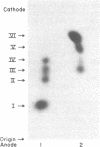
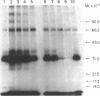
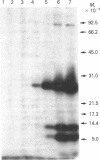
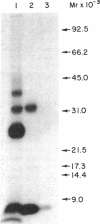
Abstract
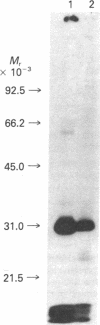
A method for the preparation of a radioisotopically labeled active-site directed reagent for proteases (125I-Tyr-Ala-Lys-ArgCH2Cl) is described, and an example of its use as a sensitive method for identifying trypsin-like proteases is provided. This high specific activity reagent was then used in an attempt to identify proteases in rat islets of Langerhans involved in the conversion of proinsulin to insulin. Previous studies have indicated that the endoprotease involved in proinsulin conversion is a cysteine proteinase and that 125I-Tyr-Ala-Lys-ArgCH2Cl affinity labels an islet crude granule fraction protein having a molecular weight of 31,500. Here we demonstrate, using a probe of higher specific activity, that the major affinity-labeled proteins of the islet crude granule fraction, when displayed by sodium dodecyl sulfate gel electrophoresis, have molecular weights of approximately 39,000 (5%), 31,500 (53%), and 5,000-6,000 (37%), with several other minor proteins (less than 5%) also labeled. The two predominant labeled proteins were mainly soluble rather than membrane bound, and they exhibited patterns of competition with various inhibitors that were similar to the pattern shown by the conversion of proinsulin to insulin in vitro. A rabbit antibody to rat liver cathepsin B immunoprecipitated both affinity-labeled 31,500 and 5,000-6,000 molecular weight proteins, and on the basis of this and structural considerations the 31,500 molecular weight cysteine protease is identified as cathepsin B. The 5,000-6,000 molecular weight peptide is an NH2-terminal, active site cysteine-containing, proteolytic fragment of the 31,500 molecular weight protein. Because cathepsin B is not per se a candidate for the proinsulin convertase because of its excessively broad substrate specificity, these studies suggest that a similar enzyme or a modified form of this enzyme is active within the secretory progranules, whereas the more typical cathepsin B may be largely confined to lysosomal contaminants in our granule preparations.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge S., Kirschke H., Friedrich K. Conversion of proinsulin into insulin by cathepsins B and L from rat liver lysosomes. Acta Biol Med Ger. 1977;36(11-12):1723–1727. [PubMed] [Google Scholar]
- Aronson N. N., Jr, Barrett A. J. The specificity of cathepsin B. Hydrolysis of glucagon at the C-terminus by a peptidyldipeptidase mechanism. Biochem J. 1978 Jun 1;171(3):759–765. doi: 10.1042/bj1710759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Blix P. M., Rubenstein A. H., Tager H. S. Iodotyrosylation of peptides using tertiary-Butyloxycarbonyl-L-[125I]iodotyrosine N-hydroxysuccinimide ester. Anal Biochem. 1980 Mar 15;103(1):70–76. doi: 10.1016/0003-2697(80)90238-9. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. S., Barrett A. J. Degradation of fructose-1,6-bisphosphate aldolase by cathepsin B. Biochem J. 1980 Jul 1;189(1):17–25. doi: 10.1042/bj1890017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Carroll R. J., Steiner D. F. Conversion of proinsulin to insulin: involvement of a 31,500 molecular weight thiol protease. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4613–4617. doi: 10.1073/pnas.79.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docherty K., Steiner D. F. Post-translational proteolysis in polypeptide hormone biosynthesis. Annu Rev Physiol. 1982;44:625–638. doi: 10.1146/annurev.ph.44.030182.003205. [DOI] [PubMed] [Google Scholar]
- Fletcher D. J., Noe B. D., Bauer G. E., Quigley J. P. Characterization of the conversion of a somatostatin precursor to somatostatin by islet secretory granules. Diabetes. 1980 Aug;29(8):593–599. doi: 10.2337/diab.29.8.593. [DOI] [PubMed] [Google Scholar]
- Fletcher D. J., Quigley J. P., Bauer G. E., Noe B. D. Characterization of proinsulin- and proglucagon-converting activities in isolated islet secretory granules. J Cell Biol. 1981 Aug;90(2):312–322. doi: 10.1083/jcb.90.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judah J. D., Quinn P. S. Calcium ion-dependent vesicle fusion in the conversion of proalbumin to albumin. Nature. 1978 Jan 26;271(5643):384–385. doi: 10.1038/271384a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loh Y. P., Gainer H. Characterization of pro-opiocortin-converting activity in purified secretory granules from rat pituitary neurointermediate lobe. Proc Natl Acad Sci U S A. 1982 Jan;79(1):108–112. doi: 10.1073/pnas.79.1.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor R. R., Hamilton J. W., Kent G. N., Shofstall R. E., Cohn D. V. The degradation of proparathormone and parathormone by parathyroid and liver cathepsin B. J Biol Chem. 1979 Jun 10;254(11):4428–4433. [PubMed] [Google Scholar]
- Pohl J., Baudys M., Tomásek V., Kostka V. Identification of the active site cysteine and of the disulfide bonds in the N-terminal part of the molecule of bovine spleen cathepsin B. FEBS Lett. 1982 Jun 1;142(1):23–26. doi: 10.1016/0014-5793(82)80210-x. [DOI] [PubMed] [Google Scholar]
- Puri R. B., Anjaneyulu K., Kidwai J. R., Mohan Rao V. K. In vitro conversion of proinsulin to insulin by cathepsin B and role of C-peptide. Acta Diabetol Lat. 1978 Sep-Dec;15(5-6):243–250. doi: 10.1007/BF02590747. [DOI] [PubMed] [Google Scholar]
- Roffman S., Sanocka U., Troll W. Sensitive proteolytic enzyme assay using differential solubilities of radioactive substrates and products in biphasic systems. Anal Biochem. 1970 Jul;36(1):11–17. doi: 10.1016/0003-2697(70)90326-x. [DOI] [PubMed] [Google Scholar]
- SCHOELLMANN G., SHAW E. Direct evidence for the presence of histidine in the active center of chymotrypsin. Biochemistry. 1963 Mar-Apr;2:252–255. doi: 10.1021/bi00902a008. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Kemmler W., Tager H. S., Peterson J. D. Proteolytic processing in the biosynthesis of insulin and other proteins. Fed Proc. 1974 Oct;33(10):2105–2115. [PubMed] [Google Scholar]
- Takahashi K., Isemura M., Ikenaka T. Isolation and characterization of three forms of cathepsin B from porcine liver. J Biochem. 1979 Apr;85(4):1053–1060. doi: 10.1093/oxfordjournals.jbchem.a132412. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Isemura M., Ono T., Ikenaka T. Location of the essential thiol of porcine liver cathepsin B. J Biochem. 1980 Jan;87(1):347–350. doi: 10.1093/oxfordjournals.jbchem.a132744. [DOI] [PubMed] [Google Scholar]
- Takio K., Towatari T., Katunuma N., Titani K. Primary structure study of rat liver cathepsin B -- a striking resemblance to papain. Biochem Biophys Res Commun. 1980 Nov 17;97(1):340–346. doi: 10.1016/s0006-291x(80)80173-2. [DOI] [PubMed] [Google Scholar]
- Trouet A. Isolation of modified liver lysosomes. Methods Enzymol. 1974;31:323–329. doi: 10.1016/0076-6879(74)31034-8. [DOI] [PubMed] [Google Scholar]
- Virji M. A., Vassalli J. D., Estensen R. D., Reich E. Plasminogen activator of islets of Langerhans: modulation by glucose and correlation with insulin production. Proc Natl Acad Sci U S A. 1980 Feb;77(2):875–879. doi: 10.1073/pnas.77.2.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoi O. O., Seldin D. C., Spragg J., Pinkus G. S., Austen K. F. Sequential cleavage of proinsulin by human pancreatic kallikrein and a human pancreatic kininase. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3612–3616. doi: 10.1073/pnas.76.8.3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zühlke H., Steiner D. F., Lernmark A., Lipsey C. Carboxypeptidase B-like and trypsin-like activities in isolated rat pancreatic islets. Ciba Found Symp. 1976;41:183–195. doi: 10.1002/9780470720233.ch10. [DOI] [PubMed] [Google Scholar]