Abstract
The Asia Pacific region is the most diverse and the most populous region in the world. Recent socioeconomic changes have resulted in an emerging epidemic of non-communicable diseases such as type 2 diabetes and nonalcoholic fatty liver disease. The prevalence of nonalcoholic fatty liver disease in Asian Pacific countries now approximates that seen in Western countries. This increase is fueled by rising obesity, partly due to adoption of Western style diets and exposure to compounds such as high fructose corn syrup that are not included in traditional diets. Furthermore, South Asian populations may be more genetically susceptible via the inheritance of polymorphisms in apolipoprotein 3 that increase insulin resistance and nonalcoholic fatty liver disease. Importantly, there remains a substantial lack of data on the incidence and natural history of nonalcoholic steatohepatitis and subsequent complications such as hepatocellular carcinoma in Asian Pacific populations. This information gap prevents estimation of current and future disease burden and impedes efforts to lobby health policymakers to improve public health measures, as given the size of Asian Pacific populations, prevention rather than treatment of non-communicable diseases remains key. This review article addresses these issues and highlights research priorities for nonalcoholic fatty liver disease within the Asia Pacific region.
Keywords: nonalcoholic steatohepatitis, fatty liver, Asia Pacific, review
Abbreviations: NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; BMI, body mass index; HCC, hepatocellular carcinoma; CURES, Chennai Urban Rural Epidemiology Study; HBV, hepatitis B virus; HCV, hepatitis C virus
Consider this. In 1988, there were 40 million people with type 2 diabetes in India.1 In 2011, India had 63 million diabetics and by 2030, that number is predicted to be a staggering 90 million.2 Nonalcoholic fatty liver disease (NAFLD) the hepatic manifestation of the metabolic syndrome is closely associated with type 2 diabetes with NAFLD present in more than 50% of type 2 diabetics. Other countries in the Asia Pacific region have faced a similar diabetes explosion; for example China had 8 million type 2 diabetics in 1996, 32 million by 2010 and is predicted to have around 60 million by 2030.2 The data for diabetes incidence in Asian Pacific countries is consistent and valid, and when considered with less robust data on NAFLD, it suggests that the Asia Pacific could face an eruption of metabolic liver disease over the next few decades.
The reasons for this increase in type 2 diabetes (and NAFLD) are partly explained by theories of socioeconomic effects on health. China and India, as developing countries, are part of the way along the nutrition transition.3 This theory represents changes in the health of a society with economic growth and urbanization (Figure 1). Developed countries of the Asia Pacific such as Australia and New Zealand have been in stage 4 for some time, where life expectancy is high but with an increased period of disability due to non-communicable diseases related to the metabolic syndrome, such as obesity and type 2 diabetes. NAFLD is now the commonest cause of abnormal liver function in stage 4 countries such as the United States and is projected to be the leading cause of liver transplantation in that country by 2020.4 The traditional cause of advanced liver disease in the Asia Pacific region has been viral hepatitis, but current socioeconomic changes suggest that metabolic liver disease may soon contribute an additional and substantial health burden.
Figure 1.
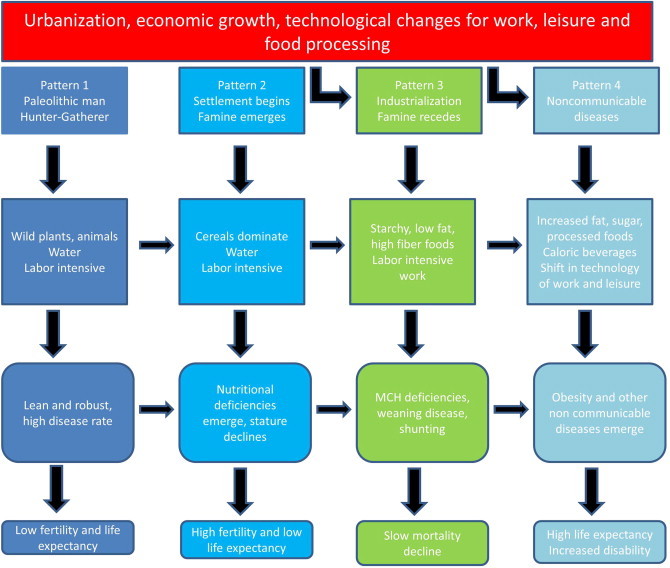
Stages of the nutrition transition. Developing countries such as India and China are entering pattern 4 (Adapted from Popkin 20023).
The Asia Pacific region extends from China and Mongolia in the north to the tip of New Zealand in the south, and includes more than 50 countries each with a unique culture, diet and economic predicament. This diversity is difficult to cover in a single paper, hence we have concentrated on the two geographically and genetically distinct areas of South Asia (covering India, Sri Lanka, Pakistan, Nepal and Bangladesh) and East Asia (including China, Korea, Japan, Taiwan). In this review, we detail published incidence and prevalence figures for NAFLD, and will outline recent findings on influential polymorphisms in South Asian populations compared with East Asian and Caucasian populations, and the implications these genetic differences may have on the natural history. We discuss what is known about the consumption of dietary components that influence progression of NAFLD, particularly high fructose corn syrup and trans fats. We highlight the synergistic effect of viral hepatitis, obesity and type 2 diabetes on the incidence of hepatocellular carcinoma in the Asia Pacific. Current gaps in data that affect our ability to model future disease burden and plan accordingly will be emphasized and to conclude, a prioritized research agenda is proposed.
Prevalence and incidence of nonalcoholic fatty liver disease in Asian Pacific countries
There are no prospective, long duration, methodologically robust studies of NAFLD incidence from the Asia Pacific, hence the true incidence is not known. In contrast, the prevalence has been reasonably well studied in general populations in a number of Asian Pacific countries and published estimates are shown in Figure 2. Ultrasound has been the principal diagnostic tool used, however ultrasound based studies that detect steatosis may underestimate prevalence as the sensitivity compared with the gold standard of liver biopsy is around 70%.5 More importantly, the advanced forms of NAFLD including nonalcoholic steatohepatitis (NASH) and fibrosis cannot be established at ultrasound and require alternative diagnostic tools of liver biopsy or noninvasive techniques such as ultrasound based elastography. Published data from the Asia Pacific with other methods has been scarce, with a recent exception.6 Overall, and in the context of these research limitations, ultrasound based studies suggest that the community prevalence of NAFLD in the Asia Pacific is at least equal to that of Western countries.
Figure 2.
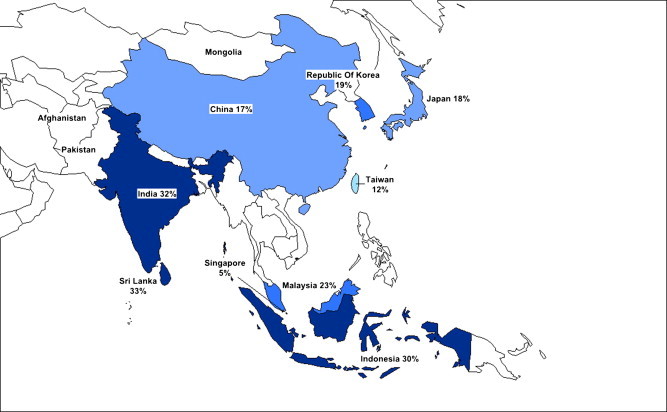
Prevalence of nonalcoholic fatty liver disease in Asian Pacific countries.
East Asia
Prevalence studies of nonalcoholic fatty liver disease in China have been available since the 1990s and indicate a median prevalence of NAFLD of 10% that varies according to location (urban or rural) and occupation.7 More recent studies show an overall increase, but maintain the environmental contrast. The highest published prevalence is 27% in urban Hong Kong Chinese using magnetic resonance spectroscopy.6 Studies from Mainland China indicate a prevalence of 25% in urban areas8 to 5% in rural areas.9 In a study from Shanghai, where ultrasound was performed on the same group of factory employees in 1995 and in 2002, diagnosis of NAFLD increased from 4% to 14%, while alcohol intake remained constant.10 Ultrasound based studies from other parts of East Asia indicate a similar prevalence. In Japan, prevalence figures from 9%11 to 18%12 to 29%13 have been reported. The prevalence in Korea is estimated at 19%14 and up to 30% in Indonesia.15
South Asia
The highest prevalence of NAFLD in the Asia Pacific region exists in India and Sri Lanka. In urban populations in India, studies suggest up to 30% may have NAFLD,16 with lower figures reported from people residing in railway colonies (16%).9 The prevalence in Sri Lanka has been recorded at 33%.17 There are no published figures for Nepal or Bangladesh.
Influential genetic polymorphisms and their importance in the Asia Pacific
South Asians may incur a ‘double-hit’ phenomenon when it comes to NAFLD, with not only a rising prevalence due to socioeconomic factors, but also a genetic predisposition that influences incidence and natural history. Insulin resistance, a key pathophysiological determinant of NAFLD18 is distinctly more common in South Asians than in other ethnic groups of comparable weight and body mass index (BMI). This ethnic specific association with insulin resistance was first reported more than 20 years ago.19 Since then, it has been shown that South Asians have a different adipokine profile than Caucasians, with lower adiponectin and higher leptin,20 and different anthropometrics with reduced lean body mass and greater visceral adiposity which is proportionately related to insulin resistance.21,22 This was quantitated in a study that compared insulin resistance in young, lean, healthy subjects of similar weight from South Asian, East Asian, Black, Hispanic and Caucasian backgrounds, and demonstrated a fourfold increase in insulin resistance in Indian males compared with any other group.23 Further genetic studies in Indian males have found that carriers of variant polymorphisms for the gene encoding apolipoprotein C3 which influences triglyceride metabolism were far more likely to have NAFLD (38% of the carriers had NAFLD compared with 0% of those who did not have these variant alleles).24 These findings were validated in a group of Caucasian males24 however other studies have not consistently reproduced this association,25 and further work is needed. Genome-wide association studies have shown that a single variant allele in PNAPLA3 also appears strongly linked with hepatic fat content, but not with measures of insulin resistance, and this was most striking in people with Hispanic ancestry.26
The salient question is whether a genetic predisposition to insulin resistance is associated with faster progression to advanced liver disease in South Asians. This has not been studied, but large scale epidemiologic studies indicate that the natural history of other conditions associated with metabolic syndrome such as cardiovascular disease is less favorable in South Asians. Indians have a higher incidence of acute coronary syndromes at an earlier age and a higher mortality from cardiovascular disease.27 This difference is not explained by conventional risk factors, and South Asian ancestry is an independent risk factor in multivariate models. South Asians have a higher cumulative burden of risk factors for cardiovascular disease at a younger age than their Caucasian counterparts.28 Given these observations, there is an urgent need to study the natural history of NAFLD in populations from the Asia Pacific, and South Asians in particular.
The “Westernization” of diet in the Asia Pacific
Epidemiologic studies suggest that two compounds in particular, trans fats and high fructose corn syrup, drive inflammation in NAFLD. These compounds are not part of traditional diets, but as Asia Pacific adopts an increasingly “Western” diet, higher consumption may play a role in progression of NAFLD from benign steatosis to an inflammatory and progressive form. Trans fats are byproducts produced during manufacture of saturated fats and are contained in processed foods including baked and fried foods. In animals, trans fats cause severe hepatic inflammation29 and excess consumption in humans increases hepatic transaminases. Furthermore, trans fats have been implicated as an independent risk factor for cardiovascular disease.30 The evidence for increased intake in Indians is limited, although trans fats are contained in the most commonly used cooking oil, Vanaspati, which is cheap and widely available, and young Indian males have a high intake of trans fats due to Vanaspati oil.31
High fructose corn syrup, primarily found in soft drinks and to sweeten highly processed foods, has been recognized to predispose to insulin resistance for some years.32 High fructose corn syrup increases hepatic lipogenesis,32 has a dense caloric content and impairs normal satiety.33 Observational data from the United States indicates that fructose consumption is a risk factor for NAFLD in this population,34 and this may be a target for public health measures in the Asia Pacific given the increasing intake of soft drink and aggressive marketing by transnational soft drink companies in developing populations.
What might be the future disease burden of nonalcoholic fatty liver disease in the Asia Pacific?
In order to calculate the future burden of liver disease due to NAFLD, and in standard units such as disability adjusted life years that can be compared with the burden due to other chronic health disorders, we need data on the annual incidence of NASH cirrhosis, NASH-related hepatocellular carcinoma (HCC) and NASH-related mortality. This figure can then be applied to susceptible populations to provide an estimate of expected new cases. Prevalence statistics alone are insufficient to model health care burden, and at present, we cannot calculate estimates that can be used to lobby policymakers and advocate for better patient care.
It is possible to estimate the current prevalence of NASH in India and China indirectly by using data on type 2 diabetes prevalence. In 2011, the current prevalence of type 2 diabetes in India was estimated as 63 million2 although some estimates are higher,35 and studies indicate that around 70% of Indian diabetics have NAFLD.36–38 This would suggest that currently, there may be over 44 million people with NAFLD in India. A well conducted population based study from India estimated that 31% of people with NAFLD had at least mild NASH,39 suggesting that there may be 13 million people with NASH in India at present. In large Indian transplant centers, 15% of liver transplants are performed for NASH-related liver disease and 5% of liver transplants for hepatocellular carcinoma (HCC) are NASH-related HCC (Dr S Gupta, Centre for Liver and Biliary Sciences, New Delhi; personal communication).
In China, assuming a conservative estimate of population prevalence of NAFLD of 10%,6 there may be 130 million Chinese with NAFLD. Recent studies on Hong Kong Chinese patients with NAFLD using elastography estimates that 4% have advanced fibrosis,7 equating to 5 million Chinese currently with advanced fibrosis who are at risk of end stage liver disease and HCC in the near future. India's population is expected to overtake that of China's in the next 2 decades, and while these figures are approximations only, to reduce disease burden in the Asia Pacific region, prevention and treatment of NAFLD and its advanced forms in South Asia particularly is an important focus.
The natural history of NAFLD and NASH in Asian Pacific patients has not been studied in long term cohort studies. A retrospective study with dual biopsies in 17 patients taken a median 6 years apart suggests that histological progression among Chinese patients is comparable with Caucasians.40 A retrospective study of Japanese cirrhotics (n = 68) showed similar survival rates between cirrhosis from NASH and HCV.41 Of importance, the natural history of NAFLD is not inexorably one way, with some studies suggesting that up to one third of Caucasian patients with NAFLD may have regression,42 particularly if patients lose weight, and this appears true for Japanese cohorts.12 Interestingly, recent data from the United States indicates that the prevalence of obesity has now leveled off at around 30%,43 and this may reflect saturation of the at-risk population in developed countries which has not yet been reached in developing countries.
To improve accuracy of future predictions, we need better data on the amount of advanced fibrosis in the Asia Pacific, and thus we need to develop and validate noninvasive tools in these populations. We need to test and validate promising biomarkers such as cytokeratin-18 in Asian populations.44 Predictive scores for fibrosis that are useful in Caucasians may be less accurate in Asians due to the inclusion of BMI45 as cut-offs for healthy BMI are lower in Asian populations46 (Table 1). Hence, an additional research priority is the development of ethnic-specific predictive scores.
Table 1.
Body mass index and corresponding risk categories for type 2 diabetes and cardiovascular disease in Asian populations.46
| Body mass index | Risk category |
|---|---|
| Less than 18.5 kg/m2 | Underweight |
| 18.5–23 kg/m2 | Increasing but acceptable risk |
| 23–27.5 kg/m2 | Increased risk |
| 27.5 kg/m2 or higher | High risk |
What is the combined impact of metabolic syndrome, nonalcoholic fatty liver disease and viral hepatitis in the Asia Pacific on the risk of advanced liver disease and primary liver cancer?
Few long term studies on the effect of nonalcoholic fatty liver disease complicating viral hepatitis in Asian Pacific populations exist. A cross sectional analysis from Chinese populations suggests that the metabolic syndrome increases progression to advanced liver disease in concurrent hepatitis B infection.47 For people with hepatitis C infection, an increased body mass index may accelerate fibrosis,48 and the cut-off at which this occurs may be lower than in Caucasians because visceral adiposity is proportionately higher in Asians.
Viral hepatitis, NASH and type 2 diabetes are all described as independent risk factors for hepatocellular carcinoma (HCC),49–51 and this combination appears synergistic in inducing HCC.52 This association is stronger in people with HCV infection than for HBV infection,52 possibly because the hepatitis C virus is directly involved in inducing insulin resistance.53 In addition, emerging data suggests that curative treatment for HCC may be less effective in the presence of visceral fat, as data from Taiwanese patients with NASH demonstrated that increased visceral fat is associated with HCC recurrence after treatment.54
What does the future hold for the prevention and treatment of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in the Asia Pacific?
Given the size of the population of the Asia Pacific region, prevention of diseases associated with obesity and insulin resistance, rather than treatment of its sequelae, is the key to controlling the epidemic. This necessitates a multifaceted, multidisciplinary approach. Interestingly, preventative interventions may be highly valuable when started in-utero. The ‘thrifty phenotype’ hypothesis suggests that intrauterine malnutrition results in smaller birth weights and predisposition to metabolic diseases in adult life.55 Clearly, proving causality in this theory is challenging, but micronutrient supplementation to mothers in China has resulted in increased birth weights in pregnant females56 and importantly, it is a low cost intervention. For prevention, lifestyle modification remains the advocated approach for NAFLD in Western countries57 and randomized trials demonstrate histological benefit proportional to weight loss.58 It is effective in reducing diabetes incidence in populations across Asia including China59 and India.60 Lifestyle modification is the primary treatment modality for NAFLD principally because it has such an attractive risk to benefit profile. However, the disadvantages are the intensive patient education and support required, and the long term outcomes can be disappointing. Even in controlled trials that enroll highly selected and educated patients who are offered multiple individualized sessions, weight loss with diets with varying macronutrient composition remains less than 4 kg at 2 years.61 Moreover, factors such as a high intake of sweetened soft drinks that are increasingly relevant in the Asia Pacific are negatively associated with durable long term weight loss.62 Individually driven lifestyle modification is also likely to be ill suited to populations where baseline education is low. Research into awareness of diabetes in a general Indian population highlights illustrates this problem. In the CURES study in India, 25% of the population did not know that a condition called diabetes existed and 88% were not aware that obesity and a sedentary lifestyle were risk factors for diabetes.63 Furthermore, less than 1 in 5 people knew that diabetes was associated with chronic ill health and complications. Physical activity alone is likely to have varying efficacy across the Asia Pacific, with recent reviews on global physical activity levels indicating that countries such as China do well in areas such as the use of active transportation to work where more than a quarter of the population cycle or walk.64 There is little published data on national physical activity levels in India, although what exists suggests that urban populations generally lead a sedentary lifestyle and physical activity is higher in rural areas.65 During this phase of rapid urbanization of the Asia Pacific, governments should use this opportunity to integrate measures of urban planning that facilitate active work and leisure time.
Pharmacologic treatments used in Western populations for advanced NASH such as thiazolidinediones have been shown to be effective in South Asians in improving insulin resistance (although no liver related data exists), and the benefit conferred may be greater than that experienced by Caucasians.66 Bariatric surgery for complications of the metabolic syndrome including NAFLD is very successful in highly selected patients, producing superior metabolic and histological benefits67,68 and the most recent, long term data suggests excellent results in prevention of type 2 diabetes incidence compared with lifestyle modification alone.69 Bariatric surgery in the Asia Pacific has become more frequent over the last 5 years70 with laparoscopic adjustable gastric banding the most common procedure. Clearly, however, the magnitude of at-risk populations in the Asia Pacific region combined with the cost and resources needed for vigilant peri-operative care, would appear to render bariatric surgery unfeasible as a population wide treatment option.
What can the academic community of hepatologists in the Asia Pacific do?
There are now a number of regional working parties that have been established with a primary research focus on NAFLD, including the Asia-Pacific Working Party on NAFLD71 and the Japan NASH study group.72 These groups are well placed to establish regionally important research priorities, with a focus on data that is critically needed to better manage NAFLD, and to steer funding bodies to support these priorities in order to make a tangible difference with limited resources. Selected research priorities for NAFLD in the Asia Pacific region are highlighted in Table 2.
Table 2.
Research priorities for the Asia Pacific region.
| Topic | What is currently known? | High priority questions for the research agenda |
|---|---|---|
| NAFLD incidence | No prospective, long duration incidence studies exist | What is the incidence of NASH-related cirrhosis, HCC and mortality in the Asia Pacific, particularly India and China? |
| Natural history | A study from Japan suggests similar outcomes for cirrhosis due to hepatitis C infection and NASH | Do South Asians, given different genetic profiles and known differences on cardiovascular disease outcomes, have accelerated disease progression and worse outcomes? |
| Diagnosis of fibrosis | A study in Hong Kong Chinese suggested a low prevalence of advanced fibrosis in the community | What is the current prevalence of early and advanced fibrosis due to NAFLD in India and Sri Lanka? |
| Predictive scores are not valid for non-Caucasian patients | Development of noninvasive predictive scores with validation in different ethnicities | |
| Knowledge of disease associated with adverse lifestyles | Community knowledge of diabetes and its sequelae is poor | How effective are interventions to modify lifestyle for Asian Pacific patients with NAFLD? |
Conclusion
The Asia Pacific region is facing an epidemic of diseases related to the metabolic syndrome, including nonalcoholic fatty liver disease. The increasing prevalence of nonalcoholic fatty liver disease in the context of established viral hepatitis and genetic susceptibility for populations within the Asia Pacific is likely to result in a significant rise in end stage liver disease over the coming decades. There remains an information gap on the incidence of advanced liver disease and end stage complications of nonalcoholic fatty liver disease, and research into these areas is urgently required to better inform health care planning.
Conflicts of interest
All authors have none to declare.
References
- 1.Ramachandran A., Jali M.V., Mohan V. High prevalence of diabetes in an urban population in south India. BMJ. 1988;297(6648):587–590. doi: 10.1136/bmj.297.6648.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shaw J.E., Sicree R.A., Zimmet P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Popkin B.M., Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes. 2004;28:S2–S9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M.R., Burns J.M., Pedersen R.A. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141(4):1249–1253. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 5.Bohte A.E., van Werven J.R., Bipat S. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21(1):87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong V.W.-S., Chu W.C.-W., Wong G.L.-H. Prevalence of non-alcoholic fatty liver disease and advanced fibrosis in Hong Kong Chinese: a population study using proton-magnetic resonance spectroscopy and transient elastography. Gut. 2012;61(3):409–415. doi: 10.1136/gutjnl-2011-300342. [DOI] [PubMed] [Google Scholar]
- 7.Fan J.-G., Farrell G.C. Epidemiology of non-alcoholic fatty liver disease in China. J Hepatol. 2009;50(1):204–210. doi: 10.1016/j.jhep.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z., Xia B., Ma C. Prevalence and risk factors of fatty liver disease in the Shuiguohu district of Wuhan city, central China. Postgrad Med J. 2007;83(977):192–195. doi: 10.1136/pgmj.2006.052258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amarapurkar D., Hashimoto E., Lesmana L.A. How common is non-alcoholic fatty liver disease in the Asia Pacific region and are there local differences? J Gastroenterol Hepatol. 2007;22:788–793. doi: 10.1111/j.1440-1746.2007.05042.x. [DOI] [PubMed] [Google Scholar]
- 10.Fan J.-G., Li F., Cai X.-B. The importance of metabolic factors for the increasing prevalence of fatty liver in Shanghai factory workers. J Gastroenterol Hepatol. 2007;22(5):663–668. doi: 10.1111/j.1440-1746.2007.04892.x. [DOI] [PubMed] [Google Scholar]
- 11.Omagari K., Kadokawa Y., Masuda J.-I. Fatty liver in non-alcoholic non-overweight Japanese adults: incidence and clinical characteristics. J Gastroenterol Hepatol. 2002;17(10):1098–1105. doi: 10.1046/j.1440-1746.2002.02846.x. [DOI] [PubMed] [Google Scholar]
- 12.Hamaguchi M., Kojima T., Takeda N. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease. Ann Intern Med. 2005;143(10):722–728. doi: 10.7326/0003-4819-143-10-200511150-00009. [DOI] [PubMed] [Google Scholar]
- 13.Jimba S., Nakagami T., Takahashi M. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 2005;22(9):1141–1145. doi: 10.1111/j.1464-5491.2005.01582.x. [DOI] [PubMed] [Google Scholar]
- 14.Park S.H., Jeon W.K., Kim S.H. Prevalence and risk factors of non-alcoholic fatty liver disease among Korean adults. J Gastroenterol Hepatol. 2006;21(1 Pt 1):138–143. doi: 10.1111/j.1440-1746.2005.04086.x. [DOI] [PubMed] [Google Scholar]
- 15.Hasan I., Gani R.A., Machmud R. Prevalence and risk factors for nonalcoholic fatty liver in Indonesia. (Abstract) J Gastroenterol Hepatol. 2002;17(suppl):A30. [Google Scholar]
- 16.Mohan V., Farooq S., Deepa M. Prevalence of non-alcoholic fatty liver disease in urban south Indians in relation to different grades of glucose intolerance and metabolic syndrome. Diabetes Res Clin Pract. 2009;84(1):84–91. doi: 10.1016/j.diabres.2008.11.039. [DOI] [PubMed] [Google Scholar]
- 17.Dassanayake A.S., Kasturiratne A., Rajindrajith S. Prevalence and risk factors for non-alcoholic fatty liver disease among adults in an urban Sri Lankan population. J Gastroenterol Hepatol. 2009;24(7):1284–1288. doi: 10.1111/j.1440-1746.2009.05831.x. [DOI] [PubMed] [Google Scholar]
- 18.Chitturi S., Abeygunasekera S., Farrell G.C. NASH and insulin resistance: insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35(2):373–379. doi: 10.1053/jhep.2002.30692. [DOI] [PubMed] [Google Scholar]
- 19.McKeigue P.M., Shah B., Marmot M.G. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet. 1991;337(8738):382–386. doi: 10.1016/0140-6736(91)91164-p. [DOI] [PubMed] [Google Scholar]
- 20.Abate N., Chandalia M., Snell P.G. Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J Clin Endocrinol Metab. 2004;89(6):2750–2755. doi: 10.1210/jc.2003-031843. [DOI] [PubMed] [Google Scholar]
- 21.Banerji M.A., Faridi N., Atluri R. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84(1):137–144. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 22.Raji A., Seely E.W., Arky R.A. Body fat distribution and insulin resistance in healthy Asian Indians and Caucasians. J Clin Endocrinol Metab. 2001;86(11):5366–5371. doi: 10.1210/jcem.86.11.7992. [DOI] [PubMed] [Google Scholar]
- 23.Petersen K.F., Dufour S., Feng J. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. Proc Natl Acad Sci USA. 2006;103(48):18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen K.F., Dufour S., Hariri A. Apolipoprotein C3 gene variants in nonalcoholic fatty liver disease. N Engl J Med. 2010;362(12):1082–1089. doi: 10.1056/NEJMoa0907295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kozlitina J., Boerwinkle E., Cohen J.C. Dissociation between APOC3 variants, hepatic triglyceride content and insulin resistance. Hepatology. 2011;53(2):467–474. doi: 10.1002/hep.24072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romeo S., Kozlitina J., Xing C. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40(12):1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheth T., Nair C., Nargundkar M. Cardiovascular and cancer mortality among Canadians of European, South Asian and Chinese origin from 1979 to 1993: an analysis of 1.2 million deaths. CMAJ. 1999;161(2):132–138. [PMC free article] [PubMed] [Google Scholar]
- 28.Joshi P., Islam S., Pais P. Risk factors for early myocardial infarction in South Asians compared with individuals in other countries. J Am Med Assoc. 2007;297(3):286–294. doi: 10.1001/jama.297.3.286. [DOI] [PubMed] [Google Scholar]
- 29.Tetri L.H., Basaranoglu M., Brunt E.M. Severe NAFLD with hepatic necroinflammatory changes in mice fed trans fats and a high-fructose corn syrup equivalent. Am J Physiol Gastrointest Liver Physiol. 2008;295(5):G987–G995. doi: 10.1152/ajpgi.90272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mozaffarian D., Katan M.B., Ascherio A. Trans fatty acids and cardiovascular disease. N Engl J Med. 2006;354(15):1601–1613. doi: 10.1056/NEJMra054035. [DOI] [PubMed] [Google Scholar]
- 31.Misra A., Sharma R., Pandey R.M. Adverse profile of dietary nutrients, anthropometry and lipids in urban slum dwellers of northern India. Eur J Clin Nutr. 2001;55(9):727–734. doi: 10.1038/sj.ejcn.1601214. [DOI] [PubMed] [Google Scholar]
- 32.Elliott S.S., Keim N.L., Stern J.S. Fructose, weight gain, and the insulin resistance syndrome. Am J Clin Nutr. 2002;76(5):911–922. doi: 10.1093/ajcn/76.5.911. [DOI] [PubMed] [Google Scholar]
- 33.Teff K.L., Elliott S.S., Tschop M. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab. 2004;89(6):2963–2972. doi: 10.1210/jc.2003-031855. [DOI] [PubMed] [Google Scholar]
- 34.Ouyang X., Cirillo P., Sautin Y. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol. 2008;48(6):993–999. doi: 10.1016/j.jhep.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohan V., Deepa M., Deepa R. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India – the Chennai Urban Rural Epidemiology Study (CURES-17) Diabetologia. 2006;49(6):1175–1178. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 36.Prashanth M., Ganesh H.K., Vima M.V. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus. J Assoc Physicians India. 2009;57:205–210. [PubMed] [Google Scholar]
- 37.Gupte P., Amarapurkar D., Agal S. Non-alcoholic steatohepatitis in type 2 diabetes mellitus. J Gastroenterol Hepatol. 2004;19(8):854–858. doi: 10.1111/j.1440-1746.2004.03312.x. [DOI] [PubMed] [Google Scholar]
- 38.Targher G., Bertolini L., Rodella S. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30(8):2119–2121. doi: 10.2337/dc07-0349. [DOI] [PubMed] [Google Scholar]
- 39.Das K., Das K., Mukherjee P.S. Nonobese population in a developing country has a high prevalence of nonalcoholic fatty liver and significant liver disease. Hepatology. 2010;51(5):1593–1602. doi: 10.1002/hep.23567. [DOI] [PubMed] [Google Scholar]
- 40.Hui A.Y., Wong V.W.-S., Chan H.L.-Y. Histological progression of non-alcoholic fatty liver disease in Chinese patients. Aliment Pharmacol Ther. 2005;21(4):407–413. doi: 10.1111/j.1365-2036.2005.02334.x. [DOI] [PubMed] [Google Scholar]
- 41.Yatsuji S., Hashimoto E., Tobari M. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24(2):248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 42.Adams L.A., Sanderson S., Lindor K.D. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42(1):132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 43.Flegal K.M., Carroll M.D., Kit B.K. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. J Am Med Assoc. 2012;307(5):491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 44.Wong V.W.-S., Wong G.L.-H., Choi P.C.-L. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59(7):969–974. doi: 10.1136/gut.2009.205088. [DOI] [PubMed] [Google Scholar]
- 45.Wong V.W.-S., Wong G.L.-H., Chim A.M.-L. Validation of the NAFLD fibrosis score in a Chinese population with low prevalence of advanced fibrosis. Am J Gastroenterol. 2008;103(7):1682–1688. doi: 10.1111/j.1572-0241.2008.01933.x. [DOI] [PubMed] [Google Scholar]
- 46.WHO expert consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 47.Wong G.L.H., Wong V.W.-S., Choi P.C.-L. Metabolic syndrome increases the risk of liver cirrhosis in chronic hepatitis B. Gut. 2009;58(1):111–117. doi: 10.1136/gut.2008.157735. [DOI] [PubMed] [Google Scholar]
- 48.Ortiz V., Berenguer M., Rayon J.M. Contribution of obesity to hepatitis C-related fibrosis progression. Am J Gastroenterol. 2002;97(9):2408–2414. doi: 10.1111/j.1572-0241.2002.05995.x. [DOI] [PubMed] [Google Scholar]
- 49.Bugianesi E., Leone N., Vanni E. Expanding the natural history of nonalcoholic steatohepatitis: from cryptogenic cirrhosis to hepatocellular carcinoma. Gastroenterology. 2002;123(1):134–140. doi: 10.1053/gast.2002.34168. [DOI] [PubMed] [Google Scholar]
- 50.Veldt B.J., Chen W., Heathcote E.J. Increased risk of hepatocellular carcinoma among patients with hepatitis C cirrhosis and diabetes mellitus. Hepatology. 2008;47(6):1856–1862. doi: 10.1002/hep.22251. [DOI] [PubMed] [Google Scholar]
- 51.Lai M.-S., Hsieh M.-S., Chiu Y.-H. Type 2 diabetes and hepatocellular carcinoma: a cohort study in high prevalence area of hepatitis virus infection. Hepatology. 2006;43(6):1295–1302. doi: 10.1002/hep.21208. [DOI] [PubMed] [Google Scholar]
- 52.Chen C.-L., Yang H.-I., Yang W.-S. Metabolic factors and risk of hepatocellular carcinoma by chronic hepatitis B/C infection: a follow-up study in Taiwan. Gastroenterology. 2008;135(1):111–121. doi: 10.1053/j.gastro.2008.03.073. [DOI] [PubMed] [Google Scholar]
- 53.Shintani Y., Fujie H., Miyoshi H. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126(3):840–848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 54.Ohki T., Tateishi R., Shiina S. Visceral fat accumulation is an independent risk factor for hepatocellular carcinoma recurrence after curative treatment in patients with suspected NASH. Gut. 2009;58(6):839–844. doi: 10.1136/gut.2008.164053. [DOI] [PubMed] [Google Scholar]
- 55.Hales C.N., Barker D.J. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35(7):595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 56.Zeng L., Dibley M.J., Cheng Y. Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomised controlled trial. BMJ. 2008;337 doi: 10.1136/bmj.a2001. a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ratziu V., Bellentani S., Cortez-Pinto H. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol. 2010;53(2):372–384. doi: 10.1016/j.jhep.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 58.Promrat K., Kleiner D.E., Niemeier H.M. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–129. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pan X.R., Li G.W., Hu Y.H. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 60.Ramachandran A., Snehalatha C., Mary S. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1) Diabetologia. 2006;49(2):289–297. doi: 10.1007/s00125-005-0097-z. [DOI] [PubMed] [Google Scholar]
- 61.Sacks F.M., Bray G.A., Carey V.J. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mozaffarian D., Hao T., Rimm E.B. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohan D., Raj D., Shanthirani C.S. Awareness and knowledge of diabetes in Chennai – the Chennai Urban Rural Epidemiology Study [CURES-9] J Assoc Physicians India. 2005;53:283–287. [PubMed] [Google Scholar]
- 64.Hallal P.C., Andersen L.B., Bull F.C. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–257. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 65.Sullivan R., Kinra S., Ekelund U. Socio-demographic patterning of physical activity across migrant groups in India: results from the Indian Migration Study. PLoS One. 2011;6(10) doi: 10.1371/journal.pone.0024898. e24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raji A., Gerhard-Herman M.D., Williams J.S. Effect of pioglitazone on insulin sensitivity, vascular function and cardiovascular inflammatory markers in insulin-resistant non-diabetic Asian Indians. Diabet Med. 2006;23(5):537–543. doi: 10.1111/j.1464-5491.2006.01843.x. [DOI] [PubMed] [Google Scholar]
- 67.Dixon J.B., Bhathal P.S., Hughes N.R. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology. 2004;39(6):1647–1654. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 68.Klein S., Mittendorfer B., Eagon J.C. Gastric bypass surgery improves metabolic and hepatic abnormalities associated with nonalcoholic fatty liver disease. Gastroenterology. 2006;130(6):1564–1572. doi: 10.1053/j.gastro.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 69.Carlsson L.M.S., Peltonen M., Ahlin S. Bariatric surgery and prevention of type 2 diabetes in Swedish obese subjects. N Engl J Med. 2012;367(8):695–704. doi: 10.1056/NEJMoa1112082. [DOI] [PubMed] [Google Scholar]
- 70.Lomanto D., Lee W.-J., Goel R. Bariatric surgery in Asia in the last 5 years (2005–2009) Obes Surg. 2012;22(3):502–506. doi: 10.1007/s11695-011-0547-2. [DOI] [PubMed] [Google Scholar]
- 71.Farrell G.C., Chitturi S., Lau G.K.K. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22(6):775–777. doi: 10.1111/j.1440-1746.2007.05002.x. [DOI] [PubMed] [Google Scholar]
- 72.Okanoue T., Umemura A., Yasui K. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis in Japan. J Gastroenterol Hepatol. 2011;26(suppl 1):153–162. doi: 10.1111/j.1440-1746.2010.06547.x. [DOI] [PubMed] [Google Scholar]


