PubMed] [Google Scholar]
- Butt T. R., Smulson M. Relationship between nicotinamide adenine dinucleotide concentration and in vitro synthesis of poly(adenosine diphosphate ribose) on purified nucleosomes. Biochemistry. 1980 Nov 11;19(23):5235–5242. doi: 10.1021/bi00564a013. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Hilz H., Stone P. Poly(ADP-ribose) and ADP-ribosylation of proteins. Rev Physiol Biochem Pharmacol. 1976;76:1-58, 177. doi: 10.1007/BFb0027686. [DOI] [PubMed] [Google Scholar]
- Hofstetter W., Mühlebach T., Lötscher H. R., Winterhalter K. H., Richter C. ATP prevents both hydroperoxide-induced hydrolysis of pyridine nucleotides and release of calcium in rat liver mitochondria. Eur J Biochem. 1981 Jul;117(2):361–367. doi: 10.1111/j.1432-1033.1981.tb06346.x. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump D. B., Smulson M. Purification and characterization of the major nonhistone protein acceptor for poly(adenosine diphosphate ribose) in HeLa cell nuclei. Biochemistry. 1980 Mar 4;19(5):1024–1030. doi: 10.1021/bi00546a030. [DOI] [PubMed] [Google Scholar]
- Kawaichi M., Oka J., Ueda K., Hayaishi O. A new method for oligo (ADP-ribose) fractionation according to chain length. Biochem Biophys Res Commun. 1981 Jul 30;101(2):672–679. doi: 10.1016/0006-291x(81)91311-5. [DOI] [PubMed] [Google Scholar]
- Kun E. Covalent modification of proteins by metabolites of NAD+. Methods Enzymol. 1980;66:168–176. doi: 10.1016/0076-6879(80)66457-x. [DOI] [PubMed] [Google Scholar]
- Kun E., Kirsten E., Piper W. N. Stabilization of mitochondrial functions with digitonin. Methods Enzymol. 1979;55:115–118. doi: 10.1016/0076-6879(79)55016-2. [DOI] [PubMed] [Google Scholar]
- Kun E., Zimber P. H., Chang A. C., Puschendorf B., Grunicke H. Macromolecular enzymatic product of NAD+ in liver mitochondria. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1436–1440. doi: 10.1073/pnas.72.4.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxide-induced loss of pyridine nucleotides and release of calcium from rat liver mitochondria. J Biol Chem. 1980 Oct 10;255(19):9325–9330. [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. Hydroperoxides can modulate the redox state of pyridine nucleotides and the calcium balance in rat liver mitochondria. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4340–4344. doi: 10.1073/pnas.76.9.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Garrison S., Oppenheimer N. J., Richardson S. H. NAD-dependent ADP-ribosylation of arginine and proteins by Escherichia coli heat-labile enterotoxin. J Biol Chem. 1979 Jul 25;254(14):6270–6272. [PubMed] [Google Scholar]
- Moss J., Stanley S. J. Amino acid-specific ADP-ribosylation. Identification of an arginine-dependent ADP-ribosyltransferase in rat liver. J Biol Chem. 1981 Aug 10;256(15):7830–7833. [PubMed] [Google Scholar]
- Moss J., Stanley S. J. Histone-dependent and histone-independent forms of an ADP-ribosyltransferase from human and turkey erythrocytes. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4809–4812. doi: 10.1073/pnas.78.8.4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss J., Stanley S. J., Watkins P. A. Isolation and properties of an NAD- and guanidine-dependent ADP-ribosyltransferase from turkey erythrocytes. J Biol Chem. 1980 Jun 25;255(12):5838–5840. [PubMed] [Google Scholar]
- Moss J., Vaughan M. Activation of adenylate cyclase by choleragen. Annu Rev Biochem. 1979;48:581–600. doi: 10.1146/annurev.bi.48.070179.003053. [DOI] [PubMed] [Google Scholar]
- Oka J., Ueda K., Hayaishi O. Snake venom phosphodiesterase: simple purification with Blue Sepharose and its application to poly(ADP-ribose) study. Biochem Biophys Res Commun. 1978 Feb 28;80(4):841–848. doi: 10.1016/0006-291x(78)91321-9. [DOI] [PubMed] [Google Scholar]
- Oppenheimer N. J., Bodley J. W. Diphtheria toxin. Site and configuration of ADP-ribosylation of diphthamide in elongation factor 2. J Biol Chem. 1981 Aug 25;256(16):8579–8581. [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr Diphtheria toxin. Annu Rev Biochem. 1977;46:69–94. doi: 10.1146/annurev.bi.46.070177.000441. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Stone P. R., Whish W. J. ADP-ribosylation of nuclear proteins. Biochem Soc Trans. 1980 Apr;8(2):215–227. doi: 10.1042/bst0080215. [DOI] [PubMed] [Google Scholar]
- Wielckens K., Bredehorst R., Adamietz P., Hilz H. Protein-bound polymeric and monomeric ADP-ribose residues in hepatic tissues. Comparative analyses using a new procedure for the quantification of poly(ADP-ribose). Eur J Biochem. 1981 Jun;117(1):69–74. doi: 10.1111/j.1432-1033.1981.tb06303.x. [DOI] [PubMed] [Google Scholar]



