Abstract
Transcription factor of the Rel/NF-κB family are known to play different roles in immunity and inflammation, although the putative role of c-Rel in transplant tolerance and GVHD remains elusive. We report here that T cells deficient for c-Rel have a dramatically reduced ability to cause acute graft-versus-host disease (GVHD) after allogeneic bone marrow transplantation (BMT) using major and minor histocompatibility mismatched murine models. In the study to understand the underlying mechanisms, we found that c-Rel-/- T cells had reduced ability to expand in lymphoid organs and to infiltrate in GVHD target organs in allogeneic recipients. c-Rel-/- T cells were defective in the differentiation into Th1 cells after encountering alloantigens, but were enhanced in the differentiation towards Foxp3+ regulatory T cells (Tregs). Furthermore, c-Rel-/- T cells had largely preserved activity to mediate graft-versus leukemia (GVL) response. Taken together, our findings indicate that c-Rel plays an essential role in T cells in the induction of acute GVHD, and suggest that c-Rel can be a potential target for therapeutic intervention in allogeneic HCT in clinic.
Keywords: c-Rel, BMT, GVHD
Introduction
Members of Rel/NF-κB family have been well recognized as key regulators of innate and adaptive immunity. c-Rel is a member of the Rel/NF-κB family of eukaryotic transcription factors, which also includes the proteins RelA (p65), RelB, NF-κB1 (p105/p50), and NF-κB2 (p100/p52) [1]. Unlike other members that are constitutively expressed in multiple cell types, c-Rel is expressed primarily in lymphoid tissues by lymphoid and myeloid cells [2]. It has been well established that c-Rel plays an important role in mediating proliferation, differentiation, and cytokine production of T cells. c-Rel regulates the production of IL-2 and expression of IL-2Rα (CD25) in T cells, which are crucial for cell division and immune function [3, 4]. In activated T cells, c-Rel signaling (downstream of TCR and CD28) may also be essential for secretion of other IL-2 dependent cytokines. IL-2 is known to be required for optimal IL-4 and IFN-γ expression by T-helper cells and for expression of granzyme and perforin by cytotoxic T lymphocytes [5]. T cells deficient for c-Rel fail to initiate autoimmune encephalomyelitis due to their inability to mount an optimal Th1 response. The Th1 deficiency appears to be caused by selective blockade of IL-12 production by c-Rel deficient antigen-presenting cells, as well as by a complete abrogation of IFN-γ expression in c-Rel–deficient T cells. Interestingly, c-Rel deficiency does not affect T-bet expression, suggesting that c-Rel may act downstream of T-bet during Th1 cell differentiation [6]. Two recent studies have provided evidence that c-Rel is required for RORγt expression in T cells [7, 8]. Therefore, c-Rel, expressed by CD4+ T cells and by myeloid cells, contributes to the differentiation and maintenance of Th17 cells [8], as well to the generation of regulatory T cells (Tregs) [9-11]. However, the role of c-Rel in regulating various effector functions in T cell response to alloantigen has not yet been characterized. Based on previous findings by us and others, both Th1 and Th17 T cells contribute to the development of graft-versus-host disease (GVHD), but either lineage is sufficient to induce GVHD [12, 13]. Disruption of T cell differentiation towards Th1 and Th17 pathways by targeting T-bet and RORγt can effectively prevent GVHD while preserving GVL effect [14]. c-Rel acts downstream of T-bet and RORγt causes defects in Th1 and Th17 immune response [7, 15]. Based on these observations, we hypothesize that c-Rel is required for the development of GVHD. Here we provide evidence that c-Rel is involved in regulating GVHD after allogeneic BMT. Our study demonstrates that c-Rel could be a novel therapeutic target to affect T-cell differentiation and subsequently reduce GVHD mortality following allogeneic bone marrow transplantation (BMT) in mice.
Results
Deficiency of c-Rel in donor T cells significantly reduces GVHD after allogeneic BMT
To examine the role of c-Rel in donor T cells in mediating acute GVHD, we first compared the ability of WT and c-Rel-/- T cells to induce GVHD using MHC mismatched BMT model: B6 (H2b) → BALB/c (H2d). Recipients were lethally irradiated and reconstituted with 5 × 106 allogeneic TCD-BM and 2 × 106 purified T cells from either WT or c-Rel-/- donors. As shown in figure 1 A and B, the recipients of c-Rel-/- T cells had significantly reduced GVHD mortality with only moderate weight loss. GVHD severity was confirmed with pathologic analysis, which showed that pathology scores were significantly lower in lung, liver, small intestine and colon of the recipients transplanted with c-Rel-/- T cells than those given WT T cells (Fig.1C).
Figure 1.
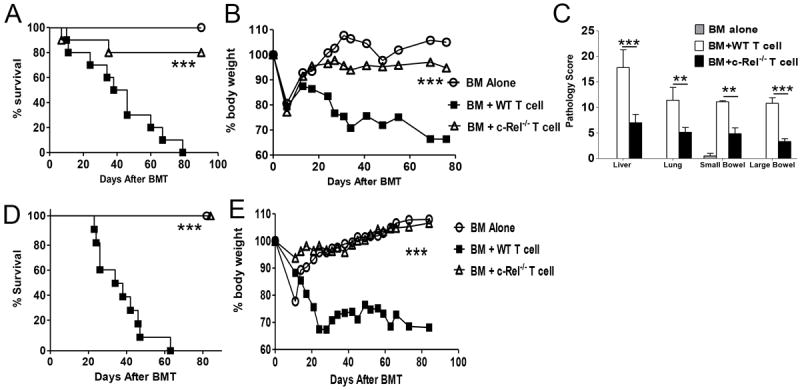
Absence of c-Rel in donor T cells reduces GVHD mortality after allogeneic BMT. Lethally irradiated BALB/c (n = 10) and BALB.B (n = 10) mice were transplanted with 5 × 106 TCD-BM alone or with 2 × 106 purified T cells from WT or c-Rel-/- B6 mice. (A) Mice survival in B6 → BALB/c BMT. (B) Percentage of original body weight over time in B6 → BALB/c BMT. (C) Pathological score mean ± SE in BALB/c recipients indicates the damage in small bowel, large bowel, liver, and lung using a semi-quantitative scoring system at day 28. (D) Mice survival in B6 → BALB.B BMT. (E) Percentage of original body weight over time in B6 → BALB.B BMT. The data showed was the combination of 2 independent experiments. Log-rank test was used to compare mice survival and 2 tailed student’s-t test was used for other analysis. Asterisk indicates statistical significance. **P < 0.01; ***P < 0.001.
In clinic HCT, most patients receive grafts from MHC-matched and multiple minor histocompatibility antigens (miHA)-mismatched donors. miHAs are peptides derived from allogeneic proteins that exist in various isoforms and can affect the fate of a graft by provoking cell mediated immune responses [16, 17]. In an effort to mimic a clinical scenario and understand the role of c-Rel in regulating T cell responses to miHAs in vivo, we used the C57BL/6 → BALB.B (both H2b) model, in which donor and recipient mice differ from one another by at least 29 different miHA loci [18]. Lethally irradiated BALB.B mice received TCD-BM alone or plus WT or c-Rel-/- T cells. As expected, the recipients of BM alone showed no signs of GVHD, whereas the recipients with WT T cells developed severe GVHD. In contrast, c-Rel-/- T cells failed to induce GVHD as 100% recipient survived long-term with little weight loss (Figure 1, D and E). Taken together, we found that the expression of c-Rel on donor T cells is required for the development of acute GVHD.
c-Rel is required for optimal expansion of alloreactive donor T cells
We performed in vitro experiments where purified T cells from c-Rel-/- and WT B6 mice were stimulated with TCD splenocytes from BALB/c. T cells deficient for c-Rel proliferated profoundly less than WT T cells (Fig. 2A). To evaluate the role of c-Rel in T-cell expansion in vivo, we isolated T cells from c-Rel-/- or WT B6 mice, labeled them with CFSE, and adoptively transferred them into lethally irradiated BALB/c recipients. At day 3, we observed that both CD4+ and CD8+ c-Rel-/- T cells significantly reduced proliferation (Fig. 2B), and furthermore CD4+ c-Rel-/- T cells had higher apoptosis rate than WT counterparts (Fig. 2C). At day 4, both WT and c-Rel-/- T cells underwent strong cell divisions in response to alloantigen, but c-Rel-/- T cells produced significantly less IFNγ than WT T cells (Fig. 2D). Although both cell types proliferated at similar levels at day 4, the total number of c-Rel-/- T cells recovered from allogeneic recipients was much lower than WT T cells (Fig. 2E). We reasoned that c-Rel-/- T cells had reduced ability to proliferate early after cell transfer, and compromised ability to survive in vivo as reported by others that c-Rel plays an important role in T-cell survival [15].
Figure 2.
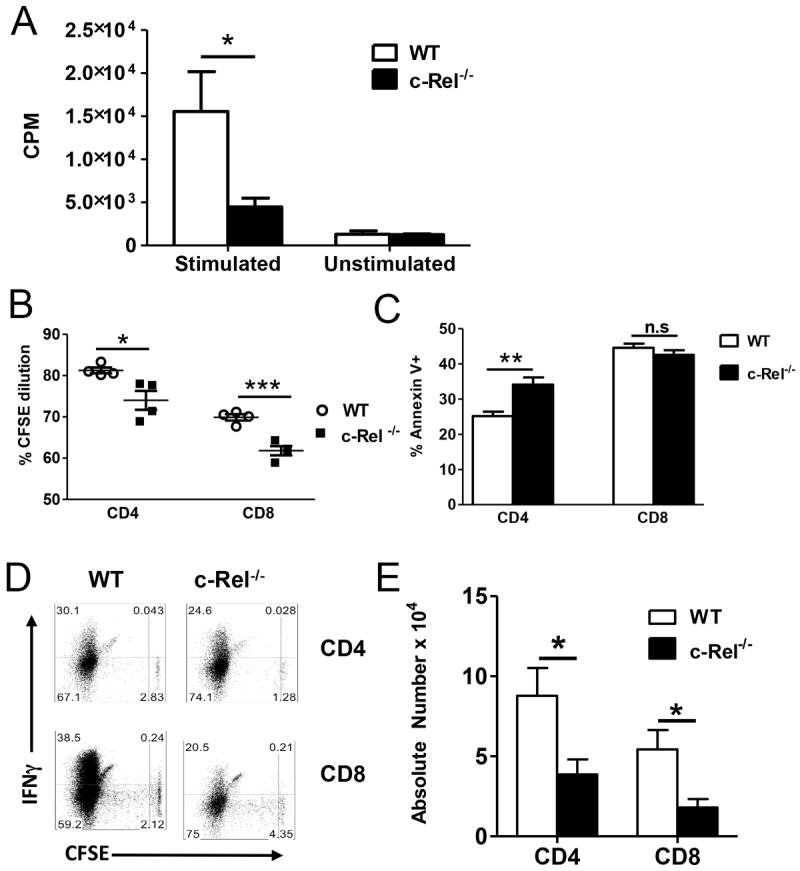
c-Rel is required for T-cell proliferation in response to alloantigen. (A) 2 × 105 purified T cells from c-Rel-/- and WT B6 mice were stimulated with 6 × 105 irradiated TCD-splenocytes from BALB/c mice. [3H]-TdR was added to cell culture 8 hours before the end of culture. Cell proliferation was measured at day 5 by [3H]-TdR incorporation. (B-E) CFSE labeled c-Rel-/- or WT T cells from B6 donors were adoptively transferred into lethally irradiated BALB/c mice. CSFE annexin V profiles and intracellular IFNg expression was measured by flow cytometry on gated live T cells 3 days (B and C) or 4 days (D and E) after BMT. One representative experiment from 3 independent experiments was shown. Panels A, C and E were presented as means ± 1SD of 4 to 5 samples per group. 2 tailed student’s-t test was used for statistical analysis. *P < 0.05; ** P < 0.01; *** P< 0.001.
c-Rel deficiency in donor T cells leads to reduced Th1 and Th17 differentiation in vitro with diminished Th1 response in vivo
To directly test whether c-Rel-/- T cells are intrinsically defective in their Th1 and Th17 differentiation, WT and c-Rel-/- T cells were polarized into Th1 and Th17 differentiation in vitro. IL-2 was added in culture to ensure sufficient activation and proliferation of c-Rel-/- T cells and thus to exclude the possibility that defect differentiation might be due to compromised activation. As shown in figure 3A, WT T cells can differentiate into either Th1 or Th17 cells depending on the culture conditions. In contrast, much less IFN-γ producing cells were generated from c-Rel-/- T cells under Th1 conditions and very few IL-17 producing cells were generated under Th17 conditions. These results suggest that c-Rel-deficient T cells are intrinsically defective in their Th1 and Th17 differentiation in vitro, which may be attributable to the reduced ability of c-Rel-/- T cells in the induction of GVHD. To further investigate the underlying mechanism by which c-Rel regulates T-cell response and GVHD development in vivo, we analyzed intracellular cytokine profiles in recipients of c-Rel-/- versus WT donor cells on day 5 after BMT (Fig. 3B). Donor T cells deficient for c-Rel produced significantly lower levels of Th1 cytokine than WT T cells in recipient spleen, liver and lung (Fig. 3C). In recipient serum, the level of TNFα was significantly lower in the recipients of c-Rel-/- than those of WT T cells (Fig. 3D). As expected, very few donor T cells differentiated into Th17 cells during the development of acute GVHD after allogeneic BMT. We did not detect significant differences of c-Rel-/- versus WT donor cells in IL-17 production. Taken together, these data demonstrate that absence of c-Rel impairs Th1 differentiation after allogenic BMT.
Figure 3.
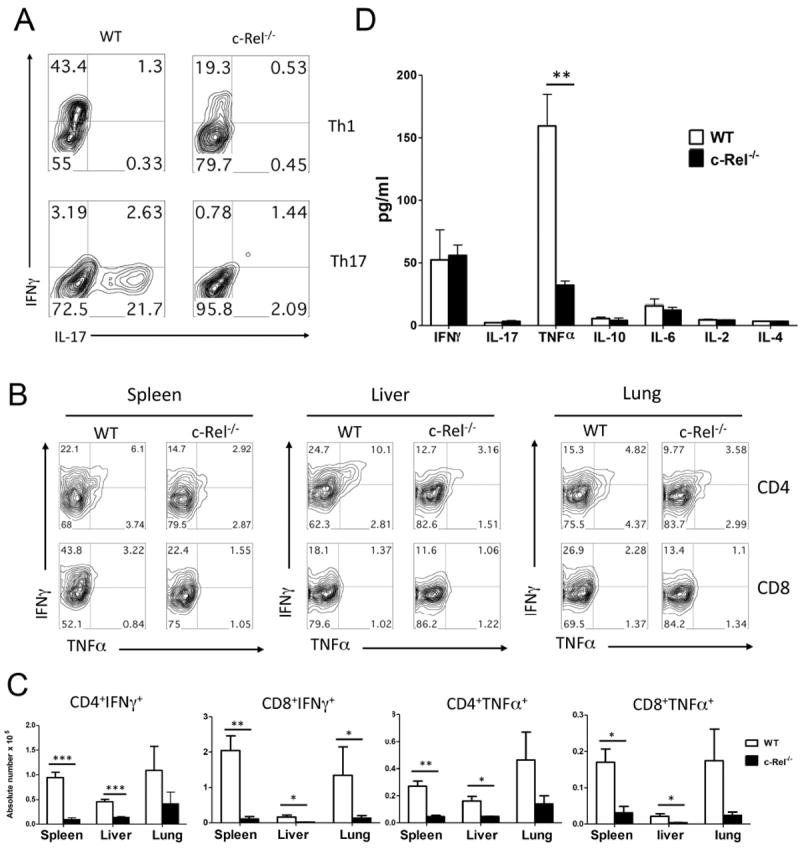
c-Rel-/- donor T cells produce lower levels of inflammatory cytokines. (A) CD4+CD25- cells isolated from WT or c-Rel-/- mice were stimulated in the presence of APCs with anti-CD3 mAb under culture conditions for Th1 and Th17 polarization as described in Material and Method. Cell phenotype was measured on day 4 with intracellular cytokine staining of IFNγ and IL-17 expression on gated live CD4+ cells. (B) Lethally irradiated (800 cGy) BALB/c mice were transplanted with 2 × 106 purified WT and c-Rel-/- T cells from B6 donors. Intracellular cytokine profiles of splenic CD4+ T cells are shown in 5 days after BMT. Representative contour plot depicts the percentages of IFN-γ- and/or TNF-α-secreting cells in the gated H-2Kb+CD4+ cells in recipient spleen, liver and lung. (C) Absolute number of IFN-γ- and/or TNF-α-secreting cells from spleen, liver and lung of recipients transplanted with WT or c-Rel-/- T cells on gated live cells. (D) Serum cytokine profile in recipients transplanted with WT and c-Rel-/- donor T cells. Lethally irradiated BALB/c mice were transplanted with TCD-BM plus 2 × 106 purified T cells from WT or c-Rel-/- B6 donors. Indicated cytokines were measured in recipient serum on day 14 after BMT. Similar data was obtained from 3 independent experiments and the result from one experiment was shown. Panel C and D were presented as means ± 1SD of 5 to 6 samples per group. 2 tailed student’s-t test was used for statistical analysis *P < 0.05; **P < 0.01; ***P < 0.001. n.s, no significant difference.
Generation of Foxp3+ regulatory T cells was increased from c-Rel-/- T cells in vivo
Regulatory T cells (Tregs, CD4+CD25+Foxp3+) have been reported to prevent or delay the onset of GVHD in animal models [19], and the presence of Tregs in GVHD target organs has been shown to correlate negatively with the severity of GVHD [20]. Since c-Rel deficiency in donor T cells resulted in reduced GVHD and impaired Th1 differentiation, we asked whether c-Rel also affected on Treg expansion or generation in vivo. CD25-depleted T cells were isolated from WT or c-Rel-/- mice and transferred together with TCD-BM into lethally irradiated BALB/c mice. We compared the presence of Tregs in the recipients 14 days after BMT, and found significantly more Tregs were derived from c-Rel-/- than WT T cells in recipient peripheral blood, spleen and liver (Fig. 4 A-C). Given Treg-depleted T cells were included in donor graft, we conclude that increased Tregs in the absence of c-Rel was due to enhanced Treg-generation from conventional CD4+ T cells under condition of allogeneic BMT. To further ask whether Tregs are functional in the absence of c-Rel, we generated TGFβ-induced Tregs from WT and c-Rel-/- CD4+CD25- T cells, and compare their activity in suppressing T-cell response to alloantigen in vitro. Allogeneic response was inhibited equally by WT or c-Rel-/- iTregs (data not shown).
Figure 4.
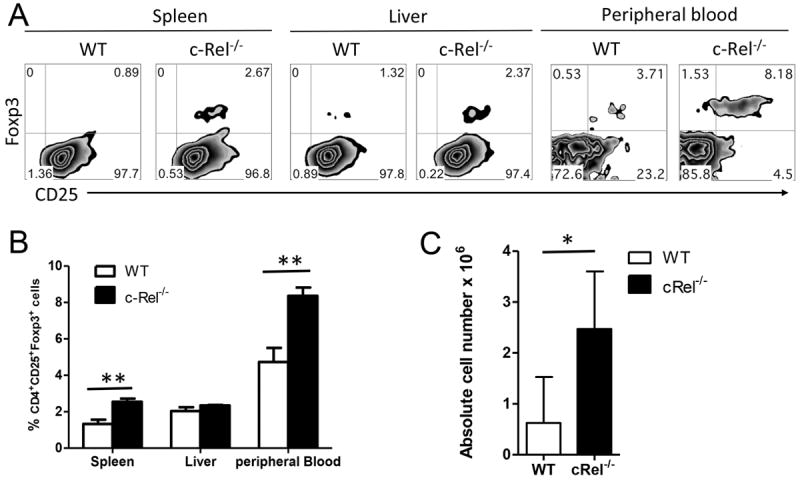
Absence of c-Rel in donor T cells leads to augmented Tregs in BMT recipient. Lethally irradiated BALB/c mice were transplanted with TCD-BM (ly5.1+) plus WT or c-Rel-/- T cells from B6 donors. Fourteen days after BMT, recipient spleen, liver and peripheral blood were harvested and measured for expression of CD4, CD25, Foxp3, ly5.1 and H-2Kb. Expression and percentage of CD25 and Foxp3 are shown on gated donor CD4+ cells (H2Kb+ly5.1-) of spleen, liver and peripheral blood (A, B). (C) Summary of absolute number of donor Tregs (CD4+CD25+Foxp+) in recipient spleen. Panel B and C were presented as means ± 1SD of 4 to 5 samples per group. 2 tailed student’s-t test was used for statistical analysis. *P < 0.05; **P < 0.01. n.s, no significant difference.
T cells deficient for c-Rel express lower levels of chemokine receptors and have decreased homing to GVHD target organs
The development of GVHD requires donor T cell-migration into target organs. We thus examined the presence of donor T cells in recipient liver and lung as well as in spleen. Consistent with the data presented in figure 3, significant lower numbers of donor CD4 or CD8 T cells were found in the recipients of c-Rel-/- T cells compared to those of WT T cells (Fig. 5 A and B). Similarly, significantly reduced numbers of donor T cells were also observed in the liver and lung of the recipients with c-Rel-/- T cells (Fig. 5 A and B). These data indicate that, in the absence of c-Rel, T cells have reduced ability to expand and likely had reduced potential to migrate into GVHD target organs as well. Migration of activated donor T cells to GVHD target organs is one of the critical steps in the pathophysiology of GVHD. G protein coupled receptors, including the chemokine receptors, play an important role in orchestrating the migration of leukocytes to peripheral organs. Thus, we measured the expression of CXCR3, CCR4, CCR6 on donor T cells as they are Th1, Th2 and Th17-associated chemokine receptors, respectively [21, 22]. c-Rel-/- donor T cells expressed much lower levels of liver-homing receptors CXCR3 in liver and lung (Fig. 5 C and D). c-Rel-/- donor T cells also expressed significantly higher levels of CCR6 than WT T cells in the lung. Because chemokine receptors are required for infiltration of alloreactive T cells into GVHD targeted organ, the distinct expression of those receptors on different types of cells likely contributed to the reduced migration of c-Rel-/- T cells in recipient liver and lung compared to WT T cells.
Figure 5.
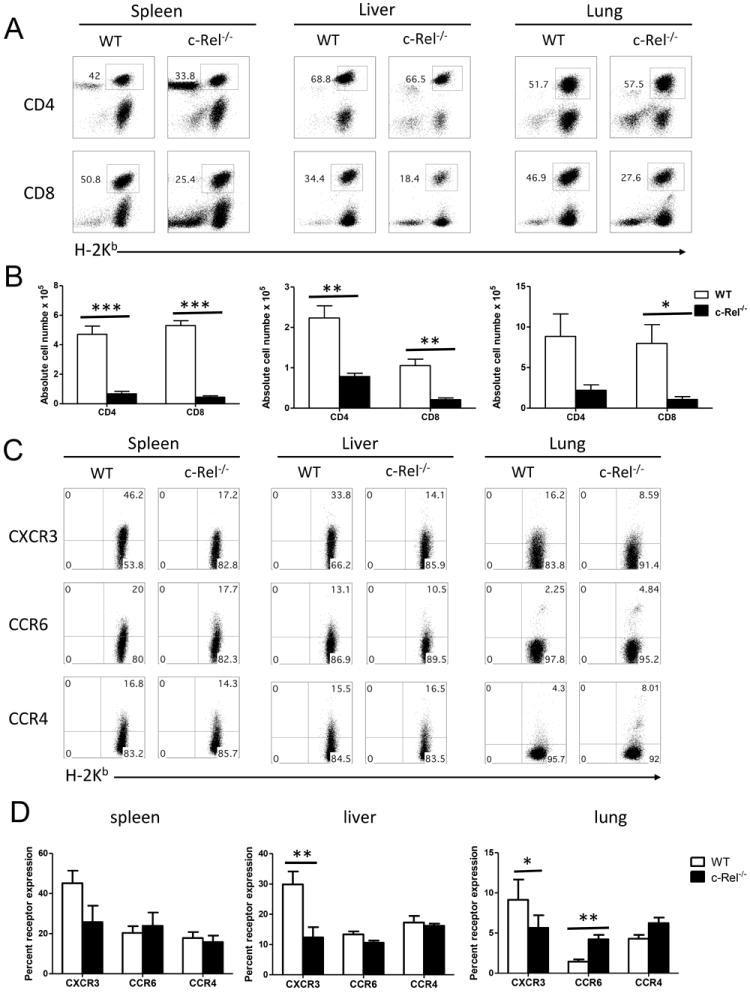
c-Rel regulates chemokine receptor expression of donor T cells after allogeneic BMT. (A) Percentage and (B) absolute number of H2Kb+CD4+ T cells and H2Kb+CD8+ T cells in spleen, liver and lung of the recipients transplanted with WT or c-Rel-/- donor T cells 14 days after BMT are shown. Mean ± SE is presented (n = 9), and data are from combined 3 replicate experiments. (C) Five days after BMT, splenocytes from recipients were stained for H-2Kb, CD4 and chemokine receptors. Gated H-2Kb+CD4+ T cells are shown in CD4 versus chemokine receptors. (D) Summary of the expression of chemokine receptors on CD4 T cells. Panel B and D were presented as means ± 1SD of 4 to 5 samples per group. 2 tailed student’s-t test was used for statistical analysis. *P < 0.05; **P < 0.01; ***P < 0.001.
c-Rel -/- T cells had partially preserved GVL activity
When HCT is used as a therapy for hematological malignances, an important role for donor T cells is to prevent relapse of the original disease through GVL effects. Therefore, it is critically important to determine whether T cells lacking c-Rel retain such beneficial GVL effect. To this end, we compared the ability of WT versus c-Rel-/- T cells in mediating GVL response against A20 B cell lymphoma in B6 → BALB/c allogeneic BMT. The A20 lymphoma cell line was transduced with luciferase reporter and lymphoma growth was monitored by in vivo bioluminescence imaging (BLI). As expected, when A20 cells were infused, all the recipients of TCD-BM alone died due to tumor relapse since these recipients had less body weight loss but very strong BLI signals (Fig. 6 A and B). The recipients of BM plus WT T cells had severe GVHD with significant weight loss but little or no BLI signals (Fig. 6). In contrast, the majority of recipients given 5 × 106 c-Rel-/- T cells survived through the observation period (Fig. 6 A) with modest weight loss but with weak BLI signal (Fig. 6 C), 2×106 c-Rel-/- T cells had relatively lower protective ability against tumor compared to 5 × 106 c-Rel-/- T cells. These results indicate that, in the absence of c-Rel, donor T cells still largely preserved GVL activity although severely impaired in the induction of GVHD.
Figure 6.
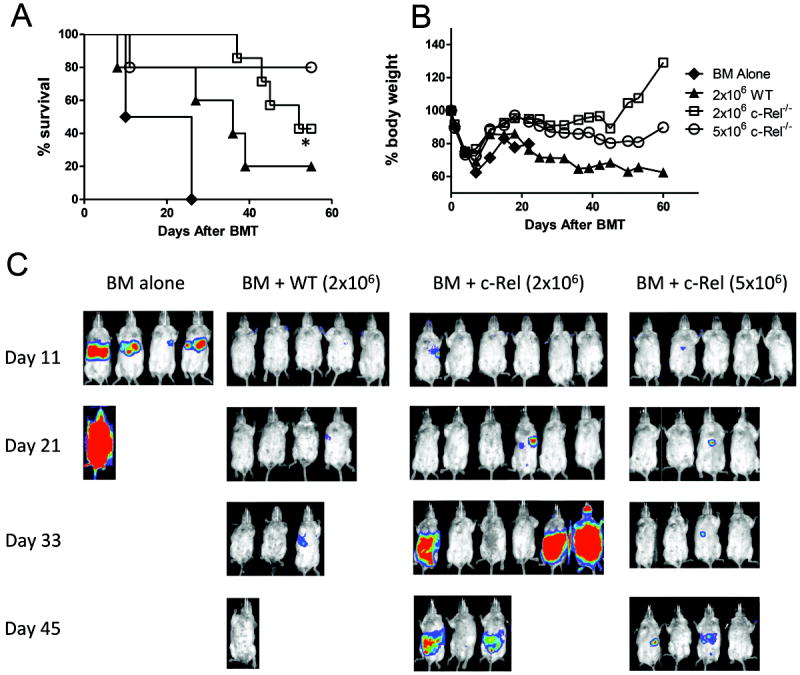
T cells deficient for c-Rel have reserved GVL activity. Lethally irradiated BALB/c mice received TCD-BM cells alone or plus 2 × 106 naïve T cells from WT or c-Rel-/- donors. Recipients were given 2 × 103 A20 tumor cells with luciferase transgene at the same time of transplantation. Overall survival (A) and the percentage of original body weight over time (B) are shown. (C) Tumor growth in recipients was monitored with in vivo BLI; data represent 1 of 2 replicate experiments. Log-rank test was used for survival analysis and 2 tailed student’s-t test was used for analyzing other experiments. Asterisk indicates statistical significance between WT and c-Rel-/- recipients. *P < 0.05.
Discussion
The transcription factor c-Rel has emerged to be an important molecule that can mediate proliferation, differentiation, and cytokine production of T cells. The extent how c-Rel regulates T-cell activation and function varies considerably. Previous studies have shown that NF-κB specific or non-specific inhibitor, PS-1145 and bortezomib significantly alleviated GVDH in allogeneic HCT [23-25]. The potential concern with bortezomib is that it may accelerate GVHD due to its gut toxicity when administered after the activation of allogeneic T cells or for a longer duration [23]. In the current study, we found that another NF-κB family member, c-Rel, also plays a key role in the pathophysiology of GVHD. c-Rel deficient T cells elicit much less GVHD than WT counterparts in both MHC and miHA mismatched BMT. Substantial evidence suggests that Th1, Th2, and Th17 CD4 T cells preferentially affect different tissues and organs in GVHD; for instance Th1 cells preferentially cause damages in intestines [14, 26]. The role for Th1 cells in the development of GVHD is supported by clinical data showing that IFN-γ-producing CD4+ T cells have been identified in patients with acute GVHD [27], and increased levels of IFN-γ protein and mRNA were also found in chronic GVHD patients [28-30]. Our current results indicate that c-Rel is a crucial transcription factor for Th1 cell differentiation after allo-HCT. However, unlike NF-κB1, which is required for Th2 cell differentiation, c-Rel appears to be required for Th1 but not Th2 cell differentiation. The mechanisms of c-Rel regulation of Th1 cell differentiation in vivo are not fully understood. Subsequent differentiation of T cells into Th1 cells is thought to occur via two pathways: 1) Upregulation of IL-12Rβ2 expression by T-bet, which amplifies the effects of IL-12 and strengthens signaling through STAT4 [31]; 2) Activation of STAT1 via IFN-γ receptor engagement, which further upregulates T-bet expression in a positive feedback loop [32]. The net effects of T-bet, STAT4, and STAT1 activation drive high-level production of IFN-γ by Th1 cells. We speculate that c-Rel, STAT-1, STAT-4, and T-bet may act in concert to regulate Th1 differentiation. Downstream c-Rel target genes that are involved in regulating Th1 cell differentiation will remain to be further studied [33].
A distinct population of CD4+ T cells, Th17 subset, has been shown to be sufficient but not necessary in the development of GVHD [12, 34]. Our data showed that c-Rel-/- T cells are defective in Th17 responses in vitro, which is consistent with the findings by others8. Given Th1-differentiation is a predominant pathway during acute GVHD, the frequency of IL-17-producing cells is typically low after allogeneic BMT. We also found low IL-17+ cells and no difference between WT and c-Rel donor T cells. In order to further investigate that Th17 cells migrated to the GVHD targeted organ, we measured expression of CCR6, a characteristic homing marker of Th17 cells [21]. Consistent with few IL-17-producing cells, the proportion of CD4+ T cells expressing CCR6 was not significantly different between WT and c-Rel-/- recipients in their liver and spleen. The higher CXCR6 expression in c-Rel-/- T cells in lung did not lead to more severe tissue damage since there were much fewer c-Rel-/- T cells infiltrated in lung.
The development of acute GVHD is often associated with reduced Tregs and thus a dramatic increase in the ratio of T effectors to Tregs that favor Th1 and Th17 differentiation. A decline in Tregs as a critical factor in the pathophysiology of chronic GVHD is also supported by several studies showing a direct correlation between a reduction in the number of Foxp3+ Tregs in both the peripheral blood and intestinal tract and the presence of chronic GVHD [35-38]. Our data showed that Foxp3+ Tregs were increased in the recipients of c-Rel-/- T cells compared to those of WT T cells, so mounting a regulatory response to the underlying inflammatory process could be a favorable factor to ameliorate GVHD development. Recent reports showed that c-Rel controls the natural Treg development in the thymus [9-11]. But in terms of the generation of induced Tregs in periphery, c-Rel is not necessary [9]. Hence, the role of c-Rel on Treg generation and expansion may differ in thymus and the periphery.
Migration of activated donor T cells to GVHD target organs is a critical step in the pathophysiology of GVHD. The interaction of chemokines and their receptors play an important role in orchestrating the migration of leukocytes to these organs [39, 40]. Donor-specific Th1 cells migrate to gastrointestinal tract and liver via chemokine receptors CXCR3, CCR9 and CCR5. Th2 cells migrate to the lung via CCR4, where they secrete IL-4, IL-5 and IL-13. IL-4 and IL-13 act upon the lung epithelium, causing inflammation and tissue remodeling that ultimately leads to pulmonary fibrosis. We found decreased levels of CXCR3 in c-Rel-/- deficient donor T cells at day 5 after BMT. CXCR3 has a major role in directing the migration of progenitors during hematopoiesis and a minor role in T cell activation, proliferation and migration to peripheral lymph nodes [41]. Thus, CXCR3 down-regulation in c-Rel-/- T cells might contribute to a reduction in the inflammatory function/phenotype and migration of these cells. CXCR3 down-regulation in donor T cells suggests that alterations in cell trafficking and homing could be one of the mechanisms by which c-Rel modulates the severity of GVHD.
Our data also indicates that TNFα production was reduced in c-Rel-/- recipient. TNFα is a key cytokine in the effector phase of GVHD following experimental and clinical allogeneic HCT [42, 43]. High level of TNFα was found in the serum of patients who developed lung injury after HCT [44] and in the lungs of animals with GVHD [45-49]. Furthermore, neutralization of TNFα by Etanercept after BMT significantly reduced the severity of experimental or clinical idiopathic pneumonia syndrome [49]. Therefore, reduced TNFα production from c-Rel-/- T cells could contribute to the diminished GVHD.
Given that allogeneic HCT is primarily utilized to treat hematologic malignancies, it is important to evaluate the contribution of donor T cells to GVL effects because transplantation would not be as beneficial for patients with a malignant disease if T cells had no activity against malignant cells. Our study shows that T cells deficient for c-Rel have preserved GVL activity against A20 lymphoma (Fig. 6). The present study indicates that T cells deficient in c-Rel induce diminished GVHD while maintaining GVL effects. Taken together, our current work provides strong evidence that c-Rel plays a pivotal role in GVHD immunopathology and set a rationale to target c-Rel for GVHD prevention or treatment after allogeneic HCT in patients.
Material and Methods
Mice
C57BL/6 (B6) (H-2b), B6.Ly5.1 and BALB/c (H-2d) mice purchased from NCI/NIH, and the founder of BALB.B (H-2b) mice were purchased from the Jackson Laboratory. c-Rel knock out B6 mice(c-Rel -/-) were generated and described previously [50]. All animals were housed in the American Association for Laboratory Animal Care–accredited Animal Resource Center at Moffitt Cancer Center. Experiments were all carried out under protocols approved by the Institutional Animal Care and Use Committee.
Antibodies and Flow cytometry
The following antibodies were purchased from eBioscience: anti-CD4–FITC, or -APC (L3T4), anti-CD8α-FITC, -APC, APC-cy7 or -Alexa Fluor 700(Ly-2), anti-CD45.1-FITC, or -APC (A20), anti-B220-PE (RA3-6B2), anti-CD25-PE (PC61.5), anti-H-2Kb-FITC, -PE, or -biotin (AF6), anti-Foxp3-PE (FJK-16s; eBioscience) and the appropriate isotype controls. Anti-CD4-Pacific blue (RM4-5), anti–IFN-γ–PE or Per-cp 5.5 (XMG1.2) and streptavidin APC-cy7 or APC, anti-TNFα-PE, or PE-Cy7 were purchased from BD Biosciences. Cells were analyzed on an LSR II (BD Biosciences). Data were analyzed using FlowJo (TreeStar). Intracelluar cytokine staining and serum cytokine analysis were carried out as described in our previously published report [51]
T cell purification, proliferation and differentiation in vitro
T cells were purified through negative selective using magnetic bead system as previously described [51]. Purified T cells isolated from WT or c-Rel-/- mice were stimulated at 2 × 105 per well with 6 × 105 irradiated T-cell depleted (TCD) splenocytes from BALB/c mice for 5 days in a 96-well plate. 1μCi per well [3H]-TdR was added to cell culture 8 hours before the end of culture. Cells were then harvested to the glass fiber filters (PerkinElmer). The [3H]-TdR incorporation was measured by liquid scintillation counting. If T cells were CFSE labeled, then the dilution of CFSE was used to indicate T cell proliferation. Differentiation of Th1 and Th17 cells was carried out by stimulating naïve CD4 T cells under polarization culture conditions as described in our previous paper [51].
BMT
Bone marrow was flushed from donor femurs and tibias with RPMI-1640 with 1% FBS and passed through sterile mesh filters to obtain single-cell suspensions. BM was T cell-depleted (TCD) in vitro with anti-Thy1.2 monoclonal antibody plus low-toxicity rabbit complement (C-6 Diagnostics). Host mice were conditioned with total body irradiation administered at 800-900 cGy (a single dose) for BALB/c and BALB.B using a Shepherd Mark I Cesium Irradiator (J.L. Shepherd and Associates). Irradiated recipients received a single intravenous injection through a lateral tail vein of 5 × 106 WT B6 BM cells with or without 2 × 106 B6 WT or c-Rel -/- T cells.
Histological analysis
Representative samples of liver, colon, small intestines and lung were obtained from transplanted recipients and histological study was done as previously described [53]
Leukemia/lymphoma models and bioluminescence imaging (BLI)
To examine the GVL effects of donor T cells, we performed the studies using the B6 → BALB/c BMT models. A20 B cell lymphoma line transduced with a luc/neo plasmid (A20-luc) was used to allow for visualization of tumor dissemination. Mice received 800 cGy TBI on day -1. On day 0, B6 recipients received grafts containing 5 × 106 BM with 2 × 106 T cells and 2 × 103 A20-Luc tumor cells. Mortality due to GVHD or tumor relapse was distinguished by BLI. The method for BLI was described in our previous work [51, 52].
Statistic analysis
For comparison of recipient survival among groups in GVHD experiments, the log-rank test was used to determine the statistical significance. To compare the engraftment and expansion of donor T cells, cytokine levels and pathology scores, a 2-tailed student’s t-test was used.
Acknowledgments
We thank Dr. S. Gerondakis for providing c-Rel KO mice. We are grateful for the technical assistance provided by Flow Cytometry and Mouse Core Facilities at the Moffitt Cancer Center. This work was supported in part by National Institutes of Health Grants R01s CA143812, CA11816 and AI 082685 to X.-Z.Y
Abbreviations
- BMT
bone marrow transplantation
- GVHD
graft-versus-host disease
- GVL
graft-versus-leukemia
- HCT
hematopoietic cell transplantation
- APC
Antigen presenting cell
- BLI
bioluminescent imaging
- TCD
T cell–depleted
- miHA
Minor histocompatibility antigen
Footnotes
Conflict of Interest: All the authors declaim no finical or commercial conflict of interest to disclose.
References
- 1.Pasparakis M. Regulation of tissue homeostasis by NF-kappa B signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 2.Brownell E, Mathieson B, Young HA, Keller J, Ihle JN, Rice NR. Detection of c-rel-related transcripts in mouse hematopoietic tissues, fractionated lymphocyte populations, and cell lines. Mol Cell Biol. 1987;7:1304–1309. doi: 10.1128/mcb.7.3.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 4.Liou H-C, Hsia CY. Distinctions between c-Rel and other NF-kappaB proteins in immunity and disease. Bioessays. 2003;25:767–780. doi: 10.1002/bies.10306. [DOI] [PubMed] [Google Scholar]
- 5.Liou H-C, Smith KA. The roles of c-rel and interleukin-2 in tolerance: a molecular explanation of self-nonself discrimination. Immunol Cell Biol. 2011;89:27–32. doi: 10.1038/icb.2010.120. [DOI] [PubMed] [Google Scholar]
- 6.Hilliard BA, Mason N, Xu LY, Sun J, Lamhamedi-Cherradi SE, Liou HC, Hunter C, et al. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843–850. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, et al. The Th17 immune response is controlled by the Rel-ROR gamma-ROR gamma T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, Hardy K, Pagler E, Ma L, Lee S, Gerondakis S, Daley S, et al. The NF-kappa B Transcription Factor c-Rel Is Required for Th17 Effector Cell Development in Experimental Autoimmune Encephalomyelitis. J Immunol. 2011;187:4483–4491. doi: 10.4049/jimmunol.1101757. [DOI] [PubMed] [Google Scholar]
- 9.Visekruna A, Huber M, Hellhund A, Bothur E, Reinhard K, Bollig N, Schmidt N, et al. c-Rel is crucial for the induction of Foxp3(+) regulatory CD4(+) T cells but not T(H)17 cells. Eur J Immunol. 2010;40:671–676. doi: 10.1002/eji.200940260. [DOI] [PubMed] [Google Scholar]
- 10.Ruan Q, Kameswaran V, Tone Y, Li L, Liou HC, Greene MI, Tone M, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruan Q, Chen YH. Nuclear factor-kappaB in immunity and inflammation: the Treg and Th17 connection. Adv Exp Med Biol. 2012;946:207–221. doi: 10.1007/978-1-4614-0106-3_12. [DOI] [PubMed] [Google Scholar]
- 12.Carlson MJ, West ML, Coghill JM, Panoskaltsis-Mortari A, Blazar BR, Serody JS. In vitro-differentiated TH17 cells mediate lethal acute graft-versus-host disease with severe cutaneous and pulmonary pathologic manifestations. Blood. 2009;113:1365–1374. doi: 10.1182/blood-2008-06-162420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, et al. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2009;113:945–952. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Wang D, Liu C, Kaosaard K, Semple K, Anasetti C, Yu X-Z. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and ROR gamma t in mice. Blood. 2011;118:5011–5020. doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pai SY, Ho IC. c-Rel delivers a one-two punch in Th1 cell differentiation. J Clin Invest. 2002;110:741–742. doi: 10.1172/JCI16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kernan NA, Dupont B. Minor histocompatibility antigens and marrow transplantation. N Engl J Med. 1996;334:323–324. doi: 10.1056/NEJM199602013340510. [DOI] [PubMed] [Google Scholar]
- 17.Robertson NJ, Chai J-G, Millrain M, Scott D, Hashim F, Manktelow E, Lemonnier F, et al. Natural regulation of immunity to minor histocompatibility antigens. J Immunol. 2007;178:3558–3565. doi: 10.4049/jimmunol.178.6.3558. [DOI] [PubMed] [Google Scholar]
- 18.Bailey DW, Mobraaten LE. Estimates of the number of loci contributing to the histoincompatibility between C57BL-6 and BALB-c strains of mice. Transplantation. 1969;7:394–400. doi: 10.1097/00007890-196905000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Edinger M, Hoffmann P, Ermann J, Drago K, Fathman CG, Strober S, Negrin RS. CD4(+)CD25(+) regulatory T cells preserve graft-versus-tumor activity while inhibiting graft-versus-host disease after bone marrow transplantation. Nat Med. 2003;9:1144–1150. doi: 10.1038/nm915. [DOI] [PubMed] [Google Scholar]
- 20.Fondi C, Nozzoli C, Benemei S, Baroni G, Saccardi R, Guidi S, Nicoletti P, et al. Increase in FOXP3(+) Regulatory T Cells in GVHD Skin Biopsies Is Associated with Lower Disease Severity and Treatment Response. Biol Blood Marrow Tr. 2009;15:938–947. doi: 10.1016/j.bbmt.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Crome SQ, Wang AY, Kang CY, Levings MK. The role of retinoic acid-related orphan receptor variant 2 and IL-17 in the development and function of human CD4(+) T cells. European J Immunol. 2009;39:1480–1493. doi: 10.1002/eji.200838908. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto Y, Bian Y, Gao P, Yashiro-Ohtani Y, Zhou XY, Ono S, Nakahara H, et al. Induction of surface CCR4 and its functionality in mouse Th2 cells is regulated differently during Th2 development. J Leukocyte Biol. 2005;78:753–761. doi: 10.1189/jlb.0305139. [DOI] [PubMed] [Google Scholar]
- 23.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107:827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun K, Welniak LA, Panoskaltsis-Mortari A, O’Shaughnessy MJ, Liu H, Barao I, Riordan W, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proc Natl Acad Sci U S A. 2004:8120–8125. doi: 10.1073/pnas.0401563101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Shaughnessy MJ, Vogtenhuber C, Sun K, Sitcheran R, Baldwin AS, Murphy WJ, Dang L, et al. Ex vivo inhibition of NF-kappaB signaling in alloreactive T-cells prevents graft-versus-host disease. Am J Transplant. 2009;9:452–462. doi: 10.1111/j.1600-6143.2008.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yi TS, Chen Y, Wang L, Du G, Huang D, Zhao DC, Johnston H, et al. Reciprocal differentiation and tissue-specific pathogenesis of Th1, Th2, and Th17 cells in graft-versus-host disease. Blood. 2009;114:3101–3112. doi: 10.1182/blood-2009-05-219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faber LM, van Luxemburg-Heijs SA, Veenhof WF, Willemze R, Falkenburg JH. Generation of CD4+ cytotoxic T-lymphocyte clones from a patient with severe graft-versus-host disease after allogeneic bone marrow transplantation: implications for graft-versus-leukemia reactivity. Blood. 1995;86:2821–2828. [PubMed] [Google Scholar]
- 28.Ochs LA, Blazar BR, Roy J, Rest EB, Weisdorf DJ. Cytokine expression in human cutaneous chronic graft-versus-host disease. Bone Marrow Transpl. 1996;17:1085–1092. [PubMed] [Google Scholar]
- 29.Ritchie D, Seconi J, Wood C, Walton J, Watt V. Prospective monitoring of tumor necrosis factor alpha and interferon gamma to predict the onset of acute and chronic graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Tr. 2005;11:706–712. doi: 10.1016/j.bbmt.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Korholz D, Kunst D, Hempel L, Sohngen D, Heyll A, Bonig H, Gobel U, et al. Decreased interleukin 10 and increased interferon-gamma production in patients with chronic graft-versus-host disease after allogeneic bone marrow transplantation. Bone Marrow Transpl. 1997;19:691–695. doi: 10.1038/sj.bmt.1700718. [DOI] [PubMed] [Google Scholar]
- 31.Mullen AC, High FA, Hutchins AS, Lee HW, Villarino AV, Livingston DM, Kung AL, et al. Role of T-bet in commitment of TH1 cells before IL-12-dependent selection. Science. 2001;292:1907–1910. doi: 10.1126/science.1059835. [DOI] [PubMed] [Google Scholar]
- 32.Afkarian M, Sedy JR, Yang J, Jacobson NG, Cereb N, Yang SY, Murphy TL, Murphy KM. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4(+) T cells. Nat Immunol. 2002;3:549–557. doi: 10.1038/ni794. [DOI] [PubMed] [Google Scholar]
- 33.Pai SY, Ho IC. c-Rel delivers a one-two punch in Th1 cell differentiation. J Clin Invest. 2002;110:741–742. doi: 10.1172/JCI16552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iclozan C, Yu Y, Liu C, Liang YM, Yi TS, Anasetti C, Yu XZ. T helper17 Cells Are Sufficient But Not Necessary to Induce Acute Graft-Versus-Host Disease. Biol Blood Marrow Tr. 2010;16:170–178. doi: 10.1016/j.bbmt.2009.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miura Y, Thoburn CJ, Bright EC, Phelps ML, Shin T, Matsui EC, Matsui WH, et al. Association of Foxp3 regulatory gene expression with graft-versus-host disease. Blood. 2004;104:2187–2193. doi: 10.1182/blood-2004-03-1040. [DOI] [PubMed] [Google Scholar]
- 36.Zorn E, Kim HT, Lee SJ, Floyd BH, Litsa D, Arumugarajah S, Bellucci R, et al. Reduced frequency of FOXP3(+) CD4(+)CD25(+) regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rieger K, Loddenkemper C, Maul J, Fietz T, Wolff D, Terpe H, Steiner B, et al. Mucosal FOXP3(+) regulatory T cells are numerically deficient in acute and chronic GvHD. Blood. 2006;107:1717–1723. doi: 10.1182/blood-2005-06-2529. [DOI] [PubMed] [Google Scholar]
- 38.Meignin V, Peffault de Latour R, Zuber J, Regnault A, Mounier N, Lemaitre F, Dastot H, et al. Numbers of Foxp3-expressing CD4+CD25high T cells do not correlate with the establishment of long-term tolerance after allogeneic stem cell transplantation. Exp Hematol. 2005;33:894–900. doi: 10.1016/j.exphem.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Sackstein R. A revision of Billingham’s tenets: The central role of lymphocyte migration in acute graft-versus-host disease. Biol Blood Marrow Tr. 2006;12:2–8. doi: 10.1016/j.bbmt.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 40.Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, Serody JS. Leukocyte migration and graft-versus-host disease. Blood. 2005;105:4191–4199. doi: 10.1182/blood-2004-12-4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jinquan T, Quan S, Jacobi HH, Jing C, Millner A, Jensen B, Madsen HO, et al. CXC chemokine receptor 3 expression on CD34(+) hematopoietic progenitors from human cord blood induced by granulocyte-macrophage colony-stimulating factor: chemotaxis and adhesion induced by its ligands, interferon gamma-inducible protein 10 and monokine induced by interferon gamma. Blood. 2000;96:1230–1238. [PubMed] [Google Scholar]
- 42.Hill GR, Teshima T, Rebel VI, Krijanovski OI, Cooke KR, Brinson YS, Ferrara JLM. The p55 TNF-alpha receptor plays a critical role in T cell alloreactivity. J Immunol. 2000;164:656–663. doi: 10.4049/jimmunol.164.2.656. [DOI] [PubMed] [Google Scholar]
- 43.Hill GR, Teshima T, Gerbitz A, Pan LY, Cooke KR, Brinson YS, Crawford JM, Ferrara JLM. Differential roles of IL-1 and TNF-alpha on graft-versus-host disease and graft versus leukemia. J Clin Invest. 1999;104:459–467. doi: 10.1172/JCI6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holler E, Kolb HJ, Moller A, Kempeni J, Liesenfeld S, Pechumer H, Lehmacher W, et al. Increased serum levels of tumor necrosis factor alpha precede major complications of bone marrow transplantation. Blood. 1990;75:1011–1016. [PubMed] [Google Scholar]
- 45.Cooke KR, Kobzik L, Martin TR, Brewer J, Delmonte J, Crawford JM, Ferrara JLM. An experimental model of idiopathic pneumonia syndrome after bone marrow transplantation. 1. The roles of minor H antigens and endotoxin. Blood. 1996;88:3230–3239. [PubMed] [Google Scholar]
- 46.Piguet PF, Grau GE, Collart MA, Vassalli P, Kapanci Y. Pneumopathies of the graft-versus-host reaction. Alveolitis associated with an increased level of tumor necrosis factor mRNA and chronic interstitial pneumonitis. Lab Invest. 1989;61:37–45. [PubMed] [Google Scholar]
- 47.Clark JG, Madtes DK, Hackman RC, Chen W, Cheever MA, Martin PJ. Lung injury induced by alloreactive Th1 cells is characterized by host-derived mononuclear cell inflammation and activation of alveolar macrophages. J Immunol. 1998;161:1913–1920. [PubMed] [Google Scholar]
- 48.Shankar G, Bryson JS, Jennings CD, Morris PE, Cohen DA. Idiopathic pneumonia syndrome in mice after allogeneic bone marrow transplantation. Am J Respir Cell Mol Biol. 1998;18:235–242. doi: 10.1165/ajrcmb.18.2.2988. [DOI] [PubMed] [Google Scholar]
- 49.Cooke KR, Hill GR, Gerbitz A, Kobzik L, Martin TR, Crawford JM, Brewer JP, et al. Tumor necrosis factor-alpha neutralization reduces lung injury after experimental allogeneic bone marrow transplantation. Transplantation. 2000;70:272–279. doi: 10.1097/00007890-200007270-00006. [DOI] [PubMed] [Google Scholar]
- 50.Kontgen F, Grumont RJ, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Gene Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 51.Yu Y, Wang D, Liu C, Kaosaard K, Semple K, Anasetti C, Yu XZ. Prevention of GVHD while sparing GVL effect by targeting Th1 and Th17 transcription factor T-bet and RORγt in mice. Blood. 2011;118:5011–5020. doi: 10.1182/blood-2011-03-340315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang Y, Liu C, Djeu JY, Zhong B, Peters T, Scharffetter-Kochanek K, Anasetti C, et al. Beta2 integrins separate graft-versus-host disease and graft-versus-leukemia effects. Blood. 2008;111:954–962. doi: 10.1182/blood-2007-05-089573. [DOI] [PMC free article] [PubMed] [Google Scholar]


