Abstract
Purpose
In vitro disintegration and dissolution are routine methods used to assess the performance and quality of oral dosage forms. The purpose of the current work was to determine the potential for interaction between capsule shell material and a green tea extract and the impact it can have on the release.
Methods
A green tea extract was formulated into simple powder-in-capsule formulations of which the capsule shell material was either of gelatin or HPMC origin. The disintegration times were determined together with the dissolution profiles in compendial and biorelevant media.
Results
All formulations disintegrated within 30 min, meeting the USP criteria for botanical formulations. An immediate release dissolution profile was achieved for gelatin capsules in all media but not for the specified HPMC formulations. Dissolution release was especially impaired for HPMCgell at pH 1.2 and for both HPMC formulations in FeSSIF media suggesting the potential for food interactions.
Conclusions
The delayed release from studied HPMC capsule materials is likely attributed to an interaction between the catechins, the major constituents of the green tea extract, and the capsule shell material. An assessment of in vitro dissolution is recommended prior to the release of a dietary supplement or clinical trial investigational product to ensure efficacy.
Keywords: Formulation, In vitro dissolution, Disintegration, Green tea extract, Hard shell capsule
Abbreviations: BA, bioavailability; BCS, biopharmaceutical classification system; C, catechin; DS, dietary supplement; EC, epicatechin; ECG, epicatechin gallate; EGCG, epigallocatechin gallate; EGC, epigallocatechin; FaSSIF, fasted state simulated intestinal fluid; FeSSIF, fed state simulated intestinal fluid; GA, gallic acid; GTE, green tea extract; HPMC, hydroxypropyl methylcellulose; HPMCcarr, hydroxypropyl methylcellulose containing carrageenan; HPMCgell, hydroxypropyl methylcellulose containing gellan gum; IR, immediate release; PIC, powder-in-capsule; SIF, simulated intestinal fluid; USP, United States Pharmacopeia
Graphical Abstract

1. Introduction
To date (assessed mid 2013) there are a total of 135 clinical trials registered with clinicaltrials.gov in which a green tea extract (GTE) has been used as an investigational product (search criteria: “green tea extract”) and 44 of these trials appear under the search “green tea extract capsules”. However, scarce public data exist on the quality of these formulations with regard to meeting in vitro disintegration and dissolution criteria and hence potential in vivo performance. The two aforementioned methods are common measures of product performance. There is increasing evidence that green and black tea consumption has beneficial health effects; such as reducing the risk for cardiovascular diseases [1], supporting weight loss [2] and preventing certain types of cancer [3]. These benefits have led to the inclusion and marketing of tea extracts in the form of dietary supplements (DS) and functional foods. The beneficial effects, e.g. anti-inflammatory, anti-oxidative, are most likely associated with the high abundance of bioactive molecules present in green and black tea, such as polyphenols and more specifically the catechins [1,4]. Though numerous DS containing GTE are commercially available, data on their actual in vitro and in vivo performance and hence efficacy are scarce. This is partly attributed to the fact that DS are not required by regulatory bodies to undergo the same stringent testing procedures as pharmaceutical formulations before they can be marketed. Therefore, unless the manufacturer makes a label claim, supplements can be marketed on the basis of safety data only. However, the same factors affecting the bioavailability (BA) and efficacy of drugs also apply to DS and hence proper formulation design and testing is a crucial step in the development of an efficacious and safe DS. The desired effect and hence dissolution profile will determine the formulation requirements e.g. immediate release (IR) if an acute benefit is desired vs. controlled release for long-term or delayed effects etc. [5]. This paper will focus on GTE powder-in-capsule (PIC) formulations intended for IR (the release of the active is not deliberately modified by a special manufacturing method or formulation design e.g. no addition of functional excipients).
Proper formulation of herbal/botanical extracts into an oral dosage form is not only critical for producing a high quality market-ready product, but also in the “research and development phases” of new functional food products. The preferred way to explore the efficacy of lead ingredient(s) is via proof-of-principle clinical intervention studies. In these early stages, clinical trials commonly employ simple standardized oral formulations of the active ingredients, such as hard-shell filled capsules. Hence, the quality and performance of such a test formulation will greatly impact the outcome of the clinical investigations and the results of these human interventions are pivotal in building a claims dossier for functional food ingredients. The outcome of human intervention studies also helps determine whether a lead ingredient will be further developed or discontinued. As mentioned earlier, PIC formulations are often the preferred choice due to their ease of formulation, the assumed reduced implications regarding stability and BA of the active ingredient(s) and volunteer/consumer compliance. Until recently gelatin has been the preferred material for capsule shells due to its gel forming characteristics and its excellent solubility in biological fluids [6], but more recently capsules made from hydroxypropyl methylcellulose (HPMC) have been introduced as an attractive vegetarian alternative. Various kinds of HPMC capsules are currently available on the market, differing mainly in whether or not a gelling agent such as carrageenan or gellan gum is added to enhance the gelation process [6]. While HPMC is known to impact the dissolution, e.g. it serves as a matrix for use in extended release tablet formulations, the potential interaction of HPMC as a capsule shell material with a botanical filling material such as polyphenols and characterization of the subsequent dissolution of such formulations has not been explained in the literature.
Even though catechins are readily soluble in the gastrointestinal fluids, their limited absorption, rapid and variable metabolism and active efflux from the enterocytes impair their BA and efficacy [7,8]. Additionally, the BA is complicated by the presence of food which can enhance or impair the absorption of the individual catechins [9]. Hence, it is critical that formulation errors do not further contribute to limitations in BA of the active(s), as the rate and extent of release from the dosage form is critical to achieve the desired benefits. Since polyphenols are known to potentially interact with certain compound classes such as proteins or cellulose derivatives [10], it is of great interest to investigate the release properties of catechins when formulated into different hard shell capsules such as gelatin or HPMC.
2. Objective
The aim of this work was to design and test three simple PIC GTE formulations, typical for use as a DS or clinical trial investigational product. As it is often assumed that there is no impact/limitation on the dissolution of the capsule contents of such formulations, IR dissolution criteria were applied. The disintegration and dissolution profiles of a commercially available GTE formulated into various capsules of gelatin or HPMC origin were tested in both compendial and biorelevant media to determine the potential for food interactions. The intent of the results presented here is to address issues of formulation and the potential for interaction between GTE ingredients and capsule shell materials.
3. Material and Methods
3.1. Materials
Hydrochloric acid (37%), glacial acetic acid (100%) and acetonitrile were obtained from VWR (Briare, France). Mono-potassium phosphate and sodium hydroxide were purchased from Sigma-Aldrich (Steinheim, Germany) and sodium acetate trihydrate was obtained from Riedel-de Haën (Seelze, Germany). Simulated intestinal fluid 148 (SIF) powder was purchased from Phares AG (Muttenz, Switzerland). Sunphenon 90 DCF-T (Lot 003191) was kindly donated by Taiyo Europe (Fiderstadt, Germany); the composition of the extract is shown in Table 1. Gelatin capsules, HPMCgell (“Vcaps”: HPMC; with gellan gum as gelling agent) and HPMC (“Vcaps Plus”: pure HPMC; no gelling agent), were obtained from Capsugel (Bornem, Belgium).
Table 1.
Composition of the green tea extract as determined by HPLC. GA = gallic acid, EGC = epigallocatechin, C = catechin, EC = epicatechin, EGCG = epigallocatechin gallate, GCG = gallocatechin gallate, ECG = epicatechin gallate.
| Component | mg/g Green tea extract |
|---|---|
| GA | 1.5 |
| EGC | 2.5 |
| C | 7.8 |
| Caffeine | 8.1 |
| EC | 59.8 |
| EGCG | 523.9 |
| GCG | 15.8 |
| ECG | 164.9 |
| Total catechins | 784.3 |
3.2. Formulations
The three different formulations tested in this study were (a) formulation “Gelatin”: 260 mg Sunphenon 90DCF-T in gelatin capsules, (b) formulation “HPMCgell”: 260 mg Sunphenon-90DCF-T in HPMC capsules with gellan gum as the gelling agent and (c) formulation “HPMC”: 260 mg Sunphenon-90DCF-T in HPMC capsules without gelling agent. All capsules were size 0 and transparent. The formulations were prepared manually using a capsule filling machine (Capsunorm 2000, Tecnyfarma, Barcelona, Spain).
3.3. Composition of compendial and biorelevant media
The compendial media – 0.1 mol/l hydrochloric acid (pH 1.2), acetate buffer (pH 4.5) and phosphate buffer (pH 6.8) – were prepared according to USP 32. Fasted state simulated intestinal fluid (FaSSIF) and fed state simulated intestinal fluid (FeSSIF) were prepared from simulated intestinal fluid (SIF) powder (Biorelevant.com, Croydon, Surrey, UK) [11].
3.4. Disintegration testing
Capsule disintegration was tested according to USP 32 chapter <2040> with a disintegration tester ZT120 and tube/rack assembly Apparatus B (Erweka GmbH, Heusenstamm, Germany). Chapter <2040> also discusses the acceptance criteria for dietary supplements. The test for hard shell capsules was applied and as the USP advises to omit the use of discs for botanical dosage forms; capsules were placed in a metal spiral capsule sinker (ProSense BV Dissolution Accessories, Oosterhout, the Netherlands) to prevent floating which is a slight modification of the description in <2040>. This modification avoids the mechanical impact discs during each stroke and at the same time keeps the capsules submerged to ensure ample fluid contact. The recorded capsule disintegration time is the time at which the capsule was visually observed to be completely disintegrated, even if some pieces of the capsule shell remained on the mesh of the test basket. Disintegration of the formulations was assessed in two immersion fluids: the USP recommended 0.05 mol/l acetate buffer as well as in demineralised water, both preheated to 37 °C. All experiments were performed as n = 6 and the mean and standard deviation were calculated.
3.5. Dissolution testing
A calibrated dissolution tester “VK 1700” (Varian Inc., Cary NC, USA) was used for all dissolution studies. The formulations were tested using the paddle method plus sinker (USP Apparatus 2), employing 900 ml of dissolution medium equilibrated to 37 ± 0.5 °C and a rotational speed of 75 rpm. Spiral capsule sinkers (ProSense BV Dissolution Accessories, Oosterhout, the Netherlands) were used to prevent capsules from floating. Samples were taken after 5, 10, 20, 30, 45, 60 and 120 min by withdrawal of 2 ml at each sampling point and the volume withdrawn was replaced with fresh pre-warmed medium. Each sample was immediately filtered through a 0.2 μm PVDF filter (Type Acrodisc LC, Pall Life Sciences, Uithoorn, The Netherlands) and directly analyzed by HPLC and/or appropriately diluted with buffer media prior to UV–vis spectrophotometer analysis. Dissolution of all three formulations was assessed using compendial media at pH 1.2 (0.1 mol/l HCl), pH 4.5 (acetate buffer) and pH 6.8 (phosphate buffer) and the experiments were performed as n = 6. HPMCgell and HPMC formulations were additionally tested in FaSSIF and FeSSIF (n = 3) due to the limitations observed in compendial media. Based on FDA guidelines, a formulation was considered to meet IR criteria if no less than 85% of the capsule content was released after 30 min [12].
3.6. Sample analysis
For the experiments performed at pH 1.2 and 4.5, the concentration of GTE released was recorded spectrophotometrically at the maximum absorbance wavelength of 274 nm on a UV–vis spectrophotometer “UV 1601” (Shimadzu, Duisburg, Germany). In both buffer solutions the maximal phenolic absorption (representative of all phenols present in the extract) was determined to be at 274 nm, hence absorption values of samples at that wavelength were used to calculate the percentage of active released. All standard curves were prepared in the respective buffers and analyzed at the same wavelength.
3.7. HPLC analyses
To confirm the results obtained by the UV–vis spectrophotometric analysis, one of the six dissolution replicates was further analyzed by HPLC employing the same method as earlier described by Wang et al. [13]. All HPLC analyses were carried out with a Shimadzu class LC VP HPLC system with LC-Solution software (Shimadzu Corporation, Kyoto, Japan). A dissolution profile was generated for six individual catechins present in the GTE: gallic acid (GA, PubChem CID: 370), epicatechin (EC, PubChem CID: 72276), epigallocatechin gallate (EGCG, PubChem CID: 65064), epicatechin gallate (ECG, PubChem CID: 107905), catechin (C, PubChem CID: 9064) and epigallocatechin (EGC, PubChem CID: 107905), as well as for the sum of all catechins and compared to the dissolution profile from the UV-spectrophotometer analysis. The results from both analyses were in excellent agreement with a mean deviation of 4.2 ± 4.8%. Hence, UV-analysis was considered appropriate for all further analyses with regard to pH 1.2 and 4.5. Due to sample instability in phosphate buffer (pH 6.8), FaSSIF and FeSSIF media, samples from those experiments were analyzed by HPLC and the dissolution profile was generated using EC as marker compound, since EC remained intact during the entire time of the analysis (stability data not shown).
4. Results
4.1. Disintegration
In both media employed – acetate buffer (pH 4.5) as well as de-mineralized water – the three formulations investigated released their content within 30 min, meeting the USP criteria for disintegration of DS. The gelatin capsules optimally disintegrated as nothing remained in the sinker or on the mesh of the basket rack assembly at the end of the test. The capsule content was also released from the two HPMC formulations, however, part of the capsule shell remained on the mesh (HPMCgell) and/or within the sinker (HPMC) after 30 min and the mean disintegration time was considerably higher compared to the gelatin capsules, see Figs. 1 and 2.
Fig. 1.
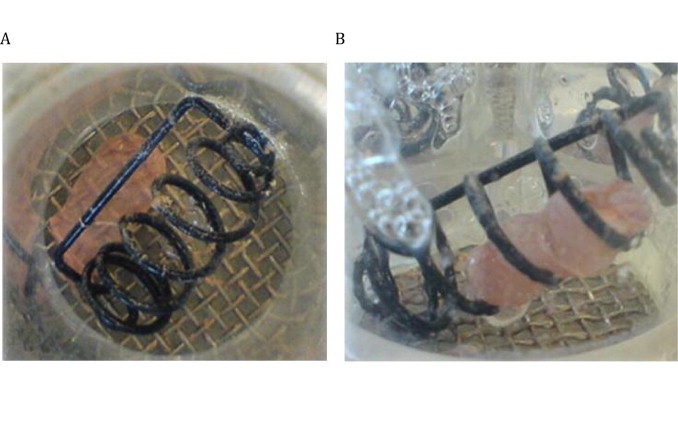
Disintegration test: HPMCgell and HPMC capsules filled with green tea extract, in acetate buffer after 30 min, (A) HPMCgell and (B) HPMC
Fig. 2.
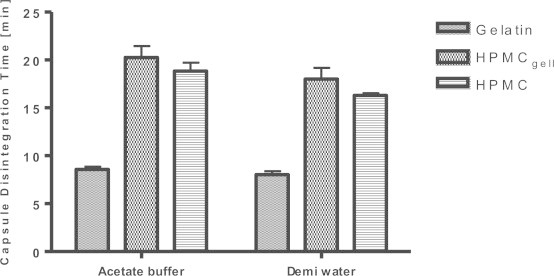
Capsule disintegration times for gelatin, HPMCgell and HPMC capsules filled with green tea extract in acetate buffer (pH 4.5) and de-mineralized water. Bars represent SD, n = 6.
4.2. Dissolution
The amounts of GTE dissolved over time in the five media employed are summarized in Table 1.
Dissolution at pH 1.2: The gelatin formulation disintegrated and dissolved rapidly, achieving complete dissolution of the active within 10 min, see Fig. 3. No residues of the capsule shell remained in the sinker at the end of the experiment after 2 h. Both HPMC formulations showed incomplete dissolution profiles. The release from the HPMC formulation was hampered and only reached a maximum release of 69% after 2 h. The HPMCgell formulation was more significantly delayed with content release beginning after 1 h and reaching a maximum release of 35% after 2 h.
Fig. 3.
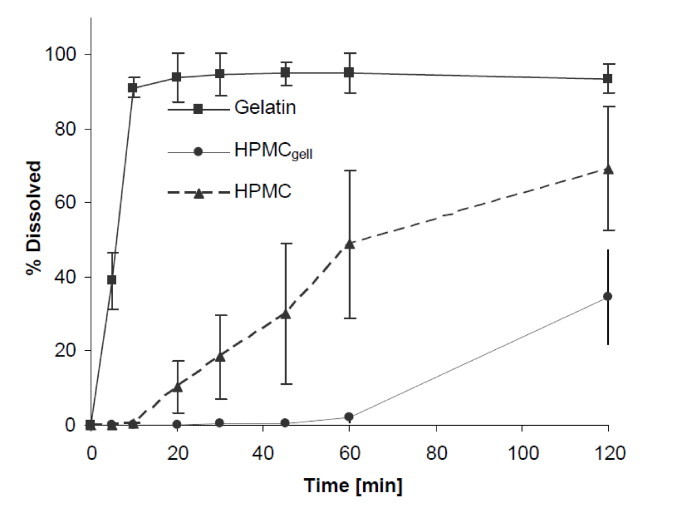
Dissolution profiles showing the mean percentage of green tea extract (GTE) released from gelatin, HPMCgell and HPMC capsules in 0.1 M HCl buffer at pH 1.2, 37 °C, 75 rpm, 2 h. Bars represent SD, n = 6.
Dissolution pH 4.5: Similar to pH 1.2, fast and complete dissolution was achieved for the gelatin formulation. As shown in Fig. 4, HPMC and HPMCgell showed a delayed release of the active and after 30 min dissolution values were 32% and 18%, respectively. At the end of the experiments with the gelatin formulation, some gluey gelatin residues adhered to the sinkers and for both HPMC formulations intact parts of the capsule shell were associated in the sinker.
Fig. 4.
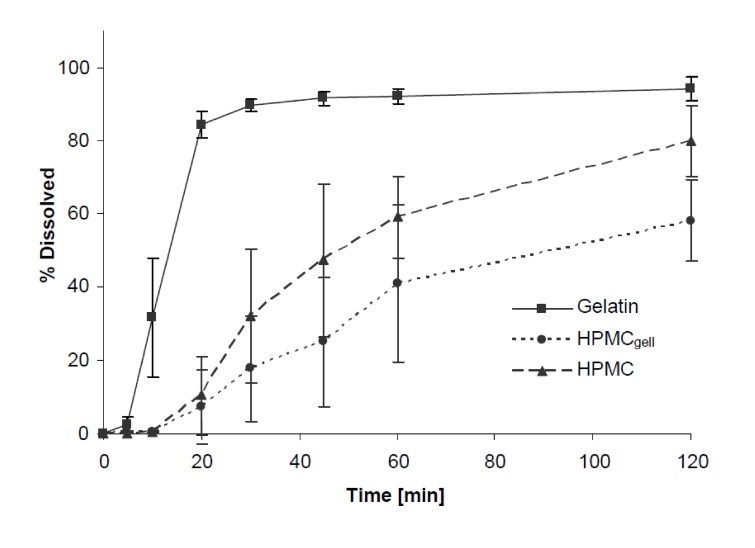
Dissolution profiles showing the mean percentage of green tea extract (GTE) released from 437 gelatin, HPMCgell and HPMC capsules in acetate buffer at pH 4.5, 37 °C, 75 rpm, 2 h. Bars represent 438 SD, n = 6.
Dissolution pH 6.8: Dissolution behaviour at pH 6.8 was similar to pH 4.5. After an initial lag time of approximately 5 min, the gelatin capsules dissolved fast and complete dissolution was achieved within 30 min. Both HMPC formulations showed again delayed release, after 30 min only 7% and 15% were dissolved from the HPMCgell and HPMC formulations, respectively (see Fig. 5).
Fig. 5.
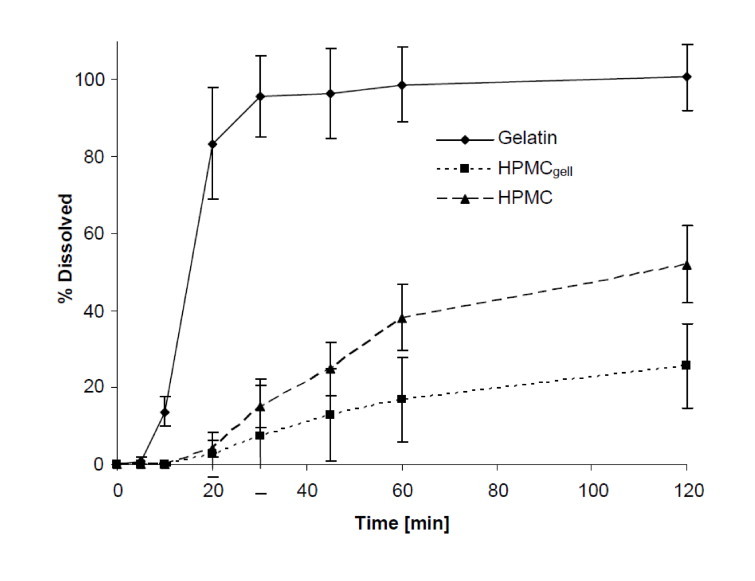
Dissolution profiles showing the mean percentage of green tea extract (GTE) released from gelatin, HPMCgell and HPMC capsules in phosphate buffer at pH 6.8, 37 °C, 75 rpm, 2 h. Bars represent SD, n = 6.
Dissolution in FeSSIF and FaSSIF: As shown in Figs. 6 and 7, dissolution in simulated intestinal fluid (fed and fasted) did not improve the release profile of the HPMC formulations compared to the compendial media. In FaSSIF, 6% and 15% of the content was released after 30 min and the maximal amount dissolved after 2 h were 33% and 61% for HPMCgell and HPMC, respectively. Dissolution in FeSSIF was further delayed with a content release after 30 min of 6% and 8%, and maximum release after 2 h of 64% and 54% for HPMCgell and HPMC, respectively.
Fig. 6.
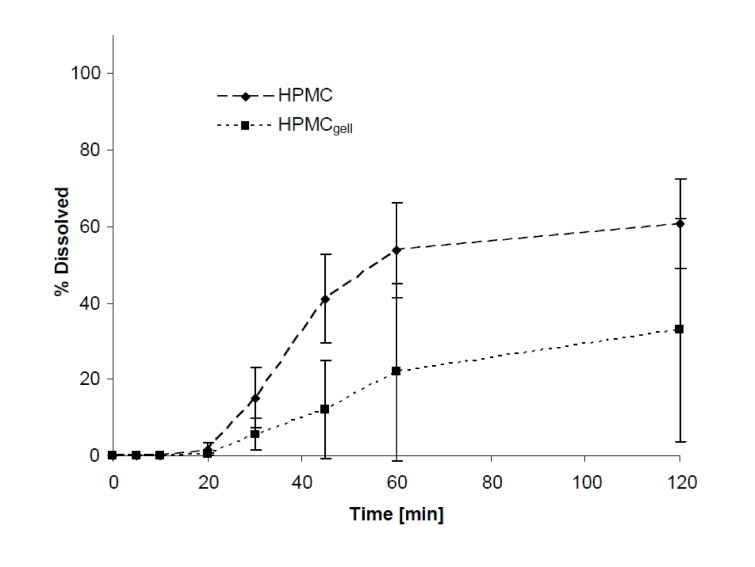
Dissolution profiles showing the mean percentage of green tea extract (GTE) released from HPMCgell and HPMC capsules in FaSSIF, pH 6.5, 37 °C, 75 rpm, 2 h. Bars represent SD, n = 3.
Fig. 7.
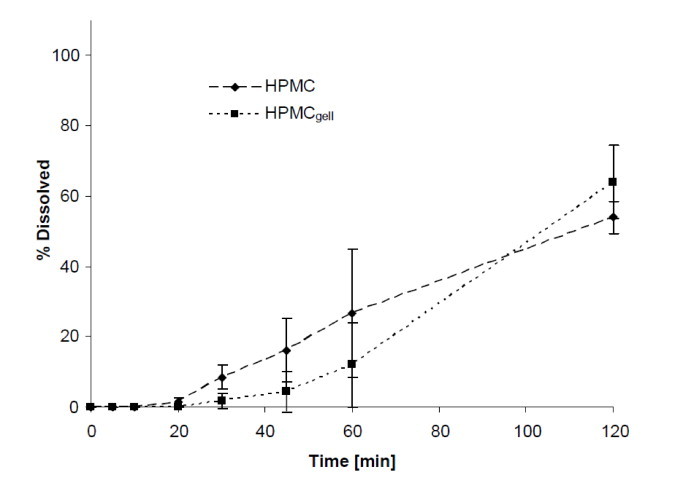
Dissolution profiles showing the mean percentage of green tea extract (GTE) released from HPMCgell and HPMC capsules in FeSSIF, pH 5, 37 °C, 75 rpm, 2 h. Bars represent SD, n = 3.
5. Discussion
The results of this study address a number of known concerns with regard to the quality and performance of marketed DS but is also intended to increase the awareness that similar issues must be dealt with in regard to clinical trial test products. A key factor dictating the efficacy of a DS or investigational product containing an active ingredient is the fraction of the ingested amount that is absorbed and reaches the target site, in a defined period of time. The design of a formulation can greatly influence the in vitro and in vivo performance and hence the efficacy/safety of oral dosage forms. Any DS or investigational product that does not disintegrate and dissolve sufficiently (in an appropriate time frame) before reaching the proximal intestine will not present the active ingredient for intestinal uptake, hence limiting absorption.
This study aimed at understanding the impact of the capsule shell material on the dissolution profile of three simple PIC GTE formulations intended for IR, such as they could be used as a DS or in a clinical trial/nutrition intervention study where an immediate effect was desired. In the tested formulations, the interference of any excipient was eliminated and purely the interaction between the capsule shell material and the active was investigated. The authors are aware that excipients could alter the interactions and subsequent work is underway to systematically investigate this further. Thus far, there are a few studies published looking at the in vitro rupture time of HPMC capsules, however most of the methods used were different from the USP guidelines applied in our experiments or focus on other active ingredients; e.g. Chiwele et al. [14] employed a ball bearing method, El-Malal and Nazzal [15] and Ku et al. [16] used the USP apparatus II with real time dissolution spectroscopy and Vardakou et al. [17] estimated capsule rupture time with a USP apparatus I as well as with a novel in vitro dynamic gastric model. From those experiments, a trend for increased rupture time in the following order was observed: Gelatin capsules < HPMCcarr < HPMC < HPMCgell. Similar trends were found in various in vivo scintigraphic measurements to estimate capsule rupture time as well as in in vitro dissolution studies. Ku et al. showed that HPMC is favourable over HPMCcarr (HPMC capsules containing carrageenan as a gelling agent) with respect to rupture time at low pH but in vitro dissolution profiles with a range of compounds at higher pH were similar [17]. The differences in rupture time between the capsules with and without gelling agent are however small (∼3 min) in relation to the time of a dissolution test (60 min). The observations from Ku et al. are subject of scientific debate with respect to their correct interpretation and meaning [18]. Cole et al. reported delayed dissolution of HPMCgell especially in acidic media and phosphate buffer when compared to gelatin [19]. Additionally, the in vivo data showed slower disintegration of HPMCgell compared to gelatin capsules in both, fasted and fed states. However, very similar in vivo disintegration times in fasted state for HPMCcarr and gelatin capsules was reported by Tuleu et al. [20].
The disintegration test is routinely used as a performance test for immediate release oral dosage forms. The data from our disintegration experiments align nicely with the aforementioned trends from the literature; regardless of the immersion fluid used, the gelatin formulations disintegrated approximately twice as fast as both HPMC formulations; nevertheless, all formulations passed the USP requirements of the disintegration test for botanical dosage forms. However, while for the gelatin capsules the content release appeared to be due to a uniform disintegration and dissolution of the capsule shell and subsequent liberation of the active, the content release from the HPMC capsules appeared to be caused by ruptures at the weakest points of the capsule shell without full disintegration of the shell itself; large portions of the shell remained on the mesh or inside the sinker after the 30 min test interval. Donauer and Löbenberg suggested that acetate buffer might not be a suitable media for in vitro disintegration testing of HPMC capsules since the presence of cations may hinder fast dissolution of the shell and the use of de-mineralized water would therefore be more appropriate [21]. However, our results showed similar disintegration times for HPMC capsules in both media, indicating that the current USP recommendation to use acetate buffer for disintegration testing of botanical dosage forms is adequate in this scenario.
Similar trends in the performance of the three formulations were observed in the dissolution experiments. As expected, the gelatin capsules disintegrated and dissolved rapidly, and achieved over 85% release after 30 min in all three compendial media, 0.1 mol/L HCl (pH 1.2), acetate buffer (pH 4.5) and phosphate buffer (pH 6.8). Capsule opening and content liberation were slower for the two HPMC formulations, both showing rather a profile of a delayed release than an IR formulation. In particular, the HPMCgell at pH 1.2 showed a very poor performance as content release was first detectable after 60 min and reached a maximum of 35% release within 2 h; this slow release was also confirmed visually with an example photograph taken after 30 min (Fig. 1). It appears as if media penetrated the capsule and wetting of the content occurred but the capsule shell remained intact, thereby trapping the contents and preventing complete release and subsequent dissolution. These findings are in line with a previous study where a slow in vitro and in vivo disintegration of HPMCgell in acidic environment was reported [19]. The delayed dissolution of those capsules was generally attributed to the ionic interactions between gellan gum in the HPMCgell and the acidic buffer, resulting in a lower solubility of gellan gels at low pH. The dissolution profile of the HPMCgell improved slightly at pH 4.5, pH 6.8, FaSSIF and FeSSIF, however, the dissolution profile deviated substantially to that what would be required of an IR formulation. The HPMC formulation performed slightly better than the HPMCgell, but still exhibited a delayed release of the content and did not meet IR criteria. This is contradictory to what could be expected as in previous studies, HPMC capsules either filled with a BCS class 1, 2 or 4 compound or a mixture of caffeine, lactose and croscarmellose were shown to dissolve rapidly at pH 1.2 and 4.5 [22]. The delayed release in our experiments can potentially be attributed to an interaction between the GTE and HPMC wall material immediately after the first signs of rupture and wetting of the GTE while inside the still largely intact capsule. As seen in the composition of the GTE (Table 1), the main constituents are the catechins EGCG (67%) and EGC (21%). Polyphenols and catechins in particular, have been shown to interact with proteins such as gelatin/collagen [23,24] but also with cellulose derivatives such as HPMC [25], resulting in insoluble complexes. Further experiments would be required to identify the specific catechin or potentially other compound(s) of the GTE causing the interaction. As mentioned earlier, the addition of a dispersant might reduce this interaction and hence improve the dissolution of the formulation. This could be especially useful for DS, however, formulations used for clinical intervention trials are often kept as simple as possible to eliminate any potential physiological effect thereof, but as shown in this study this may limit complete release.
Table 2.
Dissolution of green tea extract from gelatin, HPMCgell and HPMC capsules at pH 1.2, 4.5, 6.8, FaSSIF and FeSSIF; dissolution conditions: 37 °C, 75 rpm, sampling time points from 5 to 120 min (average % dissolved ± SD, n = 6).
| Min | Gelatin |
HPMCgell |
HPMC |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH 1.2 | pH 4.5 | pH 6.8 | pH 1.2 | pH 4.5 | pH 6.8 | FaSSIF | FeSSIF | pH 1.2 | pH 4.5 | pH 6.8 | FaSSIF | FeSSIF | |
| 5 | 39 ± 7 | 2 ± 2 | 1 ± 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | 91 ± 3 | 32 ± 16 | 14 ± 4 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| 20 | 94 ± 7 | 85 ± 4 | 83 ± 14 | 0 | 7 ± 10 | 3 ± 2 | 0 | 0 | 10 ± 7 | 10 ± 11 | 46 | 22 | 1 ± 1 |
| 30 | 95 ± 6 | 90 ± 2 | 96 ± 11 | 0 | 18 ± 14 | 7 ± 6 | 6 ± 4 | 2 ± 2 | 18 ± 11 | 32 ± 18 | 15 ± 15 | 15 ± 8 | 8 ± 4 |
| 45 | 95 ± 3 | 92 ± 2 | 96 ± 12 | 0 | 25 ± 17 | 13 ± 7 | 12 ± 13 | 4 ± 6 | 30 ± 19 | 47 ± 21 | 25 ± 12 | 41 ± 12 | 16 ± 9 |
| 60 | 95 ± 5 | 92 ± 2 | 99 ± 10 | 2 ± 1 | 41 ± 22 | 17 ± 8 | 22 ± 23 | 12 ± 12 | 49 ± 20 | 59 ± 11 | 38 ± 11 | 54 ± 13 | 27 ± 18 |
| 120 | 95 ± 4 | 94 ± 3 | 101 ± 9 | 35 ± 13 | 58 ± 11 | 25 ± 10 | 33 ± 29 | 64 ± 10 | 69 ± 17 | 80 ± 10 | 52 ± 11 | 61 ± 12 | 54 ± 5 |
The dissolution in biorelevant media showed a delayed onset and rate of dissolution in the simulated fed state compared to the simulated fasted state. These results were in good agreement with previously reported in vitro and in vivo studies [16,19]. The trend towards longer rupture times in the simulated fed state may be attributed to the slower hydration and softening rate of the tested capsule shell materials in the presence of food. Whether this would also hold for other HPMC capsule types and gelling agents is still unclear. Data from dissolution in FaSSIF and FeSSIF are especially valuable to plan the time of administration, e.g. whether to dose with or without a meal.
6. Conclusion
In conclusion, in order to properly design and guide nutrition intervention trials and to enhance the success rate of an investigational product or DS, a good understanding of the formulation's in vitro performance is crucial, as the release profiles will greatly affect the timings of clinical measurements. No- or negative-effect trials are often attributed to the active ingredient itself, rather than the formulation. Insufficient information on the quality and performance of the investigational product can lead to false negative and/or false-neutral interpretations of clinical data. GTE specifically, indicated variable disintegration and dissolution profiles depending on the capsule shell material employed. Therefore, it is recommended the performance of solid oral dosage forms intended for use as a DS or clinical trial investigational product be verified preferably with an in vitro dissolution test prior to the product being released.
Author's contribution
Natalie Glube: designed the study, supplied the materials, analysed the results and wrote the paper.
Lea von Moos: conducted the experiments, analysed the results and contributed to the manuscript.
Guus Duchateau: participated in the design, contributed to the manuscript and revised the paper.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Deka A, Vita JA. Tea and cardiovascular disease. Pharmacological Research. 2011;64(2):136–145. doi: 10.1016/j.phrs.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeukendrup AE, Randell R. Fat burners: nutrition supplements that increase fat metabolism. Obesity Reviews. 2011;12(10):841–851. doi: 10.1111/j.1467-789X.2011.00908.x. [DOI] [PubMed] [Google Scholar]
- 3.Yuan JM, Sun C, Butler LM. Tea and cancer prevention: epidemiological studies. Pharmacological Research. 2011;64(2):123–135. doi: 10.1016/j.phrs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea—a review. Journal of the American College of Nutrition. 2006;25(2):79–99. doi: 10.1080/07315724.2006.10719518. [DOI] [PubMed] [Google Scholar]
- 5.Allen LV Jr., Popovich NG, Ansel HC, editors. Ansel's pharmaceutical dosage forms and drug delivery systems. 9thed. Lippincott Williams & Wilkins; Baltimore: 2009. [Google Scholar]
- 6.Podczek F, Jones BE, editors. Pharmaceutical capsules. 2nded. The Pharmaceutical Press; London: 2004. [Google Scholar]
- 7.Yang CS, Sang S, Lambert JD, Lee MJ. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Molecular Nutrition and Food Research. 2008;52(Suppl. 1) doi: 10.1002/mnfr.200700234. S139-S151. [DOI] [PubMed] [Google Scholar]
- 8.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angewandte Chemie International Edition. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 9.Chow HH, Hakim IA, Vining DR, Crowell JA, Ranger-Moore J, Chew WM. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of Polyphenon E in healthy individuals. Clinical Cancer Research. 2005;11(12):4627–4633. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 10.Siebert KJ, Troukhanova NV, Lynn PY. Nature of polyphenol–protein interactions Journal of Agricultural and Food Chemistry. 1996;44(1):80–85. [Google Scholar]
- 11.Galia E, Nicolaides E, Horter D, Löbenberg R, Reppas C, Dressman JB. Evaluation of various dissolution media for predicting in vivo performance of class I and II drugs. Pharmaceutical Research. 1998;15(5):698–705. doi: 10.1023/a:1011910801212. [DOI] [PubMed] [Google Scholar]
- 12.Anon. Guidance for industry: Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. Rockville, MD: US Department of Health and Human Services FDA, Center for Drug Evaluation and Research (CDER); 2000.
- 13.Wang H, Wen Y, Du Y, Yan X, Guo H, Rycroft JA. Effects of catechin enriched green tea on body composition. Obesity. 2010;18(4):773–779. doi: 10.1038/oby.2009.256. [DOI] [PubMed] [Google Scholar]
- 14.Chiwele I, Jones BE, Podczeck F. The shell dissolution of various empty hard capsules. Chemical and Pharmaceutical Bulletin. 2000;48(7):951–956. doi: 10.1248/cpb.48.951. [DOI] [PubMed] [Google Scholar]
- 15.El-Malah Y, Nazzal S, Bottom CB. Hard gelatin and hypromellose (HPMC) capsules: estimation of rupture time by real-time dissolution spectroscopy. Drug Development and Industrial Pharmacy. 2007;33(1):27–34. doi: 10.1080/03639040600685183. [DOI] [PubMed] [Google Scholar]
- 16.Vardakou M, Mercuri A, Naylor TA, Rizzo D, Butler JM, Connolly PC. Predicting the human in vivo performance of different oral capsule shell types using a novel in vitro dynamic gastric model. International Journal of Pharmaceutics. 2011 doi: 10.1016/j.ijpharm.2011.07.046. [DOI] [PubMed] [Google Scholar]
- 17.Ku MS, Lu Q, Li W, Chen Y. Performance qualification of a new hypromellose capsule: part II. Disintegration and dissolution comparison between two types of hypromellose capsules. International Journal of Pharmaceutics. 2011;416(1):16–24. doi: 10.1016/j.ijpharm.2011.02.048. [DOI] [PubMed] [Google Scholar]
- 18.Jones BE, Podczeck F. Letter to the editor: on the performance qualification of hypromellose capsules. International Journal of Pharmaceutics. 2012;423(2):589–592. doi: 10.1016/j.ijpharm.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Cole ET, Scott RA, Cade D, Connor AL, Wilding IR. In vitro and in vivo pharmacoscintigraphic evaluation of ibuprofen hypromellose and gelatin capsules. Pharmaceutical Research. 2004;21(5):793–798. doi: 10.1023/b:pham.0000026430.73789.e6. [DOI] [PubMed] [Google Scholar]
- 20.Tuleu C, Khela MK, Evans DF, Jones BE, Nagata S, Basit AW. A scintigraphic investigation of the disintegration behaviour of capsules in fasting subjects:a comparison of hypromellose capsules containing carrageenan as a gelling agent and standard gelatin capsules. European Journal of Pharmaceutical Sciences. 2007;30(3–4):251–255. doi: 10.1016/j.ejps.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Donauer N, Löbenberg R. A mini review of scientific and pharmacopeial requirements for the disintegration test. International Journal of Pharmaceutics. 2007;345(1–2):2–8. doi: 10.1016/j.ijpharm.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 22.Groshens E., He X, Cade D. Hypromellose hard capsules—reproducibility of the in vitro dissolution performance in USP SGF media. Poster annual meeting & exposition of the AAPS, Los Angelos; 2009. pp. 1–4. [Google Scholar]
- 23.Yi K, Cheng G, Xing F. Gelatin/tannin complex nanospheres via molecular assembly. Journal of Applied Polymer Science. 2006;101(5):3125–3130. [Google Scholar]
- 24.Jackson JK, Zhao J, Wong W, Burt HM. The inhibition of collagenase induced degradation of collagen by the galloyl-containing polyphenols tannic acid, epigallocatechin gallate and epicatechin gallate. Journal of Materials Science: Materials. 2010;21(5):1435–1443. doi: 10.1007/s10856-010-4019-3. [DOI] [PubMed] [Google Scholar]
- 25.Patel AR, Nijsse J, Velikov KP. Novel polymer–polyphenol beads for encapsulation and microreactor applications. Soft Matter. 2011;7(9):4294–4301. [Google Scholar]


