Abstract
Background & aims
Minimal hepatic encephalopathy (MHE) has significant impact on future clinical outcomes, such as occurrence of overt HE (OHE) and survival in patients of cirrhosis. In the absence of a ‘gold standard’, psychometric hepatic encephalopathy score (PHES) is widely used for the diagnosis of MHE. This cross-sectional and prospective study was carried out to determine the usefulness of inhibitory control test (ICT) for the diagnosis of MHE.
Methods
One hundred and two patients with cirrhosis and without a history of OHE were enrolled in to the study and were subjected to PHES and ICT. MHE was diagnosed when the PHES was ≤ −5. ICT was considered abnormal when the numbers of ICT lures were more than 14.
Results
Forty-one (40.2%) patients had MHE. There were 40 patients with normal PHES and ICT, 32 with abnormal PHES and ICT, 9 with abnormal PHES and normal ICT, and 21 with abnormal ICT and normal PHES score. ICT had 78% sensitivity and 65.6% specificity and an area-under-the-curve value of 0.735 (95% CI = 0.632–0.830) for the diagnosis of MHE. In patients with cirrhosis, ICT did not correlate with severity of liver disease as measured by CTP score (r = 0.044, P = 0.658) and MELD score (r = 0.176, P = 0.077). ICT did not predict survival as well as PHES; while 6 (11.3%) patients died among those who had altered ICT compared to 4 (8.2%) patients who did not have altered ICT (P = 0.74), 8 (19.5%) patients died among those who had altered PHES compared to 2 (3.3%) patients who did not have altered PHES (P = 0.013).
Conclusion
ICT is not as useful as PHES in diagnosing MHE in patients with cirrhosis of the liver. It does not correlate with disease severity and predict survival as well as PHES.
Keywords: minimal hepatic encephalopathy; inhibitory control test, diagnosis, psychometric hepatic encepahalopahy score, natural history
Abbreviations: CTP, Child–Turcotte–Pugh; FCT, figure connection test; ICT, inhibitory control test; MELD, model for end-stage liver disease; MHE, minimal hepatic encephalopathy; NCT, number connection test; OHE, overt hepatic encephalopathy; PHES, psychometric hepatic encephalopathy score
Minimal hepatic encephalopathy (MHE) is the mild cognitive impairment of patients who have cirrhosis and/or porto-systemic shunts.1–3 MHE is considered to be one end of the clinical spectrum of HE rather than a separate phenomenon.1–3 It is associated with cognitive impairment that may have a detrimental effect on HRQOL4,5 and is likely to affect driving abilities.6–10 In view of its socio-medical relevance, simple and reproducible tests are required for routine diagnosis. But in the absence of a “gold standard”, psychometric and neurophysiological methods have been the most trusted and widely used tests to diagnose this condition.1 The psychometric hepatic encephalopathy score (PHES), which measures multiple domains of cognitive function, is a reliable test battery for the assessment of cognitive impairment in patients with MHE.1,2,11–14 The inhibitory control test (ICT) is a computerized test of attention and response inhibition that has been used to characterize attention deficit disorder, schizophrenia, and traumatic brain injury.15–18 The ICT has recently been added to the list of tests for the diagnosis of MHE. It has been validated for the diagnosis and follow-up of MHE in the USA, and has been found to be a sensitive and reliable test.19,20 However, it requires that the subject be familiar with the use of computers and needs to be validated in other populations as well. Amodio et al21 assessed the efficacy of ICT compared with diagnostic standards and found that ICT is not useful for the diagnosis of MHE, unless adjusted by target accuracy.
The study was carried out to determine the usefulness of ICT for the diagnosis of MHE and its prognostic significance on development of overt hepatic encephalopathy (OHE) and survival.
Patients and methods
The Ethics Committee of the Postgraduate Institute of Medical Education and Research (PGIMER), a tertiary level health care center in Chandigarh, India, approved the study. Each subject gave a written informed consent before inclusion in the study. The guidelines were laid down by the Indian Council of Medical Research (1994) and the Helsinki declarations (modified 1989) were adhered to in all patients in the study. A cross-sectional and prospective study was utilized.
Patient Selection
One-hundred-eighty-seven patients with cirrhosis of the liver without evidence of OHE who attended the outpatient Liver Clinic of the Department of Hepatology, Postgraduate Institute of Medical Education and Research, Chandigarh, were candidates for enrollment; 102 patients were included in the study and 85 patients were excluded as they fulfilled either one or more of exclusion criteria (Table 1, Figure 1). The diagnosis of cirrhosis of the liver was based on clinical, biochemical, and ultrasonographical, and/or liver histological data.4 Etiology work-up of cirrhosis including alcohol, chronic hepatitis B and C, autoimmune, primary biliary cirrhosis, primary sclerosing cholangitis, non-alcoholic steatohepatitis, and cryptogenic was performed as described in our previous study.4
Table 1.
Clinical and demographic characteristics of patients.
| Parameter | Patients screened (n = 187) | Patients enrolled (n = 102) | MHEa (n = 41) | NMHEa (n = 61) |
|---|---|---|---|---|
| Sex (Male)b | 168 (89.8%) | 89 (87.3%) | 37 (90.2%) | 52 (85.2%) |
| Age (in years)c | 47.57 (45.90–49.24) | 45.68 (43.38–47.97) | 46.68 (42.80–50.57) | 45 (42.11–47.89) |
| CTP Classb | ||||
| Class A | 71 (38.0%) | 54 (52.9%) | 16 (39%) | 38 (62.3%) |
| Class B | 74 (39.6%) | 35 (34.3%) | 16 (39%) | 19 (31.1%) |
| Class C | 42 (22.5%) | 13 (12.7) | 9 (22%) | 4 (6.6%) |
| MELD scorec | 13.26 (12.53–13.98) | 11.96 (11.06–12.87) | 13.29 (11.78–14.80) | 11.07 (9.97–12.17) |
| Etiologyb | ||||
| Alcohol | 120 (64.2%) | 52 (51%) | 23 (56.1%) | 29 (47.6%) |
| HBV | 22 (11.7%) | 15 (14.7%) | 9 (22.1%) | 6 (9.8%) |
| HCV | 13 (7%) | 9 (8.8%) | 4 (9.6%) | 5 (8.3%) |
| Others | 32 (17.1%) | 26 (25.5%)d | 5 (12.2%) | 21 (34.3%) |
| Education in yearsc | 11.17 (10.61–11.73) | 12.22 (11.67–12.76) | 12.39 (11.60–13.18) | 12.10 (11.34–12.86) |
CTP, Child–Turcotte–Pugh; HBV, hepatitis B virus; HCV, hepatitis C virus; MHE, minimal hepatic encephalopathy; NMHE, non-minimal hepatic encephalopathy.
MHE and NMHE columns describe patients who have been enrolled; distinction between the 2 groups was based on the results of psychometric hepatic encephalopathy score.
Number (percentage).
Mean (95% confidence interval).
Non-alcoholic steatohepatitis 5 patients, autoimmune hepatitis 5, and cryptogenic cirrhosis in 14 patients.
Figure 1.
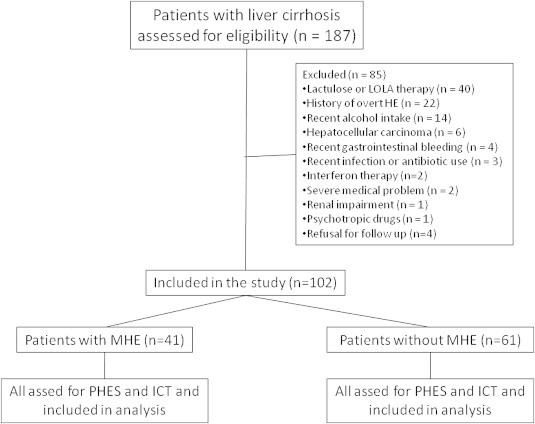
Flow chart of patients in the study.
Controls
Ninety-five healthy volunteers who were family members or friends of the patients and members of the hospital staff including ward boys, nurses, technicians or doctors and their friends were included as controls in this study. Table 2 shows details of controls and their comparison with patients. They did not have any evidence of liver disease or neurological or psychiatric disorder. None of them were on drugs that could affect psychometric performance, i.e., benzodiazepines, antiepileptics, or psychotropic agents.
Table 2.
Demographic characteristics of patients.
| Parameters | Controls (n = 95) | Cirrhotic Patients (n = 102) | P |
|---|---|---|---|
| Age (years) | 44.77 (42.3–47.2) | 45.68 (43.4–47.9) | 0.59 |
| Men:Women | 70:25 | 89:13 | 0.13 |
| Occupation | |||
| Blue collar | 63 (66.31%) | 62 (60.78%) | 0.51 |
| White collar | 32 (33.69%) | 40 (39.21%) | 0.57 |
| Education (formal education in years) | |||
| 0–8 (Junior) | 9 (9.48%) | 13 (12.74%) | 0.62 |
| 9–10 (High school) | 26 (27.37%) | 25 (24.50%) | 1.0 |
| 11–12 (Higher secondary) | 11 (11.57%) | 19 (18.62%) | 0.16 |
| >12 (Graduation/Post graduation) | 49 (51.58%) | 45 (44.11%) | 0.29 |
| Formal education in yearsa | 12.5 (11.9–13.1) | 12.2 (11.7–12.7) | 0.44 |
Mean, (95% confidence interval).
Control subjects underwent only ICT testing for determination of various cut-offs in Indian population. They did not undergo PHES because PHES has earlier been validated in Indian population and we used the cut-off for the PHES determined previously.14
Exclusion Criteria
Exclusion criteria included OHE or a history of OHE; history of recent (<3 months) alcohol intake; recent (<6 weeks) infection or antibiotic use; gastrointestinal bleeding; history of recent (<6 weeks) use of drugs such as benzodiazepines, antiepileptics, and psychotropic agents affecting psychometric performance; history of shunt surgery or transjugular intrahepatic porto-systemic shunt; electrolyte imbalance; renal impairment; presence of hepatocellular carcinoma and severe medical problems such as congestive heart failure, pulmonary disease, neurological and psychiatric disorders that could influence performance of neuropsychological tests.
Clinical and Laboratory Assessments
Clinical assessment included a thorough general physical and systemic examination, including a complete neurological assessment. The mini-mental state examination (MMSE) was performed to exclude overt cognitive impairment in all patients before PHES and ICT for the diagnosis of MHE were administered.1,2,22 West Haven criteria1 and Clinical Hepatic Encephalopathy Staging Scale (CHESS)23 for grading the mental state in patients with cirrhosis were used to differentiate between grade 0 and grade 1 HE. Laboratory investigations included a complete hemogram, serum electrolytes, renal and liver function tests, and complete coagulogram. An upper gastrointestinal endoscopy was performed in all patients for detecting the presence of esophageal varices, and the severity of liver disease was determined by Child–Turcotte–Pugh (CTP) score or class and model for end-stage liver disease (MELD) scores.
Neuropsychological Assessment
PHES has been validated in German,11 Spanish,12 Italian13 and Indian14 populations and can be performed in 15–20 min. PHES contains 6 tests: number connection test (NCT)-A, NCT-B, serial dotting test, digit symbol test, and the line tracing test for time (t) and for error (e). In the Indian version we replaced NCT-B with the figure connection test (FCT-A) because of concerns that some of our patients were not familiar with English alphabets and could not perform NCT-B.14 In principle, the FCT is similar to the NCT, except that numbers are replaced by figures (motifs). FCT is a universally applicable test to assess mental state, which transcends the barriers of linguistic differences and illiteracy. The clinical significance of these tests has been evaluated in a large number of healthy volunteers and patients with MHE.24
Diagnosis of Minimal Hepatic Encephalopathy
In Indian patients PHES ≤−5 was considered abnormal and was diagnostic of MHE.14
Inhibitory Control Test
The ICT is a computerized test of attention and response inhibition that has been used to characterize attention deficit disorder, schizophrenia, and traumatic brain injury. ICT is similar to the continuous performance test and consists of presentation of several letters at 500-ms intervals. Interspersed within these letters are the letters X and Y. The subject is instructed to respond to every X and Y during the initial part of the training run, which establishes the pre potent response. In the latter part of the training run, the subject is instructed only to respond when X and Y are alternating (called targets) and to refrain from responding when X and Y are not alternating (called lures). After the training run, 6 test runs, which last approximately 2 min each, are administered with a total of 40 lures, 212 targets, and 1728 random letters in between. At the end of the test, the lure and target response rates, lures, and target reaction times are automatically calculated. Lower lure response, higher target response, and shorter lure and target reaction times indicate good psychometric performance. The results were expressed as number and percent of incorrect lure responses, correct lure inhibitions, correct target responses, and incorrect target misses.19,20
Test–retest Reliability
A subgroup of 25 controls agreed to undergo ICT for a second time 4 weeks after the first evaluation to assess test–retest reliability.
Dietary Habits and Concurrent Therapy
There was no protein restriction and each patient was recommended a protein intake of more than 1 g/kg of body weight. Salt restriction was advised for control of ascites. Patients who were taking diuretics and β-blockers for control of ascites and portal pressure, respectively, were continued on the medications without a change.
Follow-up
Each cirrhotic patient was followed up for a minimum period of 6 months until June 30, 2010 for the external validity of the ICT. The end points were the survival and the development of OHE.
Statistical Analysis
Data were expressed as mean with 95% confidence interval (CI) and proportions with 95% CI where appropriate.25,26 Neuropsychological assessment by PHES and computerized assessment by ICT was done in all patients with cirrhosis of liver, which was correlated amongst patients in the two groups i.e. (i) without MHE and (ii) with MHE. For normally distributed data ANOVA was used and for skewed data Kruskal–Wallis test followed by Mann–Whitney test was used. Statistical analyses for categorical data were performed using χ2 test or Fisher's exact test. Cut-off value of ICT for the diagnosis of MHE was determined using ROC curves. The relationships between neuropsychological tests or PHES and ICT were assessed by Spearman-Rho rank correlation coefficient. A multivariate logistic regression analysis using the block method was performed on variables reaching a significance of P < 0.05 on univariate analysis to determine their influence on the presence of MHE. The risks estimated from the Cox regression models were expressed as hazard ratio (HR) with their respective 95% CI. The cumulative probability of death as well as the incidence of OHE in patients were analyzed using the Kaplan–Meier method. The log-rank test was used to compare Kaplan–Meier survival curves. Probability level of P < 0.05 was set for statistical significance.
Statistical analysis was performed with SPSS software for Windows, version 10.0 (SPSS Inc., Chicago, IL).
Results
Between January 1, 2009, and December 31, 2009, 187 patients with cirrhosis were screened; 102 patients (54.6%) who met the eligibility criteria were included in the study. Figure 1 shows the flow of patients into the study and reasons for the exclusion of 85 patients (45.4%). The clinical and demographic characteristics of the patients enrolled are shown in Table 1.
Of the 102 patients included in the study, 89 were men and 13 were women. Table 1 shows the etiology of cirrhosis: alcohol abuse, 52 patients (including both alcohol and hepatitis B, 2 patients; both alcohol and hepatitis C, 9 patients); chronic viral hepatitis, 24 patients (HBV, 15 patients; HCV, 9 patients); and other causes, 26 patients (non-alcoholic steatohepatitis, 7 patients; autoimmune hepatitis, 5 patients and cryptogenic cirrhosis in 14 patients).
Prevalence of Minimal Hepatic Encephalopathy
MHE was detected in 41 (40.2%) patients with cirrhosis; of 54 patients in CTP class A 16 (29.6%) had MHE, while of 35 patients in CTP class B 16 (45.7%) had MHE and of 13 patients in CTP class C 9 (69.2%) had MHE (P = 0.032). MHE was found in 23 (44.23%) of 52 patients with alcohol related cirrhosis and in 18 (36%) of 50 patients with non-alcohol related cirrhosis (P = 0.42).
PHES score correlated with CTP score (r = −0.21, P = 0.036), MELD score (r = −0.279, P = 0.005) and with ICT lures (r = −0.305, P = 0.002).
Patients with MHE had prolonged prothrombin time (P = 0.047), higher INR (P = 0.036), higher CTP (P = 0.014) and MELD scores (P = 0.020) and lower sodium values (P = 0.029). The remaining parameters—age, sex, education, etiology (alcohol versus non-alcohol), ascites (present versus absent), hemoglobin levels, white blood cells, platelets and bilirubin, aspartate transaminase, alanine transaminase, albumin, creatinine, urea and potassium levels did not differ significantly. In multivariable analysis, only CTP score was an independent predictor for presence of MHE.
Internal Validation
Sensitivity and Specificity of Inhibitory Control Test Using Psychometric Hepatic Encephalopathy Score as the Gold Standard
Although response to ICT lures was significantly higher in cirrhotic patients with MHE [19.5 (95% CI 16.7–22.3)] compared to those without MHE [12.6 (95% CI 10.6–14.6, P < 0.0001)] or compared to controls [11.3 (95% CI 10.2–12.5, P = 0.002)], there was considerable overlap between the groups (Figure 2a). The response to ICT targets was lower in cirrhotic patients with evidence of MHE [93.0 (95% CI 91.2–94.9)] compared to those without MHE [94.3 (95% CI 92.5–96.2, P = 0.049)] or compared to controls [95.7 (95% CI 94.4–97.1, P = 0.002; Figure 2b)]. ICT lures weakly correlated with percentage correct targets (r = −0.268, P = 0.007).
Figure 2.
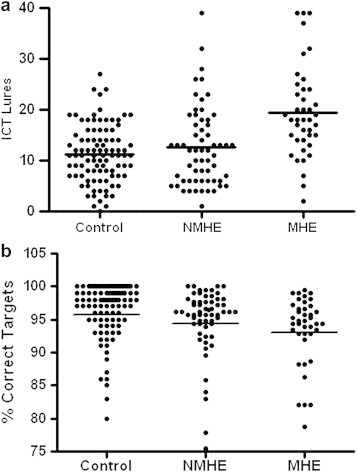
(a) Box plots showing ICT lures in cirrhotic patients with and without MHE and in controls. (b) Box plots showing response to ICT targets in cirrhotic patients with and without MHE and in controls.
ROC analysis was used to determine the cut-off value for ICT lures in Indian patients. ROC analysis of ICT lures for the diagnosis of MHE based on PHES showed an area-under-the-curve value of 0.735 (95% CI = 0.632–0.830, Figure 3) and the cut-off for ICT lures was ≥14. Using the cut-off value of 14 lures, 53 (52%) cirrhotic patients had altered ICT. There were 40 patients with normal PHES and ICT, 32 with abnormal PHES and ICT, 9 with abnormal PHES and normal ICT, and 21 with abnormal ICT and normal PHES score (Table 3). At this cut-off, ICT had 78% sensitivity and 65.6% specificity for diagnosis of MHE using PHES as a reference standard; positive predictive value was 60.4%, negative predictive value 81.6% and a diagnostic accuracy of 70.6%. In the healthy control group, a score of ≥14 ICT lures was seen in 31 of 95 (32.6%) subjects. Using the cut-off of >5 ICT lures proposed by Bajaj et al,19,20 we observed that the number of healthy patients showing altered ICT increased to 81 of 95 (88.4%). Lure weighted by target accuracy or sum of PHES score and ICT lures did not further improve the diagnostic accuracy of PHES alone.
Figure 3.
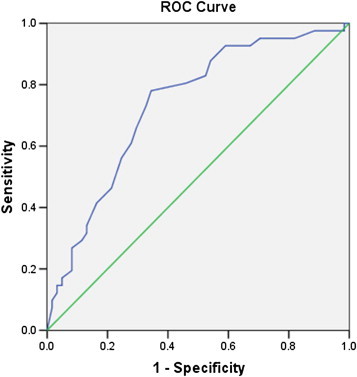
Receiver operating characteristic (ROC) curves for sensitivity and specificity of inhibitory control test in the diagnosis of MHE. With a cut-off of ICT lures ≥14, the sensitivity was 78% and specificity was 65.6%. The area-under-the curve was 0.735 (95% CI = 0.632–0.830).
Table 3.
Relationship between PHES score and ICT in 102 cirrhotics without overt hepatic encephalopathy.
| PHES | ICT |
Total | |
|---|---|---|---|
| Abnormal | Normal | ||
| Abnormal | 32 | 9 | 41 |
| Normal | 21 | 40 | 61 |
| Total | 53 | 49 | 102 |
Abbreviations: PHES, Psychometric hepatic encephalopathy score; ICT, inhibitory control test.
Factors Associated with Inhibitory Control Test
In patients with cirrhosis, response to ICT lures did not correlate with severity of liver disease as measured by CTP score (r = 0.044, P = 0.658) and MELD score (r = 0.176, P = 0.077). ICT lures did not correlate with age (r = 0.131, P = 0.189) and education (r = −0.039, P = 0.697) in cirrhotic patients.
The Spearman-Rho correlation coefficient between ICT and sum-score of PHES score was 0.305 (P = 0.002). Response to ICT lures weakly correlated with NCT-A Z score (r = 0.244, P = 0.013), FCT-A Z score (r = 0.442, P < 0.0001), DST Z score (r = 0.233, P = 0.002), and mean Z score (r = 0.327, P = 0.001). ICT lures did not correlate with SDT Z score (r = 0.185, P = 0.063), LTT for time Z score (r = 0.118, P = 0.236) and LTT for error Z score (r = 0.040, P = 0.690).
External Validation
Prediction of Death and the Risk of Developing Overt Hepatic Encephalopathy
All patients (41 with MHE and 61 without MHE) completed the study. They were followed up for at least for 6 months until June 30, 2010. The mean follow-up was 299 days (95% CI 277–321, range 61–508 and inter-quartile range 188–386 days).
Deaths
A total of 10 patients died (Table 4). There were 8 deaths (19.5%) among those who had abnormal PHES compared with 2 (3.3%) patients who did not have abnormal PHES (P = 0.013), while 6 (11.3%) patients died among those who had abnormal ICT compared with 4 (8.2%) patients who did not have abnormal ICT (P = 0.74). The number of patients who died was 5 (50.0%) when both tests indicated abnormal scores, 3 (30.0%) in patients with altered PHES and normal ICT, 1 (10.0%) in patients with normal PHES but altered ICT, and 1 (10.0%) in patients with normal values in both tests (P < 0.0001) (Table 3). All deaths except one were in patients in CTP class B and C. The only death in a patient with cirrhosis in CTP class A was because of variceal bleeding.
Table 4.
Influences of Child–Pugh–Turcotte Class, PHES, and ICT in the risk on survival.
| Test impairment | n | CTP class A (n = 54) |
CTP class B and C (n = 48) |
Total |
||
|---|---|---|---|---|---|---|
| Patients with abnormal tests [n (%)] | Deaths (n = 1) | Patients with abnormal tests [n (%)] | Deaths (n = 9) | Deaths (n = 10) | ||
| PHES abnormal | 41 | 16 (39%) | 0 | 25 (61%) | 8 | 8 |
| ICT abnormal | 53 | 26 (49.1%) | 0 | 27 (50.9%) | 6 | 6 |
| PHES and ICT abnormal | 32 | 15 (46.9) | 0 | 17 (53.1%) | 5 | 5 (50.0%) |
| PHES abnormal and ICT normal | 9 | 1 (11.1%) | 0 | 8 (88.9%) | 3 | 3 (30.0%) |
| PHES normal and ICT abnormal | 21 | 11 (52.4%) | 0 | 10 (47.6%) | 1 | 1 (10.0%) |
| PHES and ICT normal | 40 | 27 (67.5%) | 1 | 13 (32.5%) | 0 | 1 (10.0%) |
Abbreviations: PHES, Psychometric hepatic encephalopathy score; ICT, Inhibitory control test; CTP, Child–Turcotte–Pugh.
All eight deaths in MHE group patients were associated with progressive liver failure including the development of OHE in 7. In the group without MHE 1 patient died of liver failure with the development of OHE, while the other patient died of massive variceal bleeding without OHE.
Abnormal PHES was of prognostic value in predicting survival: the probability of death was higher among patients with abnormal PHES [hazard ratio 5.842 (95% CI 1.240–27.525), P = 0.026; Figure 4a]. However altered ICT (lures ≥14) did not have prognostic value for survival: the probability of death did not differ between patients with normal or abnormal ICT [hazard ratio 0.747; 95% CI 0.211–2.648, P = 0.65; Figure 4b].
Figure 4.
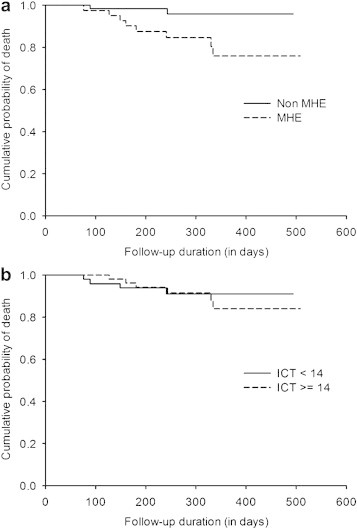
(a) Kaplan–Meier curves for prediction of death in cirrhotic patients, classified into two groups according to PHES ≤ −5 and PHES > −5 [P = 0.026, log-rank test (Mantel–Cox)]. (b). Kaplan–Meier curves for prediction of death in cirrhotic patients, classified into two groups according to ICT lures ≥14 and ICT lures <14 [P = 0.065, log-rank test (Mantel–Cox)].
Further examination of Kaplan–Meier survival curves for PHES demonstrated that they started diverging early, within100 days after initial follow-up (Figure 4a) while for ICT they started diverging late, 300 days after initial follow-up indicating independent association of PHES with patients' survival. Late divergence of curves for ICT indicate that patients with better CTP score would get a worse CTP score and then diverge; hence the CTP score rather than the cognitive tasks as assessed by ICT in this study predicts the patients' survival.
Overt Hepatic Encephalopathy
During the follow-up period, 12 patients developed OHE; 9 developed during the final event leading to death. HE was related to infection in 4 patients, upper gastrointestinal bleeding in 4 patients, diuretic use in 2 patients, renal failure in one patient, and was spontaneous in one patient. No further analysis was performed because the majority of episodes of OHE (75%) in this study were linked to death and numbers of isolated episodes of OHE were too low for meaningful analysis.
Test–retest Reliability
A randomly selected group of 25 controls underwent ICT 30 days apart to gauge the test–retest reliability. In control subjects ICT did not show any relevant change either in lures [9.2 (95% CI 6.47–11.92) vs. 10.8 (95% CI 7.9–13.7); P = 0.15] or target accuracy [97.8 (95% CI 96.8–98.8) vs. 96.4 (95%CI 94.3–94.4) P = 0.13].
Discussion
This study shows that, in Indian patients, ICT had much lower sensitivity, specificity and an area-under-the curve for MHE diagnosis compared to the figures reported earlier by Bajaj and co-workers.19,20 Though ICT is easy to administer in an outpatient setting and showed good test–retest reliability, it did not have external validity for MHE because it did not predict either survival or number of the episodes of OHE on follow-up. Thus the ICT provided cognitive measures which were not up to the recommended diagnostic standards for cognitive investigation of MHE.
This study confirms the high prevalence of MHE among patients with cirrhosis which is concordant with a previous study reporting prevalence of 48%.14 It also demonstrated that the frequency of MHE was significantly higher among patients with higher CTP score which was also found to be an independent predictor of the presence of MHE. As previously reported prevalence of MHE depends on the type of psychometric tests or neurophysiological studies used and cut-off values for these studies established inappropriate healthy controls. The prevalence of MHE has been reported to vary between 30% and 74% among patients with liver cirrhosis.2–4,27 Various risk factors for MHE as shown in the previous studies were CTP class, age, alcohol etiology, transjugular intrahepatic porto-systemic shunts, surgical porto-systemic shunts, prior episodes of OHE, and the presence of esophageal varices.2,3 None of the patients in this study had experienced a previous episode of OHE or had undergone porto-systemic shunts.
The detection of MHE is a relevant health issue, due to the impact that it has on health-related quality of life4,5,28 and driving abilities.6–10 Further treatment of MHE patients with lactulose or rifaximin show significant improvement in health-related quality of life and driving simulator performance.2,4,29,30 PHES is a reliable and preferred tool for the diagnosis of MHE which has been recommended by the working party of the 11th World Congress of Gastroenterology1 and by the consensus statement of a working party of the Indian National Association for Study of the Liver.2 The PHES, has been extensively validated in the Spanish,11 German,12 Italian13 and Indian14 populations and can be performed in 15–20 min in an outpatient setting.14–16,31 This battery of paper and pencil tests examines multiple cognitive functions such as motor speed and accuracy, visual perception, visuo-spatial orientation, visual construction, concentration, attention and working memory and relies heavily on the motor function of the patient. The weakness of this tool is the need for data related to educational level and age-adjusted distribution and it can only be administered by a qualified person. Lately, computerized tests directed toward the study of the cognitive domains have been used for the diagnosis of MHE. The ICT,19–21 together with the scan test32,33 and possibly the Cognitive Drug Research battery34 are the only computerized tests that have been expressly applied to patients with cirrhosis based on known cognitive features of MHE. ICT is a test of attention and response inhibition and has been proposed as a simple diagnostic tool for diagnosis of MHE with a high sensitivity and specificity.19,20 However, Amodio et al21 have demonstrated that the ICT is not useful for the diagnosis of MHE, unless adjusted by target accuracy. Testing inhibition (lures) does not seem to be superior to testing attention (target accuracy) for the detection of MHE. They detected a U shaped relationship between target accuracy (attention) and lures (inhibition) suggests that testing inhibition in the context of insufficient attention produces unreliable results. Authors concluded that the ICT provides cognitive measures that are related, albeit only roughly, to recommended diagnostic standards for cognitive investigation of MHE. The present study confirms observations of Amodio and co-workers.21 Although the response to ICT lures was significantly higher and the response to correct targets was significantly lower in patients with cirrhosis and MHE than in those without MHE and in controls, there was considerable overlap between the groups, limiting the ability of these ICT parameters to discriminate between these groups. Further, our results demonstrated that neither lure weighted by target accuracy nor the sum of PHES score and ICT lures could further improve upon the diagnostic accuracy of PHES alone. This can be explained by the fact that ICT examines only a small spectrum of the cognitive abnormalities seen in MHE patients where as PHES analyzes the complete spectrum. Another important point is the difference in ICT lures values in controls between different populations. The value in Indian patients in the present study (11.3 ± 5.6) was similar to the value reported in Italian patients (12.9 ± 5.8)21 but higher than in the US population (3 ± 2).19,20 Differences in age, gender and education level do not explain the difference in control values from previous studies19,20 since the study populations were comparable (Table 5). A possible explanation of this unexpected finding could be that Indian subjects are less familiar with computer use. The felicity with which a person handles the keyboard may be a key factor in deciding how many mistakes he makes.
Table 5.
Demographic characteristics of controls.
This study also addressed the external validity of the ICT by evaluating the ability of ICT to predict the cumulative probability of death and the development of OHE. The ICT did not predict either while PHES predicted both. The presence of MHE adversely affects survival of patients with liver cirrhosis. Previous studies have demonstrated strong effect of CTP score in predicting survival in patients with cirrhosis.12,14,32 This study has also demonstrated that altered PHES but not the altered ICT was associated with increased risk of death and is in accord with a previous study from this center14 which showed PHES score (≤ −6) and CTP score (≥8) independently predicted poor survival in cirrhotic patients with MHE. Amodio et al32 performed a study to assess the survival of cirrhotic patients with cognitive alterations detected by the NCT and a set of computerized psychometric tests (Scan, Choice 1, and Choice 2) measuring the reaction times and the percentage of errors in performing specific tasks. Increased risk of death was found to be associated with altered Scan test (hazard ratio = 2.4; 95% CI = 1.1–5.3), or altered Choice 2 test (hazard ratio = 2.8; 95% CI = 1.2–6.3). Multivariate regression showed that Scan and Choice 2 tests had prognostic value for survival, in addition to CTP classes in the first year of follow-up. In this study, abnormal ICT did not show any prognostic value for survival. This could be explained by the fact that ICT did not correlate with the CTP and MELD scores which are strong predictors of survival among patients with cirrhosis of liver.
During a minimum follow-up of 6 months 19.5% patients in the MHE group as compared 6.6% in the NMHE group developed OHE. These figures are lower in comparison to previous studies where the frequency of development of OHE during follow-up ranged between 22.6% and 58.6% in MHE patients and 3.9% and 17.3% in NMHE patients.35,36 Lower frequency of development of OHE may be related to the exclusion of patients with a past history of OHE and to the fact that most of the patients included in this study were in CTP class A and B.
Both inter-/intra-observer reliability and test–retest reliability are important components of the overall validation for a diagnostic test. ICT is a patient-administered instrument that has an automated, computerized analysis system. Therefore inter-/intra-observer reliability is not important. There was a high correlation of ICT lures and targets between repeated administrations, indicating excellent test–retest reliability.
A limitation of the present study was that the number of patients developing OHE was small which may be related to higher number of the cirrhotic patients in CTP class A and B and the relatively short follow-up of these patients. Future studies with a large sample size and longer follow-up are needed to determine whether the predictive ability of PHES and/or ICT for the development of OHE.
In conclusion, ICT has not been found to be useful for diagnosing MHE in patients with cirrhosis of liver.
Author roles
RKD conceived the idea for the study, performed all analyses, interpreted data, and assisted in preparing the manuscript; ST performed inhibitory control test, interpreted data, and assisted in preparing the manuscript; AK and SG performed psychometric hepatic encephalopathy score (PHES); KKT and RA performed statistical analysis and AD and YC participated in the design of the study and critically reviewed the manuscript.
Conflicts of interest
All authors have none to declare.
Acknowledgments
The paper was presented in an Oral Session at the 14th Symposium of the International Society on Hepatic encephalopathy and Nitrogen Metabolism (ISHEN), Val-David, Canada, September 14–18, 2010 and ST received ISHEN Travel Fellowship for the same.
References
- 1.Ferenci P., Lockwood A., Mullen K., Tarter R., Weissenborn K., Blei A.T. Hepatic encephalopathy-definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 2.Dhiman R.K., Saraswat V.A., Sharma B.K. Indian National Association for Study of the Liver. Minimal hepatic encephalopathy: consensus statement of a working party of the Indian National Association for Study of the Liver. J Gastroenterol Hepatol. 2010;25:1029–1041. doi: 10.1111/j.1440-1746.2010.06318.x. [DOI] [PubMed] [Google Scholar]
- 3.Dhiman R.K., Chawla Y.K. Minimal hepatic encephalopathy. Indian J Gastroenterol. 2009;28:5–16. doi: 10.1007/s12664-009-0003-6. [DOI] [PubMed] [Google Scholar]
- 4.Prasad S., Dhiman R.K., Duseja A., Chawla Y., Sharma A., Agarwal R. Lactulose improves cognitive functions and health-related quality of life in cirrhotic patients with minimal hepatic encephalopathy. Hepatology. 2007;45:549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 5.Groeneweg M., Quero J.C., De Bruijn I. Subclinical hepatic encephalopathy impairs daily functioning. Hepatology. 1998;28:45–49. doi: 10.1002/hep.510280108. [DOI] [PubMed] [Google Scholar]
- 6.Wein C., Koch H., Popp B., Oehler G., Schauder P. Minimal hepatic encephalopathy impairs fitness to drive. Hepatology. 2004;39:739–745. doi: 10.1002/hep.20095. [DOI] [PubMed] [Google Scholar]
- 7.Bajaj J.S., Hafeezullah M., Hoffmann R.G., Saeian K. Minimal hepatic encephalopathy: a vehicle for accidents and traffic violations. Am J Gastroenterol. 2007;102:1903–1909. doi: 10.1111/j.1572-0241.2007.01424.x. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj J.S., Ananthakrishnan A.N., McGinley E.L., Hoffmann R.G., Brasel K.J. Deleterious effect of cirrhosis on outcomes after motor vehicle crashes using the nationwide inpatient sample. Am J Gastroenterol. 2008;103:1674–1681. doi: 10.1111/j.1572-0241.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- 9.Bajaj J.S., Saeian K., Schubert C.M. Minimal hepatic encephalopathy is associated with motor vehicle crashes: the reality beyond the driving test. Hepatology. 2009;50:1175–1183. doi: 10.1002/hep.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajaj J.S., Hafeezullah M., Hoffmann R.G. Navigation skill impairment: another dimension of the driving difficulties in minimal hepatic encephalopathy. Hepatology. 2008;47:596–604. doi: 10.1002/hep.22032. [DOI] [PubMed] [Google Scholar]
- 11.Weissenborn K., Ennen J.C., Schomerus H., Rückert N., Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768–773. doi: 10.1016/s0168-8278(01)00026-5. [DOI] [PubMed] [Google Scholar]
- 12.Romero-Gómez M., Córdoba J., Jover R. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879–885. doi: 10.1002/hep.21586. [DOI] [PubMed] [Google Scholar]
- 13.Amodio P., Campagna F., Olianas S. Detection of minimal hepatic encephalopathy: normalization and optimization of the Psychometric Hepatic Encephalopathy Score. A neuropsychological and quantified EEG study. J Hepatol. 2008;49:346–353. doi: 10.1016/j.jhep.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 14.Dhiman R.K., Kurmi R., Thumburu K.K. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci. 2010;55:2381–2390. doi: 10.1007/s10620-010-1249-7. [DOI] [PubMed] [Google Scholar]
- 15.Epstein J.N., Johnson D.E., Varia I.M., Conners C.K. Neuropsychological assessment of response inhibition in adults with ADHD. J Clin Exp Neuropsychol. 2001;23:362–371. doi: 10.1076/jcen.23.3.362.1186. [DOI] [PubMed] [Google Scholar]
- 16.Konrad K., Gauggel S., Manz A., Schöll M. Inhibitory control in children with traumatic brain injury (TBI) and children with attention deficit/hyperactivity disorder (ADHD) Brain Inj. 2000;14:859–875. doi: 10.1080/026990500445691. [DOI] [PubMed] [Google Scholar]
- 17.Garavan H., Ross T.J., Stein E.A. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci USA. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pliszka S.R., Liotti M., Woldorff M.G. Inhibitory control in children with attention-deficit/hyperactivity disorder: event-related potentials identify the processing component and timing of an impaired right-frontal response-inhibition mechanism. Biol Psychiatry. 2000;48:238–246. doi: 10.1016/s0006-3223(00)00890-8. [DOI] [PubMed] [Google Scholar]
- 19.Bajaj J.S., Saeian K., Verber M.D., Hischke D., Hoffmann R.G., Franco J. Inhibitory control test is a simple method to diagnose minimal hepatic encephalopathy and predict development of overt hepatic encephalopathy. Am J Gastroenterol. 2007;102:754–760. doi: 10.1111/j.1572-0241.2007.01048.x. [DOI] [PubMed] [Google Scholar]
- 20.Bajaj J.S., Hafeezullah M., Franco J. Inhibitory control test for the diagnosis of minimal hepatic encephalopathy. Gastroenterology. 2008;135:1591–1600. doi: 10.1053/j.gastro.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Amodio P., Ridola L., Schiff S. Improving the inhibitory control task to detect minimal hepatic encephalopathy. Gastroenterology. 2010;139:510–518. doi: 10.1053/j.gastro.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 22.Folstein M., Folstein S.E., McHugh P.R. “Mini-Mental State” a practical method for grading the cognitive state of patients for the clinician. J Psych Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz M., Córdoba J., Doval E. Development of a clinical hepatic encephalopathy staging scale. Aliment Pharmacol Ther. 2007;26:859–867. doi: 10.1111/j.1365-2036.2007.03394.x. [DOI] [PubMed] [Google Scholar]
- 24.Dhiman R.K., Saraswat V.A., Verma M., Naik S.R. Figure connection test: a universal test for assessment of mental state. J Gastroenterol Hepatol. 1995;10:14–23. doi: 10.1111/j.1440-1746.1995.tb01041.x. [DOI] [PubMed] [Google Scholar]
- 25.Armitage P., Berry G., Matthews J.N.S. Statistical Methods in Medical Research. 4th ed. Oxford: Blackwell Science; 2002. Analysing means and proportions; pp. 83–146. [Google Scholar]
- 26.Newcombe R. Two-sided confidence intervals for the single proportion: a comparative evaluation of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Das A., Dhiman R.K., Saraswat V.A., Naik S.R. Prevalence and natural history of subclinical hepatic encephalopathy in cirrhosis. J Gastroenterol Hepatol. 2001;16:531–535. doi: 10.1046/j.1440-1746.2001.02487.x. [DOI] [PubMed] [Google Scholar]
- 28.Schomerus H., Hamster W. Quality of life in cirrhotics with minimal hepatic encephalopathy. Metab Brain Dis. 2001;16:37–41. doi: 10.1023/a:1011610427843. [DOI] [PubMed] [Google Scholar]
- 29.Sidhu S.S., Goyal O., Mishra B.P., Sood A., Chhina R.S., Soni R.K. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (The RIME Trial) Am J Gastroenterol. 2010;106:307–316. doi: 10.1038/ajg.2010.455. [DOI] [PubMed] [Google Scholar]
- 30.Bajaj J.S., Heuman D.M., Wade J.B. Rifaximin improves driving simulator performance in a randomized trial of patients with minimal hepatic encephalopathy. Gastroenterology. 2011;140:478–487. doi: 10.1053/j.gastro.2010.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissenborn K. PHES: one label, different goods?! J Hepatol. 2008;49:308–312. doi: 10.1016/j.jhep.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 32.Amodio P., Del Piccolo F., Marchetti P. Clinical features and survival of cirrhotic patients with subclinical cognitive alterations detected by the number connection test and computerized psychometric tests. Hepatology. 1999;29:1662–1667. doi: 10.1002/hep.510290619. [DOI] [PubMed] [Google Scholar]
- 33.Amodio P., Marchetti P., Del Piccolo F. Study on the Sternberg paradigm in cirrhotic patients without overt hepatic encephalopathy. Metab Brain Dis. 1998;13:159–172. doi: 10.1023/a:1020665431411. [DOI] [PubMed] [Google Scholar]
- 34.Mardini H., Saxby B.K., Record C.O. Computerized psychometric testing in minimal encephalopathy and modulation by nitrogen challenge and liver transplant. Gastroenterology. 2008;135:1582–1590. doi: 10.1053/j.gastro.2008.06.043. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann I.J., Groeneweg M., Quero J.C. The prognostic significance of subclinical hepatic encephalopathy. Am J Gastroenterol. 2000;95:2029–2034. doi: 10.1111/j.1572-0241.2000.02265.x. [DOI] [PubMed] [Google Scholar]
- 36.Saxena N., Bhatia M., Joshi Y.K., Garg P.K., Dwivedi S.N., Tandon R.K. Electrophysiological and neuropsychological tests for the diagnosis of subclinical hepatic encephalopathy and prediction of overt encephalopathy. Liver. 2002;22:190–197. doi: 10.1034/j.1600-0676.2002.01431.x. [DOI] [PubMed] [Google Scholar]


