Abstract
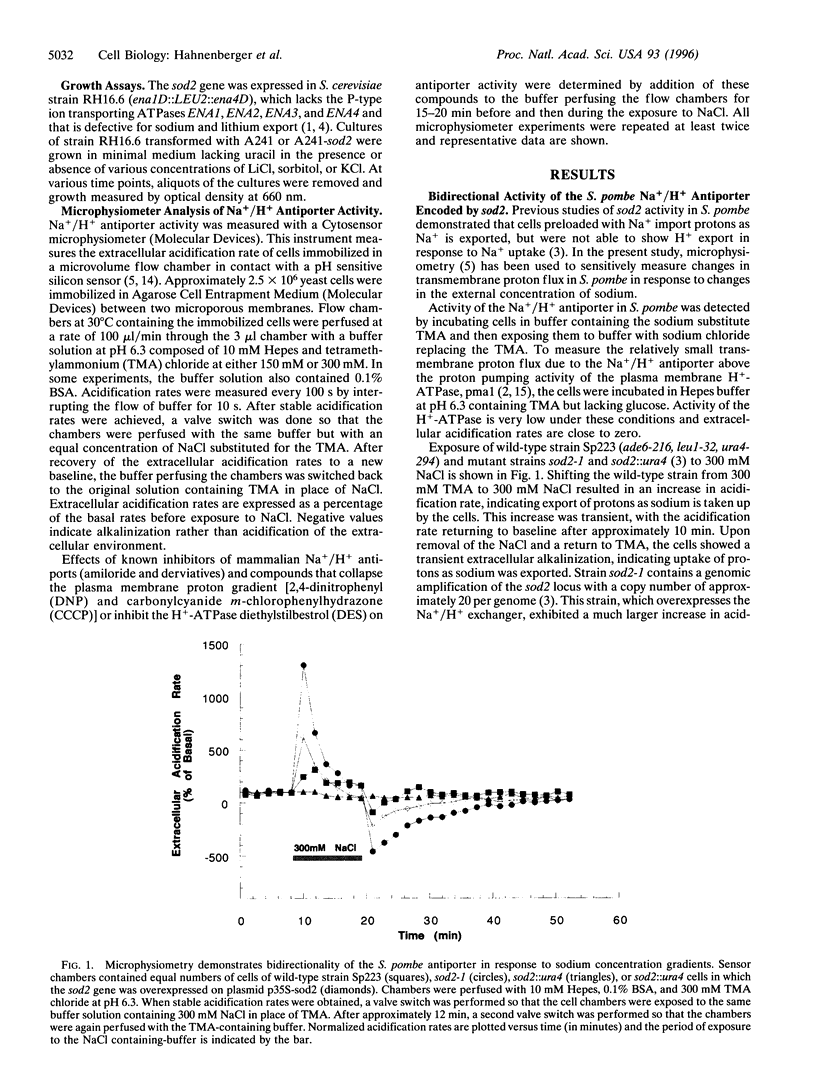
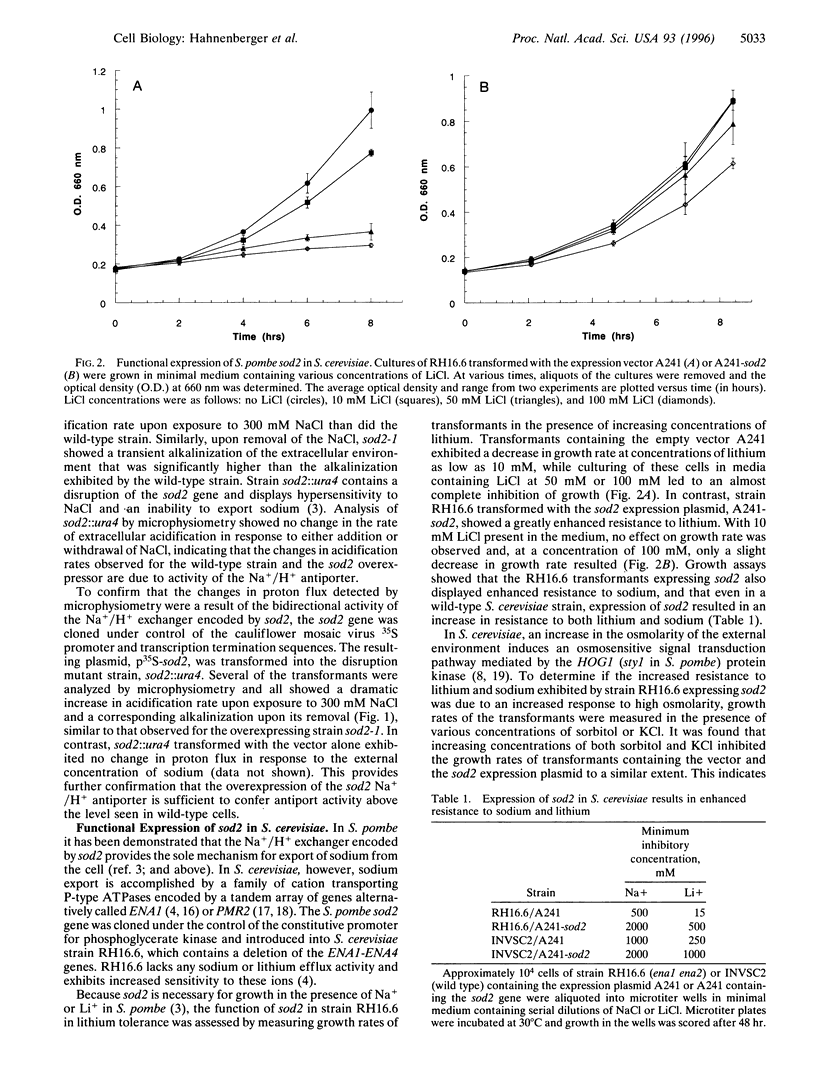
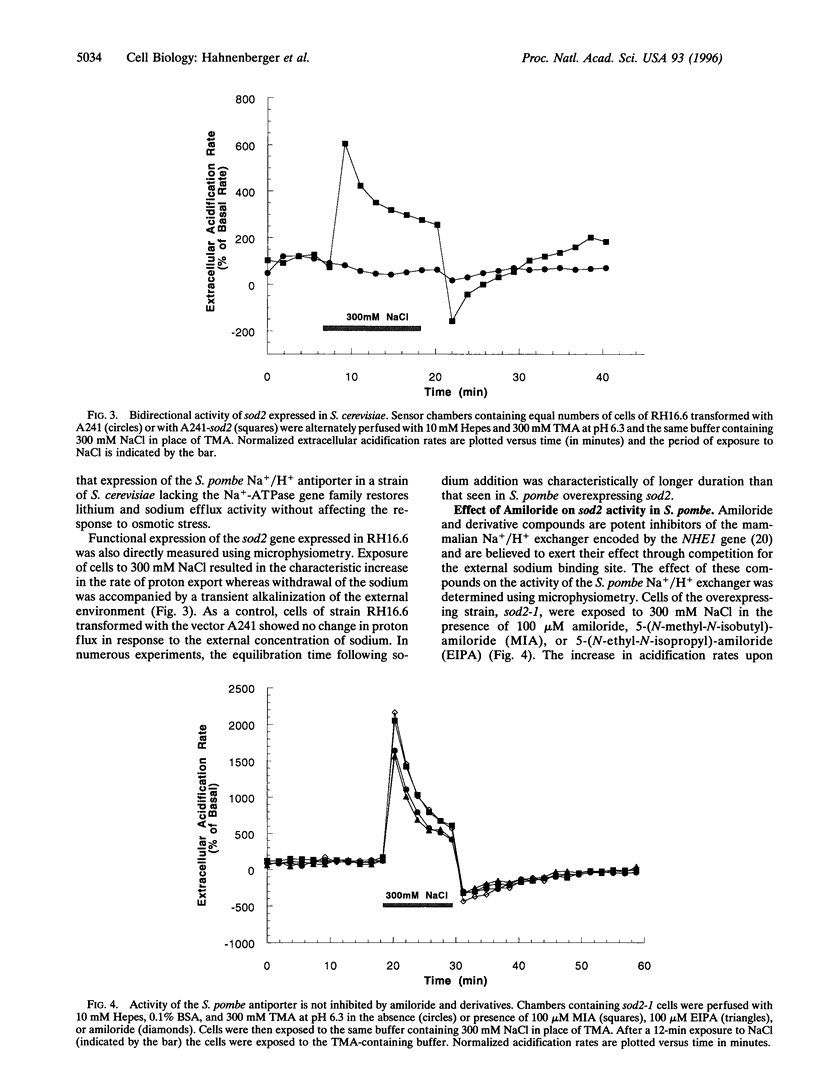
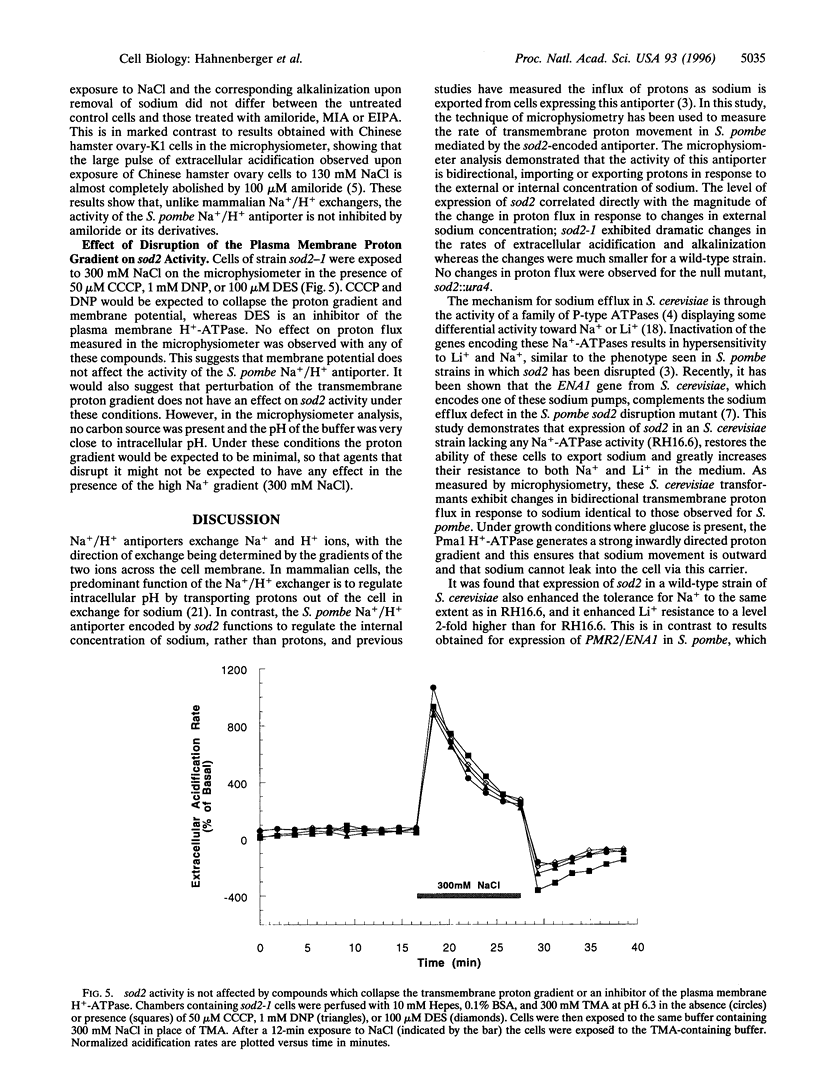
In the fission yeast, Schizosaccharomyces pombe, tolerance to high sodium and lithium concentrations requires the functioning of the sod2, Na+/H+ antiporter. We have directly measured the activity of this antiporter and demonstrated reconstitution of the activity in gene deletion strains. In addition, we have shown that it can be transferred to, and its antiporter activity detected in, the budding yeast, Saccharomyces cerevisiae, where it also confers sodium and lithium tolerance. Proton flux through the S. pombe Na+/H+ antiporter was directly measured using microphysiometry. The direction of transmembrane proton flux mediated by this antiporter was reversible, with protons being imported or exported in response to the external concentration of sodium. This bidirectional activity was also detected in S. cerevisiae strains expressing sod2 and expression of this gene complemented the sodium and lithium sensitivity resulting from inactivation of the ENA1/PMR2 encoded Na+-exporting ATPases. This suggests that antiporters or sodium pumps can be utilized interchangeably by S. cerevisiae to regulate internal sodium concentration. Potent inhibitors of mammalian Na+/H+ exchangers were found to have no effect on sod2 activity. The proton flux mediated by sod2 was also found to be unaffected by perturbation of membrane potential or the plasma membrane proton gradient.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bañuelos M. A., Quintero F. J., Rodríguez-Navarro A. Functional expression of the ENA1(PMR2)-ATPase of Saccharomyces cerevisiae in Schizosaccharomyces pombe. Biochim Biophys Acta. 1995 Apr 26;1229(2):233–238. doi: 10.1016/0005-2728(95)00006-5. [DOI] [PubMed] [Google Scholar]
- Brewster J. L., de Valoir T., Dwyer N. D., Winter E., Gustin M. C. An osmosensing signal transduction pathway in yeast. Science. 1993 Mar 19;259(5102):1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Counillon L., Franchi A., Pouysségur J. A point mutation of the Na+/H+ exchanger gene (NHE1) and amplification of the mutated allele confer amiloride resistance upon chronic acidosis. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4508–4512. doi: 10.1073/pnas.90.10.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliegel L., Fröhlich O. The Na+/H+ exchanger: an update on structure, regulation and cardiac physiology. Biochem J. 1993 Dec 1;296(Pt 2):273–285. doi: 10.1042/bj2960273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garciadeblas B., Rubio F., Quintero F. J., Bañuelos M. A., Haro R., Rodríguez-Navarro A. Differential expression of two genes encoding isoforms of the ATPase involved in sodium efflux in Saccharomyces cerevisiae. Mol Gen Genet. 1993 Jan;236(2-3):363–368. doi: 10.1007/BF00277134. [DOI] [PubMed] [Google Scholar]
- Gmünder H., Kohli J. Cauliflower mosaic virus promoters direct efficient expression of a bacterial G418 resistance gene in Schizosaccharomyces pombe. Mol Gen Genet. 1989 Dec;220(1):95–101. doi: 10.1007/BF00260862. [DOI] [PubMed] [Google Scholar]
- Hafeman D. G., Parce J. W., McConnell H. M. Light-addressable potentiometric sensor for biochemical systems. Science. 1988 May 27;240(4856):1182–1185. doi: 10.1126/science.3375810. [DOI] [PubMed] [Google Scholar]
- Haro R., Garciadeblas B., Rodríguez-Navarro A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991 Oct 21;291(2):189–191. doi: 10.1016/0014-5793(91)81280-l. [DOI] [PubMed] [Google Scholar]
- Jia Z. P., McCullough N., Martel R., Hemmingsen S., Young P. G. Gene amplification at a locus encoding a putative Na+/H+ antiporter confers sodium and lithium tolerance in fission yeast. EMBO J. 1992 Apr;11(4):1631–1640. doi: 10.1002/j.1460-2075.1992.tb05209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleyman T. R., Cragoe E. J., Jr Amiloride and its analogs as tools in the study of ion transport. J Membr Biol. 1988 Oct;105(1):1–21. doi: 10.1007/BF01871102. [DOI] [PubMed] [Google Scholar]
- Kurtz S., Luo G., Hahnenberger K. M., Brooks C., Gecha O., Ingalls K., Numata K., Krystal M. Growth impairment resulting from expression of influenza virus M2 protein in Saccharomyces cerevisiae: identification of a novel inhibitor of influenza virus. Antimicrob Agents Chemother. 1995 Oct;39(10):2204–2209. doi: 10.1128/aac.39.10.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T., Takekawa M., Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995 Jul 28;269(5223):554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993 Jan 15;123(1):127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- McConnell H. M., Owicki J. C., Parce J. W., Miller D. L., Baxter G. T., Wada H. G., Pitchford S. The cytosensor microphysiometer: biological applications of silicon technology. Science. 1992 Sep 25;257(5078):1906–1912. doi: 10.1126/science.1329199. [DOI] [PubMed] [Google Scholar]
- Millar J. B., Buck V., Wilkinson M. G. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995 Sep 1;9(17):2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Navarro A., Quintero F. J., Garciadeblás B. Na(+)-ATPases and Na+/H+ antiporters in fungi. Biochim Biophys Acta. 1994 Aug 30;1187(2):203–205. doi: 10.1016/0005-2728(94)90111-2. [DOI] [PubMed] [Google Scholar]
- Rudolph H. K., Antebi A., Fink G. R., Buckley C. M., Dorman T. E., LeVitre J., Davidow L. S., Mao J. I., Moir D. T. The yeast secretory pathway is perturbed by mutations in PMR1, a member of a Ca2+ ATPase family. Cell. 1989 Jul 14;58(1):133–145. doi: 10.1016/0092-8674(89)90410-8. [DOI] [PubMed] [Google Scholar]
- Tang W., Ruknudin A., Yang W. P., Shaw S. Y., Knickerbocker A., Kurtz S. Functional expression of a vertebrate inwardly rectifying K+ channel in yeast. Mol Biol Cell. 1995 Sep;6(9):1231–1240. doi: 10.1091/mbc.6.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y., Miwa S., Tamai Y. Characterization of Na+/H(+)-antiporter gene closely related to the salt-tolerance of yeast Zygosaccharomyces rouxii. Yeast. 1995 Jul;11(9):829–838. doi: 10.1002/yea.320110905. [DOI] [PubMed] [Google Scholar]
- Wieland J., Nitsche A. M., Strayle J., Steiner H., Rudolph H. K. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 1995 Aug 15;14(16):3870–3882. doi: 10.1002/j.1460-2075.1995.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


