Abstract
When fed 10 ppm of one of the following sterols: cholesterol (cholest-5-en-3β-ol), wingsterol (21-isopentylcholesterol), desmosterol [cholesta-5,24(25)-dien-3β-ol], 24-methylenecholesterol [ergosta-5,24(28)-dien-3β-ol], or fucosterol [stigmasta-5,24(28)-dien-3β-ol], the pathogenic fungus Phytophthora cactorum, which is naturally unable to epoxidize squalene, accumulated each of the test compounds to similar levels. Fucosterol, the only sterol metabolized, was reduced to yield 24-ethylcholesterol. All the sterols tested induced the formation of sex structures. Fertilization and subsequent maturation of oospores capable of germination occurred only with the naturally occurring sterols. Wingsterol treatments resulted in aborted oospores. None of the sterols tested was inhibitory to growth, measured as changes in the 21-day mycelial dry weight. The results are consistent with the view that the accumulated sterol functions to regulate the life cycle of P. cactorum. However, the metabolism and kinds of recognition of the sterol molecule, in terms of uptake and effects on growth and induction of the various sexual events, contrast sharply with what is known for other oomycetous fungi such as Achlya and Saprolegnia. This implies that the evolutionary histories of the Oomycetes may be different.
Keywords: Oomycetes, Pythiaceae, fucosterol metabolism, reproduction
Full text
PDF


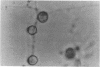
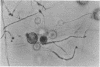
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowman R. D., Mumma R. O. The lipids of Phythium ultimum. Biochim Biophys Acta. 1967 Dec 5;144(3):501–510. doi: 10.1016/0005-2760(67)90038-0. [DOI] [PubMed] [Google Scholar]
- Elliott C. G., Hendrie M. R., Knights B. A. The sterol requirement of Phytophthora cactorum. J Gen Microbiol. 1966 Mar;42(3):425–435. doi: 10.1099/00221287-42-3-425. [DOI] [PubMed] [Google Scholar]
- Elliott C. G. Sterols in fungi: their functions in growth and reproduction. Adv Microb Physiol. 1977;15:121–173. doi: 10.1016/s0065-2911(08)60316-1. [DOI] [PubMed] [Google Scholar]
- Hendrix J. W. Differential uptake and metabolism of sitosterol and cholesterol by Achlya, Pythium, and Phytophthora species. Can J Microbiol. 1975 Jun;21(6):735–737. doi: 10.1139/m75-108. [DOI] [PubMed] [Google Scholar]
- Knights B. A., Elliott C. G. Metabolism of delta7- and delta5,7-sterols by Phytophthora cactorum. Biochim Biophys Acta. 1976 Aug 23;441(2):341–346. doi: 10.1016/0005-2760(76)90178-8. [DOI] [PubMed] [Google Scholar]
- Nes W. D., Patterson G. W., Bean G. A. Effect of Steric and Nuclear Changes in Steroids and Triterpenoids on Sexual Reproduction in Phytophthora cactorum. Plant Physiol. 1980 Nov;66(5):1008–1011. doi: 10.1104/pp.66.5.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes W. R., Adler J. H., Billheimer J. T., Erickson K. A., Joseph J. M., Landrey J. R., Marcaccio-Joseph R., Ritter K. S., Conner R. L. A comparison of the biological properties of androst-5-en-3 beta-ol, a series of (20R)-n-alkylpregn-5-en-3 beta-ols and 21-isopentylcholesterol with those of cholesterol. Lipids. 1982 Mar;17(3):257–262. doi: 10.1007/BF02535113. [DOI] [PubMed] [Google Scholar]
- Parks L. W., McLean-Bowen C., Bottema C. K., Taylor F. R., Gonzales R., Jensen B. W., Ramp J. R. Aspects of sterol metabolism in the yeast Saccharomyces cerevisiae and in Phytophthora. Lipids. 1982 Mar;17(3):187–196. doi: 10.1007/BF02535102. [DOI] [PubMed] [Google Scholar]
- Richards J. B., Hemming F. W. Dolichols, ubiquinones, geranylgeraniol and farnesol as the major metabolites of mevalonate in Phytophthora cactorum. Biochem J. 1972 Aug;128(5):1345–1352. doi: 10.1042/bj1281345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sietsma J. H., Haskins R. H. Further studies on sterol stimulation of sexual reproduction in pythium. Can J Microbiol. 1967 Apr;13(4):361–367. doi: 10.1139/m67-048. [DOI] [PubMed] [Google Scholar]
- Warner S. A., Eierman D. F., Sovocool G. W., Domnas A. J. Cycloartenol-derived sterol biosynthesis in the Peronosporales. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3769–3772. doi: 10.1073/pnas.79.12.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. G., Gottlieb D. Evidence from cell-free systems for differences in the sterol biosynthetic pathway of Rhizoctonia solani and Phytophthora cinnamomi. Biochem J. 1978 Feb 15;170(2):355–363. doi: 10.1042/bj1700355. [DOI] [PMC free article] [PubMed] [Google Scholar]