Abstract
With advancements in immunosuppressive strategies and availability of better immunosuppressive agents, survival rate following liver transplantation has improved significantly in the recent times. Besides improvements in surgical techniques, the most important factor that has contributed to this better outcome is the progress made in the field of immunosuppression. Over the last several years, the trend has changed to tailored immunosuppression with the aim of achieving optimal graft function while avoiding its undesirable side effects. Induction agents are no longer used routinely and the aim is to provide minimal immunosuppression in the maintenance phase. The present review discusses the various types of immunosuppressive agents, their mechanism of action, clinical utility, advantages and disadvantages, and their side effects in short and long-term. It also discusses about tailoring immunosuppression in presence of various situations such as renal dysfunction, metabolic syndrome, hepatitis C recurrence, cytomegalovirus infections and so on. The issue of chronic kidney disease and the available renal sparing immunosuppressive strategies has been particularly stressed upon. Finally, it discusses about the practical aspects of various immunosuppression regimens including drug monitoring.
Keywords: liver transplantation, immunosuppression, metabolic syndrome
Abbreviations: HLA, human leukocyte antigen; MAP, mitogen activated protein; NF-kB, nuclear factor kappa B; IL-2, interleukin-2; CNI, Calcineurin inhibitor; NFAT, nuclear factor of activated T cells; FKBP12, FK506 binding protein; MPA, mycophenolic acid; mTORC1, mammalian target of rapamycin complex 1; CKD, chronic kidney disease; MS, metabolic syndrome; HCV, hepatitis C virus; ACR, acute cellular rejection; PTLD, post-transplant lymphoproliferative disease; ATP, adenosine triphosphate
While earlier attempts of liver transplantation in the era of radiation or Azathioprine and steroids resulted in poor patient survival (30–35% 1 year survival in 1960s and 1970s), use of Cyclosporine A led to acceptable survival rate and changed the scenario for liver transplantation.1,2 With the advances in immunosuppression, now a days liver transplantation has 1, 3 and 5 year survival rates 88%, 80% and 75% respectively.3 The present goal of immunosuppression is to maintain optimal graft function while avoiding its undesirable side effects. Over the last several years, practice has been changed to tailored immunosuppression- induction agents are no longer used routinely and aim is to provide minimal immunosuppression in maintenance phase. Liver is a relatively immunoprotective organ and has less rejection rates as compared to other solid organs. Also, the immunosuppression used following liver transplantation is lesser as compared to other organ transplants. In addition, some centers accept lower levels of calcineurin inhibitors in living donor liver transplants.4 Also acute cellular rejection episode does not affect liver graft survival.5 The potential mechanisms for lesser rejection in liver includes production of soluble MHC1 by liver (blocks preformed antibodies and inhibits T cell activation) and good regeneration capacity of liver.1 Human leukocyte antigen (HLA) typing is not used to select the liver donor6 and in combined or simultaneous organ transplants, liver transplant can provide protection to other organs transplanted along with liver.7
There are three main immune signals involved in host immunity against liver allograft as shown in Figure 1 (adapted from reference 1). Host antigen presenting cells present graft antigens to reactive host T cells and cause lymphocyte activation, which is mediated through the T cell receptor (TCR; CD3 complex), named as signal 1. Along with signal 1, T cells activation also needs costimulatory signal 2, provided by interaction of CD80/CD86 (surface of antigen presenting cells) and CD28 (surface of T lymphocytes).8–11 Signals 1 and 2 activate downstream signal transduction pathways, which include calcium/calcineurin pathway (target for calcineurin inhibitors), RAS mitogen activated protein (MAP) kinase and nuclear factor kappa B (NF-kB) pathways which in turn leads to transcription of cytokines including interleukin-2 (IL-2). Interleukin-2 acts on other immune cells providing signal 3 trigger for cell proliferation. This activation pathway is blocked by mTOR inhibitors (target for Sirolimus and Everolimus).11,12 Lymphocyte proliferation requires nucleotide synthesis.13 Antimetabolites (Azathioprine and Mycophenolate) block nucleotide synthesis and thus work as immunosuppressants.
Figure 1.
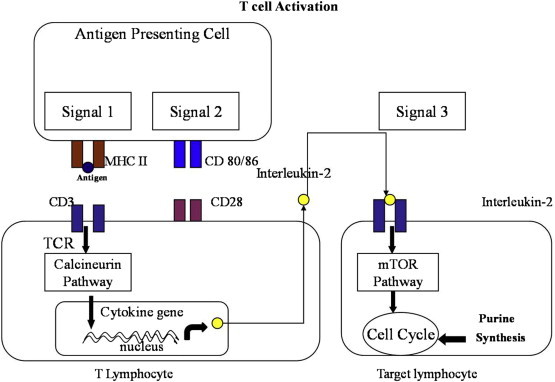
Immune signals involved in host immunity.
The different classes of immunosuppressant drugs used in liver transplantation are shown in Table 1. Various drugs have different mechanism of action and different toxicity profile, so present practice is to combine drugs from various classes. The most commonly used combination is to use Calcineurin inhibitor (CNI), Mycophenolate and steroids following transplantation.12 The standard immunosuppression protocol at author's center consists of triple immunosuppression including Calcineurin inhibitors (Tacrolimus mainly), Mycophenolate and short-term steroids (tapered over three months except in hepatitis C and auto-immune hepatitis patients).
Table 1.
Classes of immunosuppressive drugs.
| Class | Drugs | Mechanism of action | |
|---|---|---|---|
| Pharmacological immunosuppressive agents | Corticosteroids | Inhibit cytokine transcription by antigen presenting cells, broad spectrum of effects | |
| Calcineurin inhibitors | Cyclosporin, tacrolimus | Inhibition of signal 2 transduction | |
| Antimetabolites | Azathioprine, mycophenolate | Inhibition of purine and DNA synthesis and prevention of T cell proliferation | |
| mTOR inhibitors | Sirolimus, Everolimus | Inhibition of signal 3 transduction and prevention of T cell proliferation | |
| Biological immunosuppressive agents | T cell-depleting agents | Anti-CD3 (monoclonal): OKT3 | Interference with signal 1 |
| ATG/ALG: horse and rabbit | Interference with signals 1–3 | ||
| Anti-CD52 Campath-1H (Alemtuzumab): | Depletion of thymocytes, T cell, B cells, monocytes | ||
| Non-T cell-depleting agents | Anti–IL-2 receptors, basiliximab, daclizumab | Inhibition of T cell proliferation and signal 3 | |
| belatacept | Inhibition of signal 2 |
Adapted from reference12.
Calcineurin inhibitors (CNI)
Tacrolimus and Cyclosporine belong to the group of cycloneurine inhibitors, although they act on different targets. Cyclosporine binds to cyclophilin which interferes with calcineurin's dephosphorylation of nuclear factor of activated T cells (NFAT), thus preventing it's translocation into nucleus and proinflammatory cytokine production.13 On the other hand, Tacrolimus binds to FK506 binding protein (FKBP12) and leads to calcineurin inhibition.14 Tacrolimus is considered to be 100 times potent on molar level than Cyclosporine.1 Several studies found less incidence of acute cellular rejection with Tacrolimus when compared to Cyclosporine, however, a multicentre study by Levy et al involving 250 and 145 patients in Cyclosporine and Tacrolimus arms respectively found no difference in acute rejection rates between the two arms (26% versus 24% at 3 months).14–17 Adverse events related to CNI use are shown in Table 2; diabetes and neurotoxicity are more common with Tacrolimus, whereas hypertension and hyperlipidemia are more common with Cyclosporine use. Gingival hyperplasia and hypertrichosis are typically caused by Cyclosporine only.18 Both Tacrolimus and Cyclosporine are metabolized by cytochrome p450 system in liver, so their serum levels are affected by various drugs acting on cytochrome p450 as shown in Table 3.18 Doses and target levels of these drugs are shown in Table 4.19,20
Table 2.
Side effects of immunosuppression drugs.
| CNIs | Nephrotoxicity, neurotoxicity, diabetes, hyperkalemia, metabolic acidosis, hypertension, hyperlipidemia, hypertension, metabolic syndrome gingival hyperplasia and hypertrichosis (only with Cyclosporine) |
| MMF | Myelosuppression, gastrointestinal side effects, viral infections (CMV, HSV), spontaneous abortions in pregnant women |
| Sirolimus | Hyperlipidemia, metabolic syndrome, myelosuppression, proteinuria, poor wound healing, pneumonitis, skin rash |
| Corticosteroids | Diabetes, metabolic syndrome, hypertension, obesity, osteoporosis, avascular necrosis, growth retardation, cushingoid features, psychosis, poor wound healing, adrenal suppression, cataract |
Adapted from reference 18.
Table 3.
Drug interactions of immunosuppression.
| Drugs that increase CNI levels | Macrolides: clarithromycin, erythromycin, azithromycin |
| Antifungals: azole drugs | |
| Calcium channel blockers: verapamil, diltiazem, nifedipine | |
| Others: cisapride, metaclopramide, amiodarone, danazol, cimetidine, protease inhibitors | |
| Drugs that decrease CNI | Antibiotics: rifabutin, rifampin |
| Anticonvulsants: carbamazepine, phenobarbital, phenytoin, fosphenytoin |
Adapted from reference 18.
Table 4.
Doses and target trough levels of immunosuppressants.
| Drug | Dose | Target level (ng/ml) |
|---|---|---|
| Cyclosporine | 8 ± 4 mg/kg/day (two divided doses) | 0–3 months: 200–250 |
| 3–6 months: 150–200 | ||
| 6–12 months: 120–150 | ||
| > 12 months: 80–120 | ||
| Tacrolimus | 0.1–0.3 mg/kg/day(two divided doses) | 0–6 months: 7–10 |
| >6 months: 5–7 | ||
| Sirolimus | 2 mg once a day | 5–10 |
| Mycophenolate | 500–1000 mg twice a day | Monitoring not being practiced |
Adapted from reference19,20.
Antimetabolites
Mycophenolic acid (MPA) is the active compound of Mycophenolate mofetil and Mycophenolate sodium. MPA inhibits inosine 5′ monophosphate dehydrogenase (rate limiting enzyme in the de novo synthesis of guanosine nucleotides).21 Thus administration of MPA results in blockade of lymphocyte proliferation.12 Main advantage of MPA is lack of nephrotoxicity, so it can be combined with CNI, thus permitting lower doses of CNIs.22,23 When given alone (without CNIs), use of MPA resulted in higher rates of rejection compared to the CNIs.23 Adverse events related to MPA use are gastrointestinal and bone marrow suppression (leucopenia and thrombocytopenia).24 The use of Azathioprine is associated with higher incidence of acute cellular rejection, but similar graft survival at 1 year when compared to Mycophenolate.5
mTOR inhibitors
Sirolimus and Everolimus belong to the group of mTOR inhibitors. They act on signal three to suppress immunity. They also bind to FK506 binding protein inside cells and inhibit mammalian target of rapamycin complex 1 (mTORC1).25 It subsequently inhibits IL-2 signaling to T cells, thus preventing T cell proliferation. Sirolimus is also metabolized by the cytochrome p450 system.24 In a study including 15 patients, Watson et al in 1999 showed effectiveness of Sirolimus monotherapy for maintenance immunosuppression.26 Some initial studies showed higher incidence of hepatic artery thrombosis with use of Sirolimus, but it was not subsequently substantiated.27,28 Conversion of CNI to Sirolimus is also useful for patients with CNI induced nephrotoxicity,29–31 but it causes proteinuria and may worsen renal function in patients with pre-existing renal disease.32,33 Sirolimus impairs wound healing as it impairs action of TGFβ,34 hence it is usually avoided in the early post operative period. In addition, it may cause interstitial pneumonitis, peripheral edema and dermatological side effects (acne, mouth ulcers).18
Use of Sirolimus as anti-tumor agent
Sirolimus has also been shown to have additional anti-tumor properties.35 In a meta-analysis by Liang et al, including five studies (total 2950 patients), use of Sirolimus was associated with better 1, 3 and 5 year survival rates and odds ratio of 0.42 for hepatocellular carcinoma recurrence as compared to Sirolimus free regimens, without statistically significant increased incidence of hepatic artery thrombosis and acute cellular rejection.28
Steroids
Steroids have multiple targets for inhibition of immune system. They cause stabilization of lysosomal membranes, inhibitory effects on antigen presentation by dendritic cells, decrease in the number of circulating CD4 + T cells, IL-1 transcription and IL-1-dependent lymphocyte activation.1,13 Adverse events related to use of steroids are shown in Table 3. The current practice is to taper steroids early to minimize their side effects.12
Induction agents
-
i)
Polyclonal and monoclonal antibodies: Polyclonal antibodies cause lymphocyte depletion.18 Use of these agents allow delayed introduction of CNI, thus preserving renal function.36 Polyclonal antibodies also have a role in treatment of steroid resistant acute cellular rejection.37 Side effects of polyclonal antibodies include first dose reaction due to pyrogen release,38 thrombocytopenia, anemia, cytomegalovirus infection, post-transplant lymphoproliferative disease, pruritic skin rashes, serum sickness and anaphylaxis.39 Monoclonal antibodies have fewer side effects compared to the antilymphocyte globulins, do not cause first dose reactions and are associated with less risk of opportunistic infections and post transplant lymphoproliferative disease.18 Use of induction agents is far less common in liver transplantation as compared to other solid organ or hematologic transplants as acute cellular rejection is less common with present combined immunosuppression protocols including CNIs and there is no adverse effect of acute cellular rejection on graft survival.
-
ii)
Interleukin-2 receptor antibodies: These agents block the IL2 receptor (CD25) and two formulations are available – basiliximab (Novartis) and daclizumab (Hoffman La Roche). Basiliximab is a chimeric protein (murine variable regions are conjugated to the human immunoglobulin constant region of IgG1) whereas daclizumab is humanized molecule (withdrawn from market recently).1 These antibodies permit delayed introduction of CNIs.40,41 One theoretical concern with their use is elimination of T regulatory cells (which promote tolerance) as these cells also express CD25.42
Other new drugs
Alemtuzumab and Belatacept: Alemtuzumab is anti-CD52, humanized antibody (Campath-1H) that targets human lymphocytes, as well as monocytes, macrophages, natural killer cells and thymocytes. It targets lymphocytes from both the blood and the peripheral lymph nodes with sparing of memory T cells.43 In a study comparing Alemtuzumab induction (55 liver graft recipients) with control group (85 recipients), Levitsky et al found less number of rejection episodes and new onset hypertension at cost of more infections in Alemtuzumab group.44 Belatacept is co-stimulation signal blocker, used in kidney transplantation. It blocks stimulatory signal between CD28 receptor on T cells and CD80 and CD86 on the antigen presenting cell by simulating CTLA-4 activity (CTLA-4 is negative regulator by competing with CD28 for CD80/86 binding).45 A recent abstract showed comparable efficacy of Belatacept as compared to Tacrolimus alone (inferior to Tacrolimus and Mycophenolate combination) with fewer metabolic, neurological and renal side effects.46
Problems with current immunosuppression
Kidney Injury and Immunosuppression
Chronic kidney disease (CKD) is a common problem in liver transplant recipients. In an analysis of 36,849 liver graft recipients, Ojo et al found 18% prevalence of chronic renal failure at 5 years (defined as a glomerular filtration rate ≤ 29 ml per minute per 1.73 m2 body-surface area or end stage renal disease) and 4.5 times higher risk of mortality in patients with renal failure as compared to patients without renal failure.47 Chronic kidney disease can occur in liver transplant recipients either due to pre-transplant kidney disease or more commonly, be an effect of post-transplant immunosuppressant nephrotoxicity. The calcineurin inhibitors (Tacrolimus and Cyclosporine) are cornerstone of maintenance immunosuppression in liver transplantation and CNI free regimens had more chances of rejection as compared to regimens with CNI.48,49 Calcineurin inhibitors are associated with nephrotoxicity which has both acute and chronic components. Acute reduction of GFR by CNIs is caused by afferent arteriolar vasoconstriction which is probably mediated by endothelin, rennin angiotensin aldosterone system and reduced activity of vasodilators. The chronic form of CNI nephrotoxicity is characterized by development of irreversible structural damage—obliterative arteriolopathy, glomerular collapse and scarring, tubular vacuolization, tubular atrophy and interstitial fibrosis, which are probably the long-term consequences of renal hypoxia secondary to renal vasoconstriction.50–52 With concomitant use with CNIs, mTOR inhibitors such as Sirolimus may worsen nephropathy possibly because of inhibition of renal tubular cell proliferation (part of tubular repair) and increase in TGF-β expression in experimental CsA nephrotoxicity. A characteristic cast nephropathy lesion has also been reported with use of mTOR inhibitors.53–55
Renal Sparing Strategies
The various strategies that can be used to halt or improve CNI induced renal changes include reduction or complete withdrawal of CNI, addition of Mycophenolate, initial induction therapy followed by delayed use of CNI and avoiding combination of Sirolimus and CNIs. Besides, one should aim for adequate control of diabetes and hypertension in these patients by use of insulin, calcium channel blockers to counteract renal vasoconstriction and use of angiotensin converting enzyme inhibitors or angiotensin receptors blockers.55,56 As regards conversion to Sirolimus in patients with CNI induced nephrotoxicity, several studies did show better GFR at 3 months following conversion to Sirolimus, but there was no difference between Sirolimus versus low dose CNI arm at 1 year.57–59 Use of Sirolimus is not advised in presence of proteinuria of more than 0.8 g/day because of risk of further worsening of renal function.56 Also, in presence of significant kidney disease (GFR < 40 ml/min), Sirolimus may not be reno-protective and use of low dose CNI and MMF combination is probably the best strategy.56,57,60 Two recently published studies have shown a better glomerular filtration rate in Everolimus group as compared to CNIs.61,62
Metabolic Syndrome (MS)
Metabolic syndrome is defined as a cluster of interconnected factors that directly increase the risk of coronary heart disease, other forms of cardiovascular atherosclerotic diseases and diabetes mellitus type 2.63 Metabolic syndrome (MS) is very common after liver transplantation (43%–58% in various series as compared to a pre-transplant prevalence of 5–29%).64 It has been associated with increased risks of cardiovascular disease, cardiovascular death, liver-related death, and overall mortality.65–67 Among the various causative factors for development of post liver transplant metabolic syndrome, effects of immunosuppression (calcineurin inhibitors, mTOR inhibitors and steroids) are the most important. All these drugs cause hyperglycemia (gluconeogenesis or less peripheral glucose utilization), less insulin production and dyslipidemia.67
Hepatitis C Virus (HCV) Recurrence
Hepatitis C progression is associated with immunosuppression, but the type of CNI used probably does not matter. Steroid boluses are associated with severe recurrence and should be avoided,68 also the current practice include low dose steroids with slow taper. In a meta-analysis, Berenguer et al did not find statistically significant differences between Tacrolimus and Cyclosporine based therapies as regards mortality, graft survival, biopsy proven acute rejection or fibrosing cholestatic hepatitis.69 In the multicenter LIS2T study, Levy et al found no difference between Cyclosporine (250 patients) or Tacrolimus (245 patients) regarding HCV recurrence, although mean time for histological diagnosis of HCV recurrence was significantly longer in the Cyclosporine group.70 Steroid free regimens are not associated with less hepatitis C recurrence as compared to steroids based regimens. However, use of steroid boluses is clearly associated with more severe recurrence and should be avoided.68
Neurological Complications
Neurological complications of liver transplantation are often drug related. Calcineurin inhibitors can cause tremors, encephalopathy, seizures, paresthesias, psychosis, hallucinations and ataxia, while steroids are linked to abnormal behavior and psychosis.71 CNIs cause neurotoxicity by several mechanisms such as cerebral vasoconstriction, interruption of cellular physiologic processes (by binding to immunophilins which are involved in protein folding, transport and stability), toxic effect on oligodendrocytes (white matter changes on imaging) and effect on selective neurotransmission systems (inhibition of GABA, glutaminergic N-methyl-d-aspartate receptor and serotonin depletion).71–73 Treatment of CNI related neurotoxicity includes dose reduction or substituting an alternative CNI. Side effects due to steroids are reversible with dose reduction or cessation of intravenous therapy.71
Immunosuppression and CMV Infection
CMV infection is common after liver transplantation. CMV increases morbidity and mortality by both direct and indirect effects. Its direct effects include fever, myelosuppression, and organ invasive disease (gastrointestinal, hepatitis, central nervous system and pneumonitis). Its indirect effects are related to graft rejection, propensity to other infections and HCV recurrence. Patients with donor positive and recipient negative status, use of biologic agents, rejection and sepsis are at higher risk for developing CMV infection. In presence of active CMV infection, the immunosuppression should be reduced along with instituting specific therapy such as Ganciclovir/Valganciclovir.74,75
Rejection
Acute cellular rejection (ACR) presents with a histological triad of portal tract infiltrates, bile duct injury, and venous endothelialitis,76 typically biopsy proven acute rejection is treated with pulse intravenous steroid boluses. In many cases, mild to moderated rejection can be treated with increasing the levels of CNI alone.77 As compared to Cyclosporine, use of Tacrolimus has been shown to reduce the incidence of both acute cellular rejection and steroid resistant rejection.78 Chronic rejection is characterized by progressive bile duct loss (defined as ductopenia in at-least 50% of portal tracts) and by arteriopathy with foamy cell infiltration (may not be picked up by liver biopsy). Chronic ductopenic rejection is not related to acute cellular rejection and is associated with etiology of liver disease, human leukocyte antigen–matching profiles and CMV infection. As pathogenesis of chronic ductopenic rejection is complex, it responds uncommonly to increase in immunosuppression.77
Development of de novo Malignancies and Post-Transplant Lymphoproliferative Disease
Patients after liver transplantation are at increased risk for development of de novo malignancies and it constitutes second major cause of death after cardiovascular events. Studies have shown incidence of de novo malignancies from 2.3% at 24 months to 12.5% at 93 months follow-up. Malignancies associated with chronic viral infection are more common such as EBV associated post-transplant lymphoproliferative disease (PTLD).79 Also, there is a differential risk of post-transplant malignancies based on pre-transplant cause with alcoholic patients at a greater risk.80 PTLD occurs due to uncontrolled lymphoproliferation of EBV infected cells in immunocompromised individual. The spectrum of PTLD ranges from reactive, polyclonal hyperplasia to high-grade monoclonal lymphoma. Most of PTLD are of B cell in origin and EBV is present in majority.81,82 EBV negative PTLD is associated with later onset, monomorphic histology and aggressive clinical behavior similar to lymphomas in immunocompetent patients.83 Risk factors for PTLD include Epstein–Barr virus seronegativity at transplant, younger age, intensity of immunosuppression and the first year post-transplant. In patients diagnosed with PTLD, management options include reduction of immunosuppression, rituximab, combination chemotherapy, and adoptive immunotherapy.81,82
Steroid Free Immunosuppression
Regimens without steroids are desirable in several situations such as diabetes, hypertension, metabolic syndrome, children and obesity. Present practice includes steroid taper over several months. The available evidence shows feasibility of steroid free immunosuppression but it does not unequivocally establish the benefits of corticosteroid-free immunosuppression for either hepatitis C or other group of patients.84
Disease Specific Approach
Steroids are generally tapered off by three to six months in majority of indications for liver transplant.20 For hepatitis C, role of low dose steroids with slow taper is advocated by some studies85–87 Most centers would continue prolonged low dose steroids in patients transplanted for auto-immune liver diseases.20
Drug Monitoring
To keep drug levels in therapeutic range, trough level monitoring is done conventionally for CNIs and Sirolimus. However, trough levels alone have little clinical utility for assessing the overall immunosuppression status and they need to be seen in the context of clinical background. Also, many centers prefer doing C2 levels (Cyclosporine levels after 2 h of dose) for those on cyclosporine. Monitoring of Mycophenolate level has not gained acceptance due to lack of its clinical utility and high cost.88 The Cylex ImmuKnow assay may provide a global assessment of immune function. This assay is based on the amount of adenosine triphosphate (ATP) produced by CD4þ T cells in response to non–donor-specific mitogenic stimulation (phytohemagglutinin-L) in vitro. This assay may be helpful in assessing over-immunosuppressed state which is a known risk factor for viral hepatitis recurrence and infections following transplantation.89,90
Discontinuation of Immunosuppression
Discontinuation of immunosuppression in stable liver transplant recipients may be possible in almost 20% of recipients, but there is no way to identify these individuals at present. Also, there is no proof that discontinuation of low dose immunosuppression in long-term survivors after liver transplantation leads to lesser morbidity or mortality. With the currently available knowledge and clinical experience, withdrawal of immunosuppression in clinical practice is not recommended.91,92
Conflicts of interest
All authors have none to declare.
References
- 1.Geissler E.K., Schlitt H.J. Immunosuppression for liver transplantation. Gut. 2009;58:452–463. doi: 10.1136/gut.2008.163527. [DOI] [PubMed] [Google Scholar]
- 2.Borel J.F., Feurer C., Gubler H.U., Stahelin H. Biological effects of cyclosporin A: a new antilymphocytic agent. Agents Actions. 1976;6:468–475. doi: 10.1007/BF01973261. [DOI] [PubMed] [Google Scholar]
- 3.Murray K.F., Carithers R.L., Jr., AASLD AASLD practice guidelines: evaluation of the patient for liver transplantation. Hepatology. 2005;41:1407–1432. doi: 10.1002/hep.20704. [DOI] [PubMed] [Google Scholar]
- 4.Saigal S., Shah S.R. Liver transplantation-economics in the less developed world. Indian J Gastroenterol. 2012;31:13–14. doi: 10.1007/s12664-011-0159-8. [DOI] [PubMed] [Google Scholar]
- 5.Wiesner R., Rabkin J., Klintmalm G. A randomized double-blind comparative study of mycophenolate mofetil and azathioprine in combination with cyclosporine and corticosteroids in primary liver transplant recipients. Liver Transpl. 2001;7:442–450. doi: 10.1053/jlts.2001.23356. [DOI] [PubMed] [Google Scholar]
- 6.Navarro V., Herrine S., Katopes C. The effect of HLA class I (A and B) and class II (DR) compatibility on liver transplantation outcomes: an analysis of the OPTN database. Liver Transpl. 2006;12:652–658. doi: 10.1002/lt.20680. [DOI] [PubMed] [Google Scholar]
- 7.Rasmussen A., Davies H.F., Jamieson N.V. Combined transplantation of liver and kidney from the same donor protects the kidney from rejection and improves kidney graft survival. Transplantation. 1995;59:919–921. [PubMed] [Google Scholar]
- 8.Choudhuri K., Wiseman D., Brown M.H., Gould K., van der Merwe P.A. T-cell receptor triggering is critically dependent on the dimensions of its peptide-MHC ligand. Nature. 2005;436:578–582. doi: 10.1038/nature03843. [DOI] [PubMed] [Google Scholar]
- 9.Zheng H., Jin B., Henrickson S.E., Perelson A.S., von Andrian U.H., Chakraborty A.K. How antigen quantity and quality determine T-cell decisions in lymphoid tissue. Mol Cell Biol. 2008;28:4040–4051. doi: 10.1128/MCB.00136-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang D., Matsumoto R., You Y. CD3/CD28 costimulation-induced NF-jB activation is mediated by recruitment of protein kinase C-y, Bcl10, and IjB kinase b to the immunological synapse through CARMA1. Mol Cell Biol. 2004;24:164–171. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez O.M., Rosen H.R. Basic concepts in transplant immunology. Liver Transpl. 2005;11:370–381. doi: 10.1002/lt.20406. [DOI] [PubMed] [Google Scholar]
- 12.Wiesner R.H., Fung J.J. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transpl. 2011;17:S1–S9. doi: 10.1002/lt.22410. [DOI] [PubMed] [Google Scholar]
- 13.Taylor A.L., Watson C.J., Bradley J.A. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23–46. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 14.Pichlmayr R., Winkler M., Neuhaus P. Three-year follow-up of the European Multicenter Tacrolimus (FK506) Liver Study. Transplant Proc. 1997;29:2499–2502. doi: 10.1016/s0041-1345(97)00464-8. [DOI] [PubMed] [Google Scholar]
- 15.Wiesner R.H. A long-term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation. 1998;66:493–499. doi: 10.1097/00007890-199808270-00014. [DOI] [PubMed] [Google Scholar]
- 16.O'Grady J.G., Burroughs A., Hardy P., Elbourne D., Truesdale A. Tacrolimus versus microemulsifiedciclosporin in liver transplantation: the TMC randomised controlled trial. Lancet. 2002;360:1119–1125. doi: 10.1016/s0140-6736(02)11196-2. [DOI] [PubMed] [Google Scholar]
- 17.Levy G., Villamil F., Samuel D. Results of lis2t, a multicenter, randomized study comparing cyclosporine microemulsion with C2 monitoring and tacrolimus with C0 monitoring in de novo liver transplantation. Transplantation. 2004;77:1632–1638. doi: 10.1097/01.tp.0000129095.51031.42. [DOI] [PubMed] [Google Scholar]
- 18.Pillai A.A., Levitsky J. Overview of immunosuppression in liver transplantation. World J Gastroenterol. 2009;15:4225–4233. doi: 10.3748/wjg.15.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muir A.J. Immunosuppressive medications. In: Killenberg P.G., Clavien P.A., editors. Medical Care of the Liver Transplant Patient. 10th ed. Blackwell Publishing Ltd; Malden, Massachusetts: 2007. pp. 505–521. [Google Scholar]
- 20.Menon K.R., Wiesner R.H. Immunosuppression: the global picture. In: Schiff E.R., Sorrell M.F., Maddrey W.C., editors. Schiff's Diseases of the Liver. 10th ed. Lippincott Williams and Wilkins; Philadelphia: 2007. pp. 1467–1477. [Google Scholar]
- 21.Barkmann A., Nashan B., Schmidt H.H. Improvement of acute and chronic renal dysfunction in liver transplant patients after substitution of calcineurin inhibitors by mycophenolate mofetil. Transplantation. 2000;69:1886–1890. doi: 10.1097/00007890-200005150-00025. [DOI] [PubMed] [Google Scholar]
- 22.Stewart S.F., Hudson M., Talbot D., Manas D., Day C.P. Mycophenolate mofetil monotherapy in liver transplantation. Lancet. 2001;357:609–610. doi: 10.1016/s0140-6736(00)04065-4. [DOI] [PubMed] [Google Scholar]
- 23.Dharancy S., Iannelli A., Hulin A. Mycophenolate mofetil monotherapy for severe side effects of calcineurin inhibitors following liver transplantation. Am J Transplant. 2009;9:610–613. doi: 10.1111/j.1600-6143.2008.02529.x. [DOI] [PubMed] [Google Scholar]
- 24.Sollinger H.W. Mycophenolates in transplantation. Clin Transplant. 2004;18:485–492. doi: 10.1111/j.1399-0012.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 25.Mita M.M., Mita A., Rowinsky E.K. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol Ther. 2003;2:S169–S177. [PubMed] [Google Scholar]
- 26.Watson C.J., Friend P.J., Jamieson N.V. Sirolimus: a potent new immunosuppressant for liver transplantation. Transplantation. 1999;67:505–509. doi: 10.1097/00007890-199902270-00002. [DOI] [PubMed] [Google Scholar]
- 27.McAlister V.C., Peltekian K.M., Malatjalian D.A. Orthotopic liver transplantation using low-dose tacrolimus and sirolimus. Liver Transpl. 2001;7:701–708. doi: 10.1053/jlts.2001.26510. [DOI] [PubMed] [Google Scholar]
- 28.Liang W., Wang D., Ling X. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012;18:62–69. doi: 10.1002/lt.22441. [DOI] [PubMed] [Google Scholar]
- 29.Kniepeiss D., Iberer F., Grasser B., Schaffellner S., Tscheliessnigg K.H. Sirolimus and mycophenolate mofetil after liver transplantation. Transpl Int. 2003;16:504–509. doi: 10.1007/s00147-003-0579-1. [DOI] [PubMed] [Google Scholar]
- 30.Fairbanks K.D., Eustace J.A., Fine D., Thuluvath P.J. Renal function improves in liver transplant recipients when switched from a calcineurin inhibitor to sirolimus. Liver Transpl. 2003;9:1079–1085. doi: 10.1053/jlts.2003.50183. [DOI] [PubMed] [Google Scholar]
- 31.Nair S., Eason J., Loss G. Sirolimus monotherapy in nephrotoxicity due to calcineurin inhibitors in liver transplant recipients. Liver Transpl. 2003;9:126–129. doi: 10.1053/jlts.2003.50026. [DOI] [PubMed] [Google Scholar]
- 32.Bumbea V., Kamar N., Ribes D. Long term results in renal transplant patients with allograft dysfunction after switching from calcineurin inhibitors to sirolimus. Nephrol Dial Transplant. 2005;20:2517–2523. doi: 10.1093/ndt/gfh957. [DOI] [PubMed] [Google Scholar]
- 33.Diekmann F., Gutierrez-Dalmau A., Lopez S. Influence of sirolimus on proteinuria in de novo kidney transplantation with expanded criteria donors: comparison of two CNI-free protocols. Nephrol Dial Transplant. 2007;22:2316–2321. doi: 10.1093/ndt/gfm181. [DOI] [PubMed] [Google Scholar]
- 34.Dean P.G., Lund W.J., Larson T.S. Wound-healing complications after kidney transplantation: a prospective, randomized comparison of sirolimus and tacrolimus. Transplantation. 2004;77:1555–1561. doi: 10.1097/01.tp.0000123082.31092.53. [DOI] [PubMed] [Google Scholar]
- 35.Guertin D.A., Sabatini D.M. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Soliman T., Hetz H., Burghuber C. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transpl. 2007;13:1039–1044. doi: 10.1002/lt.21185. [DOI] [PubMed] [Google Scholar]
- 37.Midtvedt K., Fauchald P., Lien B. Individualized T cell monitored administration of ATG versus OKT3 in steroid resistant kidney graft rejection. Clin Transplant. 2003;17:69–74. doi: 10.1034/j.1399-0012.2003.02105.x. [DOI] [PubMed] [Google Scholar]
- 38.Guttmann R.D., Caudrelier P., Alberici G., Touraine J.L. Pharmacokinetics, foreign protein immune response, cytokine release, and lymphocyte subsets in patients receiving thymoglobulin and immunosuppression. Transplant Proc. 1997;29:24S–26S. [PubMed] [Google Scholar]
- 39.Ducloux D., Kazory A., Challier B. Long-term toxicity of antithymocyte globulin induction may vary with choice of agent: a single-center retrospective study. Transplantation. 2004;77:1029–1033. doi: 10.1097/01.tp.0000116442.81259.60. [DOI] [PubMed] [Google Scholar]
- 40.Liu C.L., Fan S.T., Lo C.M. Interleukin-2 receptor antibody (basiliximab) for immunosuppressive induction therapy after liver transplantation: a protocol with early elimination of steroids and reduction of tacrolimus dosage. Liver Transpl. 2004;10:728–733. doi: 10.1002/lt.20144. [DOI] [PubMed] [Google Scholar]
- 41.Ramirez C.B., Doria C., Di Francesco F. Anti-IL2 induction in liver transplantation with 93% rejection-free patient and graft survival at 18 months. J Surg Res. 2007;138:198–204. doi: 10.1016/j.jss.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 42.Li W., Kuhr C.S., Zheng X.X. New insights into mechanisms of spontaneous liver transplant tolerance: the role of Foxp3-expressing CD25 + CD4 + regulatory T cells. Am J Transplant. 2008;8:1639–1651. doi: 10.1111/j.1600-6143.2008.02300.x. [DOI] [PubMed] [Google Scholar]
- 43.Magliocca J.F., Knechtle S.J. The evolving role of alemtuzumab (Campath-1H) for immunosuppressive therapy in organ transplantation. Transpl Int. 2006;19:705–714. doi: 10.1111/j.1432-2277.2006.00343.x. [DOI] [PubMed] [Google Scholar]
- 44.Levitsky J., Thudi K., Ison M.G., Wang E., Abecassis M. Alemtuzumab induction in non-hepatitis C positive liver transplant recipients. Liver Transpl. 2011;17:32–37. doi: 10.1002/lt.22180. [DOI] [PubMed] [Google Scholar]
- 45.Vincenti F., Larsen C., Durrbach A. Costimulation blockade with belatacept in renal transplantation. N Engl J Med. 2005;353:770–781. doi: 10.1056/NEJMoa050085. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Valdecasas J.C., Feng S., Lake J.R. Belatacept based immunosuppression in de novo liver transplant recipients: 1-year experience from a phase II study. Liver Transpl. 2011;17(suppl 1):S79. doi: 10.1111/ajt.12810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ojo A.O., Held P.J., Port F.K. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931–940. doi: 10.1056/NEJMoa021744. [DOI] [PubMed] [Google Scholar]
- 48.Schlitt H.J., Barkmann A., Boker K.H. Replacement of calcineurin inhibitors with mycophenolate mofetil in liver-transplant patients with renal dysfunction: a randomised controlled study. Lancet. 2001;357:587–591. doi: 10.1016/s0140-6736(00)04055-1. [DOI] [PubMed] [Google Scholar]
- 49.Watson C.J., Gimson A.E., Alexander G.J. A randomized controlled trial of late conversion from calcineurin inhibitor-based to sirolimus-based immunosuppression in liver transplant recipients with impaired renal function. Liver Transpl. 2007;13:1694–1702. doi: 10.1002/lt.21314. [DOI] [PubMed] [Google Scholar]
- 50.Naesens M., Kuypers D.R.J., Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 51.Shihab F.S., Andoh T.F., Tanner A.M., Yi H., Bennett W.M. Expression of apoptosis regulatory genes in chronic cyclosporine nephrotoxicity favors apoptosis. Kidney Int. 1999;56:2147–2159. doi: 10.1046/j.1523-1755.1999.00794.x. [DOI] [PubMed] [Google Scholar]
- 52.Wolf G. Renal injury due to renin-angiotensin-aldosterone system activation of the transforming growth factor-beta pathway. Kidney Int. 2006;70:1914–1919. doi: 10.1038/sj.ki.5001846. [DOI] [PubMed] [Google Scholar]
- 53.Anglicheau D., Pallet N., Rabant M. Role of P-glycoprotein in cyclosporine cytotoxicity in the cyclosporine–sirolimus interaction. Kidney Int. 2006;70:1019–1025. doi: 10.1038/sj.ki.5001649. [DOI] [PubMed] [Google Scholar]
- 54.Shihab F.S., Bennett W.M., Yi H., Choi S.O., Andoh T.F. Sirolimus increases transforming growth factor-beta1 expression and potentiates chronic cyclosporine nephrotoxicity. Kidney Int. 2004;65:1262–1271. doi: 10.1111/j.1523-1755.2004.00498.x. [DOI] [PubMed] [Google Scholar]
- 55.Charlton M.R., Wall W.J., Ojo A.O. Report of the first international liver transplantation society expert panel consensus conference on renal insufficiency in liver transplantation. Liver Transpl. 2009;15:S1–34. doi: 10.1002/lt.21877. [DOI] [PubMed] [Google Scholar]
- 56.Duvoux C., Pageaux G.P. Immunosuppression in liver transplant recipients with renal impairment. J Hepatol. 2011;54:1041–1054. doi: 10.1016/j.jhep.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 57.DuBay D., Smith R.J., Qiu K.G., Levy G.A., Lilly L., Therapondos G. Sirolimus in liver transplant recipients with renal dysfunction offers no advantage over low-dose calcineurin inhibitor regimens. Liver Transpl. 2008;14:651–659. doi: 10.1002/lt.21429. [DOI] [PubMed] [Google Scholar]
- 58.Shenoy S., Hardinger K.L., Crippin J. Sirolimus conversion in liver transplant recipients with renal dysfunction: a prospective, randomized, single-center trial. Transplantation. 2007;83:1389–1392. doi: 10.1097/01.tp.0000261630.63550.41. [DOI] [PubMed] [Google Scholar]
- 59.Campbell M.S., Rai J., Kozin E. Effects of sirolimus vs. calcineurin inhibitors on renal dysfunction after orthotopic liver transplantation. Clin Transplant. 2007;21:377–384. doi: 10.1111/j.1399-0012.2006.00653.x. [DOI] [PubMed] [Google Scholar]
- 60.Schena F.P., Pascoe M.D., Alberu J., Sirolimus CONVERT Trial Study Group Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation. 2009;87:233–242. doi: 10.1097/TP.0b013e3181927a41. [DOI] [PubMed] [Google Scholar]
- 61.De Simone P., Nevens F., De Carlis L. Everolimus with reduced tacrolimus improves renal function in de novo liver transplant recipients: a randomized controlled trial. Am J Transplant. 2012;12:3008–3020. doi: 10.1111/j.1600-6143.2012.04212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fischer L., Klempnauer J., Beckebaum S. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation–PROTECT. Am J Transplant. 2012;12:1855–1865. doi: 10.1111/j.1600-6143.2012.04049.x. [DOI] [PubMed] [Google Scholar]
- 63.Kassi E., Pervanidou P., Kaltasa G., Chrousos G. Metabolic syndrome: definitions and controversies. BMC Med. 2011;9:48. doi: 10.1186/1741-7015-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tellez-Avila F.I., Sanchez-Avila F., Garcia-Saenz-de-Sicilia M. Prevalence of metabolic syndrome, obesity and diabetes type 2 in cryptogenic cirrhosis. World J Gastroenterol. 2008;14:4771–4775. doi: 10.3748/wjg.14.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Motillo S., Filion K.B., Genest J. The metabolic syndrome and cardiovascular risk a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56:1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 66.Pagdala M., Dasarathy S., Eghtesad B., McCullough A.J. Post transplant metabolic syndrome: an epidemic waiting to happen. Liver Transpl. 2009;15:1662–1670. doi: 10.1002/lt.21952. [DOI] [PubMed] [Google Scholar]
- 67.Watt K.D. Metabolic syndrome: is immunosuppression to blame? Liver Transpl. 2011;(suppl 3):S38–S42. doi: 10.1002/lt.22386. [DOI] [PubMed] [Google Scholar]
- 68.Berenguer M. Hot topic in hepatitis C virus research: the type of immunosuppression does not matter. Liver Transpl. 2011;17:S24–S28. doi: 10.1002/lt.22347. [DOI] [PubMed] [Google Scholar]
- 69.Berenguer M., Royuela A., Zamora J. Immunosuppression with calcineurin inhibitors with respect to the outcome of HCV recurrence after liver transplantation: results of a meta-analysis. Liver Transpl. 2007;13:21–29. doi: 10.1002/lt.21035. [DOI] [PubMed] [Google Scholar]
- 70.Levy G., Grazi G.L., Sanjuan F. 12-month follow-up analysis of a multicenter, randomized, prospective trial in de novo liver transplant recipients (LIS2T) comparing cyclosporine microemulsion (C2 monitoring) and tacrolimus. Liver Transpl. 2006;12:1464–1472. doi: 10.1002/lt.20802. [DOI] [PubMed] [Google Scholar]
- 71.Senzolo M., Ferronato C., Burra P. Neurologic complications after solid organ transplantation. Transpl Int. 2009;22:269–278. doi: 10.1111/j.1432-2277.2008.00780.x. [DOI] [PubMed] [Google Scholar]
- 72.Dawson F.M. Immunosoppressants, immunophilins and the nervous system. Ann Neurol. 1996;40:559. doi: 10.1002/ana.410400403. [DOI] [PubMed] [Google Scholar]
- 73.Stoltenburg-Didinger G., Boegner F. Glia toxicity in dissociates cell cultures induced by cyclosporine. Neurotoxicology. 1992;13:179. [PubMed] [Google Scholar]
- 74.Razonable R.R. Cytomegalovirus infection after liver transplantation: current concepts and challenges. World J Gastroenterol. 2008;14:4849–4860. doi: 10.3748/wjg.14.4849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lautenschlager I. CMV infection, diagnosis and antiviral strategies after liver transplantation. Transpl Int. 2009;22:1031–1040. doi: 10.1111/j.1432-2277.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- 76.Neil D.A., Hübscher S.G. Current views on rejection pathology in liver transplantation. Transpl Int. 2010;23:971–983. doi: 10.1111/j.1432-2277.2010.01143.x. [DOI] [PubMed] [Google Scholar]
- 77.O’Grady J.G. The immunoreactive patient: rejection and autoimmune disease. Liver Transpl. 2011;17:S29–S33. doi: 10.1002/lt.22413. [DOI] [PubMed] [Google Scholar]
- 78.Haddad E.M., McAlister V.C., Reouf E. Cyclosporine versus tacrolimus for liver transplanted patients. Cochrane Database Syst Rev. 2006 doi: 10.1002/14651858.CD005161.pub2. CD005161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fung J.J., Jain A., Kwak E.J., Kusne S., Dvorchik I., Eghtesad B. De novo malignancies after liver transplantation: a major cause of late death. Liver Transpl. 2001;7:S109–S118. doi: 10.1053/jlts.2001.28645. [DOI] [PubMed] [Google Scholar]
- 80.Saigal S., Norris S., Muiesan P., Rela M., Heaton N., O'Grady J. Evidence of differential risk for posttransplantation malignancy based on pretransplantation cause in patients undergoing liver transplantation. Liver Transpl. 2002;8:482–487. doi: 10.1053/jlts.2002.32977. [DOI] [PubMed] [Google Scholar]
- 81.Allen U., Alfieri C., Preiksaitis J. Epstein–Barr virus infection in transplant recipients: summary of a workshop on surveillance, prevention and treatment. Can J Infect Dis. 2002;13:89–99. doi: 10.1155/2002/634318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kamdar K.Y., Rooney C.M., Heslop H.E. Post-transplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant. 2011;16:274–280. doi: 10.1097/MOT.0b013e3283465715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dotti G., Fiocchi R., Motta T. Epstein-Barr virus-negative lymphoproliferate disorders in long term survivors after heart, kidney, and liver transplant. Transplantation. 2000;69:827–833. doi: 10.1097/00007890-200003150-00027. [DOI] [PubMed] [Google Scholar]
- 84.O'Grady J.G. Corticosteroid-free immunosuppression in liver transplantation. Trends Transpl. 2009;3:77–84. [Google Scholar]
- 85.Brillanti S., Vivarelli M., De Ruvo N. Slowly tapering off steroids protects the graft against hepatitis C recurrence after liver transplantation. Liver Transpl. 2002;8:884–888. doi: 10.1053/jlts.2002.34640. [DOI] [PubMed] [Google Scholar]
- 86.Berenguer M., Aguilera V., Prieto M. Significant improvement in the outcome of HCV-infected transplant recipients by avoiding rapid steroid tapering and potent induction immunosuppression. J Hepatol. 2006;44:717–722. doi: 10.1016/j.jhep.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 87.Vivarelli M., Burra P., La Barba G. Influence of steroids on HCV recurrence after liver transplantation: a prospective study. J Hepatol. 2007;47:793–798. doi: 10.1016/j.jhep.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 88.Levitsky Josh. Next level of immunosuppression: drug/Immune monitoring. Liver Transpl. 2011;17:S60–S65. doi: 10.1002/lt.22385. [DOI] [PubMed] [Google Scholar]
- 89.Kowalski R.J., Post D.R., Mannon R.B. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation. 2006;82:663–668. doi: 10.1097/01.tp.0000234837.02126.70. [DOI] [PubMed] [Google Scholar]
- 90.Xue F., Zhang J., Han L. Immune cell functional assay in monitoring of adult liver transplantation recipients with infection. Transplantation. 2010;89:620–626. doi: 10.1097/TP.0b013e3181c690fa. [DOI] [PubMed] [Google Scholar]
- 91.Sánchez-Fueyo A. Hot-topic debate on tolerance: immunosuppression withdrawal. Liver Transpl. 2011;17:S69–S73. doi: 10.1002/lt.22421. [DOI] [PubMed] [Google Scholar]
- 92.Porrett P., Shaked A. The failure of immunosuppression withdrawal: patient benefit is not detectable, inducible, or reproducible. Liver Transpl. 2011;17:S66–S68. doi: 10.1002/lt.22377. [DOI] [PubMed] [Google Scholar]


