Abstract
Background
Compared to HIV-negative women, HIV-infected women have increased risk of low birth weight (LBW) and preterm delivery (PTD). We assessed whether severity of maternal HIV-1 disease was associated with LBW or PTD.
Methods
Secondary analysis of The Malaria and HIV in Pregnancy prospective cohort, which enrolled HIV-positive, pregnant Malawian women from 2000–2004. Included participants (n=809) were normotensive, antiretroviral treatment naïve women who delivered a live, singleton infant. Binomial regression models were used to assess the unadjusted and adjusted prevalence ratios (PRs) and 95% confidence intervals (CI) of the effect of severity of HIV-1 disease, defined by viral load and CD4-positive T-cell counts, on prevalence of LBW and PTD.
Results
In unadjusted analyses, among those with malaria (n=198), there was no association between severity of HIV-1 infection and LBW, while among women without malaria (n=611), we observed a harmful association between both increasing peripheral viral load and LBW (PR: 1.44 per one-log10 increase, 95% CI: 1.12, 1.86) and placental viral load and LBW (PR: 1.24 per one-log10 increase, 95% CI: 1.00, 1.53). We observed a similar association between increasing placental viral load and PTD (PR: 1.33 per one-log10 increase, 95% CI: 1.04, 1.69). These associations persisted in multivariate models adjusted for residence, maternal education, primigravid status, and maternal anemia.
Conclusions
In malaria-negative women, maternal HIV-1 disease severity was significantly associated with increased prevalence of LBW and PTD. Such an association was not found in the malaria-infected women.
Keywords: Human immunodeficiency virus type 1 (HIV-1), adverse birth outcomes, low birth weight, preterm delivery, placenta, sub-Saharan Africa
INTRODUCTION
Women of child-bearing age comprise approximately half of the estimated 33.3 million people living with HIV-1. Children born to HIV-infected women have an increased risk of adverse birth outcomes, including congenital HIV-1 infection, and low birth weight (LBW)1–3. Malaria is also prevalent among pregnant women, and children born to women with malaria also have an increased risk of LBW4. LBW is defined as “infants born weighing 2500 grams or less, regardless of gestational age or cause of LBW”, and it can be caused by preterm delivery (PTD; birth before 37 weeks’ gestation), intrauterine growth restriction (IUGR), or congenital/genetic abnormalities. LBW is a significant cause of infant morbidity and mortality5. In 2010, PTD was the second leading cause of child death worldwide6, and Malawi had the highest prevalence of PTD in the world7.
The placenta provides fetal nourishment and serves as a barrier to fetal infection, and deficiencies in placental function are causally linked to obstetric complications8. Infections that colonize the placenta, such as Plasmodium falciparum, are associated with adverse birth outcomes9, 10. Plasmodium falciparum parasites can be sequestered in the intervillous space of the placenta 11, and placental malaria has been associated with higher HIV-1 concentration in both peripheral and placental plasma 12. Our group13 and others14 have shown that HIV-1 replicates in the placenta, and it has also been shown that HIV-1 infection alters the cytokine profile in the placenta15–17. Thus, when evaluating risk factors for adverse birth outcomes, potential interactions between malaria and HIV-1 coinfection should be considered 18. The objective of this study was to determine if the severity of HIV-1 infection is associated with LBW or PTD.
METHODS
Participant enrollment
We describe a secondary analysis of data collected through The Malaria and HIV-1 in Pregnancy (MHP) prospective cohort study. The primary purpose of the parent study was to determine the association between malaria and HIV-1 mother-to-child transmission (MTCT)19. From December 2000 until March 2004, women who presented for delivery to the Antenatal Ward at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi, were screened for eligibility for MHP. Women were ineligible if they were in the active phase of labor, were participating in other research studies, lived outside Blantyre district, were less than 15 years of age, were hypertensive, had altered consciousness, delivered a stillbirth, or twins. HIV-infected women and their offspring received nevirapine according to the HIVNET 012 protocol. Only women who delivered at QECH were included in this study.
Definitions
Gestational age at delivery was self-reported. Maternal CD4+ T-cells were quantified by FACScan and HIV-1 RNA was quantified using Amplicor HIV-1 Monitor v1.5 (Roche Diagnostics). Peripheral and placental thick blood films were Giemsa-stained and examined for P. falciparum parasites (malaria) by two trained microscopists. Thick smears were considered P. falciparum negative if parasites were not observed after counting 200 leukocytes. Full-thickness placenta sections were formalin-fixed and paraffin embedded according to standard procedures. After Giemsa staining, placenta sections were examined for malaria parasites19. Women were considered syphilis seroreactive if they were both RPR and TPHA positive, as described20. Anemia was defined as hemoglobin <11 g/dL; “any malaria” was defined as a positive malaria diagnosis on peripheral blood smear or placental blood smear or histology. Infant HIV-1 status at birth was determined by real-time polymerase chain reaction19. Chorioamnionitis was assessed by histopathological examination according to published methods21.
Statistical analysis
Statistical analysis was performed with STATA (Version 10.1) and SAS (Version 9.2). Using binomial regression models, we assessed the unadjusted and adjusted prevalence ratios (PRs) and 95% confidence intervals (CI) of the effect of HIV-1 severity on prevalence of adverse birth outcomes. We characterized HIV-1 severity through three separate primary exposure variables: a) continuous placental HIV-1 viral load; b) continuous maternal peripheral HIV-1 viral load and c) continuous maternal CD4+ T-cell count. We characterized adverse birth outcomes through two separate, primary, dichotomously-coded outcome variables: LBW and PTD. We separately assessed each exposure-outcome relationship to produce unadjusted and adjusted PRs. We selected binomial regression for multivariable modeling because the prevalence of each outcome was greater than 10%, the cut-off value above which logistic regression is not recommended 22. All three HIV-1 severity variables met the assumption of linearity in the log risk for each outcome. Multivariable models were constructed as follows. Using product-interaction terms, we evaluated whether any of the exposure-outcome associations under investigation varied by maternal malaria status or maternal anemia. A p-value for any interaction term of <0.20 was interpreted as evidence of meaningful modification. We developed a starting list of potential confounders of each exposure-outcome association using Directed Acyclic Graphs23. Variables assessed for possible confounding included those previously associated with either HIV-1 severity or adverse birth outcomes, and included demographic characteristics (age, education, rural/urban residence), clinical factors (intermittent preventative therapy for malaria during pregnancy or IPTp, maternal syphilis, anemia, chorioamnionitis, primigravid status) and infant factors (gender). To construct final models, we used a manual, backward elimination, change-in-estimate strategy24, with a 10% threshold for variable retention. To ease interpretability, any variable surviving backward elimination for any of the models was retained in all multivariable models.
Sensitivity analyses
Our primary analyses evaluated the effect of HIV-1 severity separately on prevalence of LBW and PTD. We undertook three sensitivity analyses to assess the robustness of our findings. First, because preterm infants will naturally have lower birthweight because of their shorter gestation, we repeated our LBW analyses after removing from the dataset all observations with PTD. We then reran our unadjusted and adjusted multivariable models (using the same set of adjustment variables as in the main analysis) to see whether any increase in prevalence of LBW due to increased severity of HIV-1 disease persisted in term infants. Second, because fetal HIV-1 status may be associated with adverse birth outcomes, we repeated both the LWB and PTD analyses after restricting the dataset to only children confirmed to be HIV-negative at delivery. We again reran the unadjusted and adjusted models to determine whether the associations between HIV-1 disease severity and adverse birth outcomes changed. Third, to further assess potential modification of the exposure-outcome association by malaria status, we examined separately malaria diagnosed by peripheral blood smear versus placental blood smear versus placental histology.
Ethical approval
MHP was approved by the College of Medicine Research Committee at the University of Malawi and the Institutional Review Boards (IRB) of the University of Michigan and the University of North Carolina at Chapel Hill. The analyses presented in this manuscript also received IRB approval from the Ohio State University.
RESULTS
Characteristics of HIV-positive participants who delivered live, singleton infants in the Malaria and HIV-1 in Pregnancy study, Malawi
Among the HIV-infected women in the parent Malaria and HIV-1 in Pregnancy (MHP) cohort, 809 live, singleton deliveries occurred, 80% via vaginal delivery. Participants’ median age at enrollment was 24 (interquartile range (IQR): 21 to 28 years) and the median gravidity was two (IQR: 1 to 4 pregnancies) (Table 1). A majority of the participants had attended primary school (91%), were unemployed (75%) and lived in an urban setting (76%). At the time of delivery, 56% of the participants had anemia, 26% had histological evidence of chorioamnionitis, and seven percent were syphilis seropositive. One-quarter of the participants were primigravid. Most (86%) participants took iron-folate supplements during the pregnancy and 93% received at least one dose of IPTp for malaria (Table 1). With regard to malaria, ten percent of the women were peripheral blood smear positive, eight percent were placental blood smear positive, and 18% had histological evidence of malaria in a placental biopsy. Because all HIV-positive women in the MHP study received their HIV-1 diagnosis at enrollment, all participants were antiretroviral treatment naïve; 95% of the HIV-infected participants received single-dose nevirapine to prevent HIV-1 mother-to-child transmission.
Table I.
Characteristics of HIV-positive participants who delivered live, singleton infants in the Malaria and HIV-1 in Pregnancy study, Malawi, 2000–2004.
| CHARACTERISTIC | N= 809 |
|---|---|
| Median (IQR), n | |
| Age (years) | 24 (21, 28), 638 |
| Blood pressure | |
| Systolic | 110 (100, 120), 809 |
| Diastolic | 70 (60, 70), 809 |
| Body mass index at delivery | 24 (22, 26), 788 |
| CD4+ T-cell count, cells/µL | 343 (198, 516), 755 |
| Gestational age, weeks | 39 (38, 40), 772 |
| Gravidity | 2 (1, 4), 809 |
| Hemoglobin concentration [g/dL] | 11 (9, 12), 805 |
| Infant birth weight, kilograms | 2·9 (2·5, 3·2), 808 |
| Placental weight, grams | 520 (440, 600), 770 |
| Peripheral HIV-1 concentration, log10 copies/mL | 4·5 (4·0, 5·0), 539 |
| Placental HIV-1 concentration, log10 copies/mL | 3·7 (2·8, 4·4), 275 |
| n (%) | |
| Anemia (hemoglobin < 11 g/dL) | |
| No | 353 (44) |
| Yes | 452 (56) |
| Missing | 4 (1) |
| Chorioamnionitis | |
| No | 469 (57) |
| Yes | 207 (26) |
| Missing | 143 (17) |
| Education level (maternal) | |
| None | 70 (9) |
| Primary school | 477 (59) |
| Secondary school | 260 (32) |
| University | 1 (0) |
| Missing | 1 (0) |
| Employment | |
| No | 603 (75) |
| Yes | 186 (23) |
| Missing | 20 (2) |
| Infant gender | |
| Female | 395 (49) |
| Male | 414 (51) |
| Iron-folate tablets (during antenatal period) | |
| No | 111 (14) |
| Yes | 698 (86) |
| Missing | 0 (0) |
| Low birth weight (<2·5 kilograms) | |
| No | 637 (79) |
| Yes | 171 (21) |
| Missing | 1 (0) |
| Malaria | |
| Peripheral blood smear positive | |
| No | 728 (90) |
| Yes | 80 (10) |
| Missing | 1 (0) |
| Placental blood smear positive | |
| No | 710 (88) |
| Yes | 62 (8) |
| Missing | 37 (5) |
| Placental histology positive | |
| No | 580 (72) |
| Yes | 147 (18) |
| Missing | 82 (10) |
| Any malaria | |
| No | 611 (76) |
| Yes | 198 (24) |
| Missing | 0 (0) |
| Intermittent Presumptive Treatment | |
| None | 55 (7) |
| 1 Dose | 261 (32) |
| 2 Doses | 314 (39) |
| 3 Doses | 175 (22) |
| Missing | 4 (0) |
| Mode of delivery | |
| Elective cesarean section | 21 (3) |
| Emergency cesarean section | 137 (17) |
| Vaginal, breech | 12 (1) |
| Vaginal, vacuum extraction | 38 (5) |
| Vaginal, vertex | 601 (74) |
| Missing | 0 (0) |
| Preterm delivery (<37 weeks) | |
| No | 648 (80) |
| Yes | 124 (16) |
| Missing | 37 (5) |
| Residence | |
| Urban | 612 (76) |
| Periurban | 94 (12) |
| Rural | 88 (11) |
| Missing | 15 (2) |
| Syphilis seropositivity | |
| No | 746 (92) |
| Yes | 60 (7) |
| Missing | 3 (0) |
Definitions: n/a= not available; any malaria= a positive malaria diagnosis on peripheral blood smear or placental blood smear or placental histology.
Low birth weight and preterm delivery are common in Malawi
In this cohort of HIV-infected women, 21% of participants delivered a LBW infant and 16% delivered preterm. Age, malaria status, and gravidity were not associated with prevalence of LBW or PTD (Table 2). Anemia was more common among women with infants with LBW (68%) and PTD (68%) compared to women with normal weight, term infants (52%). Chorioamnionitis was also more commonly observed in the LBW (33%) and PTD (37%) groups compared to the normal weight, term delivery group (23%). Female sex was more prevalent among LBW infants (57%) compared to normal weight, term infants (46%). Finally, syphilis seropositivity was more common among mothers of PTD infants (11%) than normal weight, term infants (6%) (Table 2).
Table 2.
Characteristics of HIV-infected women, by adverse birth outcome (n=809).
| None N=570 |
Low birth weight N=171 |
Preterm delivery N=124 |
|
|---|---|---|---|
| CHARACTERISTIC | Median (IQR), n | Median (IQR), n | Median (IQR), n |
| Age, years | 24 (21,27), 457 | 24 (20, 28), 128 | 24 (20, 29), 99 |
| Body mass index at delivery | 24 (23, 26), 554 | 22 (21, 24), 167 | 23 (21, 25), 124 |
| Gravidity | 2 (1, 4), 570 | 2 (2, 4), 171 | 3 (2, 4), 124 |
| [HIV-1], (periphery)* | 4·5 (3·8, 5·0), 387 | 4·6 (4·2, 5·2), 110 | 4·6 (4·0, 5·1), 86 |
| [HIV-1] (placenta)* | 3·5 (2·7, 4·3), 187 | 4·1 (2·9, 4·8), 65 | 4·0 (3·2, 4·7), 54 |
| Placental weight, grams | 540 (470, 620), 548 | 420 (370, 500), 162 | 450 (390, 520), 119 |
| n (%) | n (%) | n (%) | |
| Anemia (hemoglobin <11 g/dL) | |||
| No | 269 (47) | 55 (32) | 40 (32) |
| Yes | 297 (52) | 116 (68) | 84 (68) |
| Missing | 4 (1) | 0 (0) | 0 (0) |
| Chorioamnionitis | |||
| No | 337 (59) | 87 (51) | 65 (52) |
| Yes | 131 (23) | 57 (33) | 46 (37) |
| Missing | 102 (18) | 27 (16) | 13 (10) |
| CD4+ T-cells <200 cells/µL | |||
| No | 418 (73) | 100 (59) | 76 (61) |
| Yes | 119 (21) | 55 (32) | 41 (33) |
| Missing | 33 (6) | 16 (9) | 7 (6) |
| Infant gender | |||
| Female | 261 (46) | 98 (57) | 63 (51) |
| Male | 309 (54) | 73 (43) | 61 (49) |
| Iron-folate tablets | |||
| No | 73 (13) | 31 (18) | 21 (17) |
| Yes | 497 (87) | 140 (82) | 103 (83) |
| Length of Gestation (duration) | |||
| Extremely preterm (<28 weeks) | 0 (0) | 0 (0) | 0 (0) |
| Very preterm (28 – 32 weeks) | 0 (0) | 12 (8) | 12 (10) |
| Moderately preterm (33 – 34 weeks) | 0 (0) | 22 (13) | 33 (27) |
| Late preterm (35 – 36 weeks) | 0 (0) | 45 (26) | 79 (64) |
| Term (≥ 37 weeks) | 570 (100) | 78 (46) | 0 (0) |
| Missing | 0 (0) | 14 (8) | 0 (0) |
| Malaria (peripheral or placental) | |||
| No | 437 (77) | 125 (73) | 86 (69) |
| Yes | 133 (23) | 46 (27) | 38 (31) |
| Malaria Intermittent Preventative Therapy | |||
| No | 33 (6) | 16 (9) | 13 (10) |
| Yes | 534 (94) | 155 (91) | 110 (89) |
| Missing | 3 (1) | 0 (0) | 1 (1) |
| Primigravidity | |||
| No | 425 (75) | 129 (75) | 95 (77) |
| Yes | 145 (25) | 42 (25) | 29 (23) |
| Missing | 0 (0) | 0 (0) | 0 (0) |
| Syphilis seroreactivity | |||
| No | 531 (93) | 157 (92) | 110 (89) |
| Yes | 37 (6) | 14 (8) | 14 (11) |
| Missing | 2 (0) | 0 (0) | 0 (0) |
[HIV-1]= HIV-1 RNA concentration, in log10 copies/mL
Placental viral load correlates with birth weight and gestational age
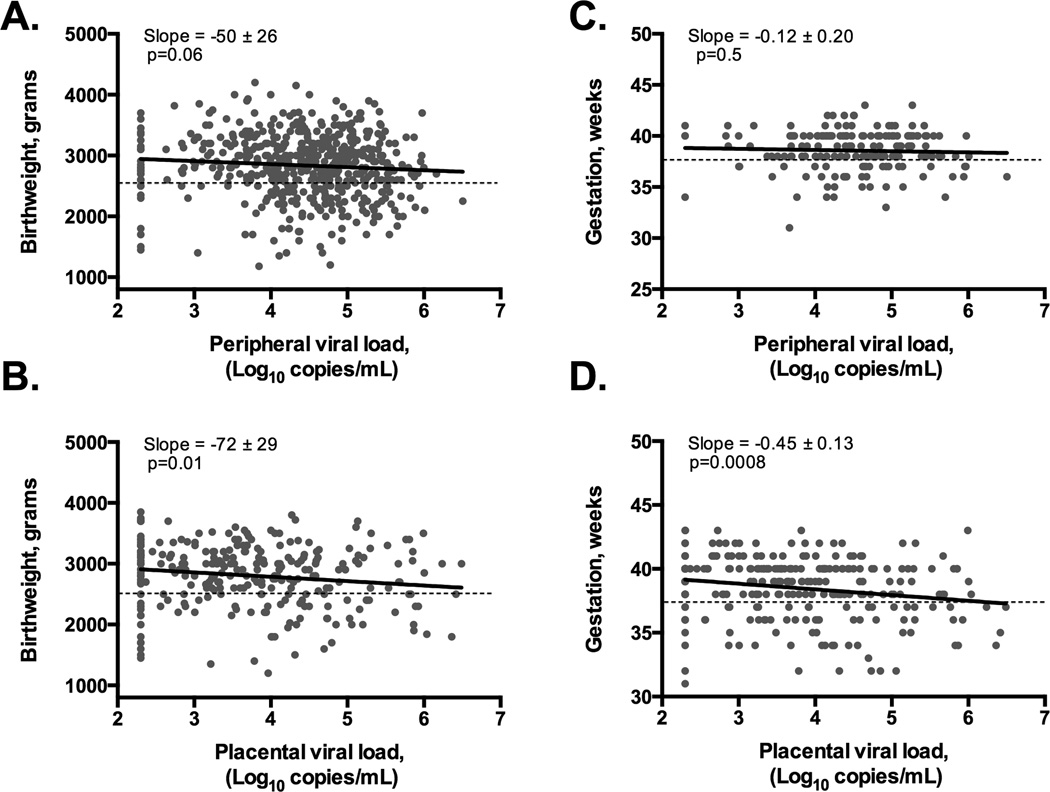
Maternal peripheral HIV-1 viral load was inversely correlated with continuous infant birthweight (correlation coefficient = −0.08, p=0.06), but not with continuous duration of gestation (correlation coefficient= −0.05, p=0.5) (Figures 1A & 1C). HIV-1 concentration in the placenta was inversely correlated with both birth weight (correlation coefficient= −0.15, p=0.01) and duration of gestation (correlation coefficient= −0.20, p=0.0008) (Figures 1B & 1D).
Figure 1. Correlation between viral load and continuous birth weight or gestational age.
Birthweight is inversely correlated with HIV-1 concentration in peripheral plasma (panel A), placental plasma (panel B). Gestational age does not correlate with HIV-1 concentration in peripheral plasma (panel C), but is inversely correlated with HIV-1 concentration in placental plasma (panel D). P-values calculated by pairwise correlation. Horizontal, black dotted lines indicate the cutoff for low birthweight (<2500 grams) or preterm birth (<37 weeks).
Low birthweight
The effect of HIV-1 disease severity on prevalence of LBW differed meaningfully by maternal malaria status. Residence, education level, primigravid status and anemia were retained as confounders in adjusted multivariable models. Among women with malaria, we observed no significant association between any measure of HIV-1 disease severity and prevalence of LBW in unadjusted or adjusted analyses (Table 3). Among malaria-negative women, for all measures of HIV-1 disease severity, more severe HIV-1 disease was significantly associated with increased prevalence of LBW in both unadjusted and adjusted models. The adjusted PR for a 1-log increase in placental HIV-1 viral load was 1.22 (95% CI: 1.00, 1.48); for a 1-log increase in peripheral HIV-1 viral load was 1.38 (95% CI: 1.08, 1.77); and for a 100-cell/ µl decrease in CD4+ T-cells was 1.12 (95% CI: 1.05, 1.21) (Table 3).
Table 3.
Unadjusted and adjusted effect of placental viral load, peripheral viral load, and CD4+ T-cell count on prevalence of low birthweight, stratified by maternal malaria status.
| Malaria-positive | Malaria-negative | |||||||
|---|---|---|---|---|---|---|---|---|
| METRIC | uPR | 95% CI | aPR | 95% CI | uPR | 95% CI | aPR | 95% CI |
| Placental viral load | ||||||||
| Per 1·0-log10 increase | 1·18 | 0·82, 1·70 | 1·25 | 0·87, 1·82 | 1·24 | 1·00, 1·53 | 1·23 | 1·02, 1·49 |
| Peripheral viral load | ||||||||
| Per 1·0- log10 increase | 0·92 | 0·61, 1·38 | 0·86 | 0·56, 1·32 | 1·44 | 1·12, 1·86 | 1·41 | 1·10, 1·82 |
| CD4+ T-cell count | ||||||||
| Per 100-cell/µl decrease | 0·98 | 0·85, 1·12 | 0·98 | 0·86, 1·11 | 1·13 | 1·05, 1·23 | 1·12 | 1·04, 1·21 |
uPR: unadjusted prevalence ratio
aPR: adjusted prevalence ratio; all adjusted models control for residence, maternal education, primigravid status and maternal anemia
CI: confidence interval
Preterm delivery
The effect of HIV-1 disease severity on prevalence of PTD also differed by maternal malaria status (Table 4). Among malaria-positive women, neither placental viral load nor CD4+ T-cell count were significantly associated with PTD in unadjusted or adjusted analyses, but a 1-log increase in peripheral viral load was significantly protective against PTD in adjusted analyses (PR: 0.56, 95% CI: 0.47, 0.85). Among women without malaria, higher placental viral load and lower CD4+ T-cell count were both significantly associated with increased prevalence of PTD (adjusted PR for a 1-log increase in placental viral load: 1.29, 95% CI: 1.02, 1.63; adjusted PR for 100-cell/µl decrease in CD4+ T-cells: 1.16, 95% CI: 1.05, 1.28). Increases in peripheral HIV-1 viral load did not significantly increase prevalence of PTD in malaria-negative women (Table 4).
Table 4.
Unadjusted and adjusted effect of placental viral load, peripheral viral load, and CD4+ T-cell count on prevalence of preterm delivery, stratified by maternal malaria status.
| Malaria-positive | Malaria-negative | |||||||
|---|---|---|---|---|---|---|---|---|
| METRIC | uPR | 95% CI | aPR | 95% CI | uPR | 95% CI | aPR | 95% CI |
| Placental viral load | ||||||||
| Per 1·0- log10 increase | 1·09 | 0·73, 1·62 | 1·05 | 0·70, 1·56 | 1·33 | 1·04, 1·69 | 1·29 | 1·02, 1·64 |
| Peripheral viral load | ||||||||
| Per 1·0- log10 increase | 0·71 | 0·49, 1·03 | 0·55 | 0·36, 0·85 | 1·21 | 0·90, 1·62 | 1·16 | 0·86, 1·55 |
| CD4+ T-cell count | ||||||||
| Per 100-cell/µl decrease | 1·04 | 0·89, 1·21 | 1·02 | 0·88, 1·18 | 1·18 | 1·07, 1·30 | 1·16 | 1·05, 1·28 |
uPR: unadjusted prevalence ratio
aPR: adjusted prevalence ratio; all adjusted models control for residence, maternal education, primigravid status and maternal anemia
CI: confidence interval
The association between HIV-1 and LBW remains after exclusion of PTD and HIV-infected infants
When the dataset was restricted to include only infants born after 37 weeks’ gestation (n=662), the associations between HIV-1 severity and prevalence of LBW among women with malaria were strengthened, but remained not statistically significant: the adjusted PR for a 1-log10 increase in placental viral load was 1.64 (95% CI: 0.98, 2.74), compared to 1.26 (95% CI: 0.87, 1.83) in the primary analysis (Supplemental Table 1). The PR for LBW for a 1-log10 increase in peripheral HIV-1 viral load was 1.66 (95% CI: 0.80, 3.47), compared to 0.88 (95% CI: 0.57, 1.35) in the primary analysis. However, excluding these observations had almost no affect on the magnitude of the associations between HIV-1 severity and prevalence of LBW in women without malaria. Given the reduced sample size, many of the PRs are no longer statistically significant, but the strength of the associations was essentially unchanged (Supplemental Table 1).
When the dataset was restricted only to observations with infants known to be HIV-negative at birth (n=686), we observed no meaningful changes in the associations between HIV-1 severity and prevalence of LBW or PTD, for malaria-positive or malaria-negative women (Supplemental Tables 2, 3). Thus, the observed associations in our primary analysis between HIV-1 severity and LBW or PTD are unlikely to be explained by infant HIV-1 status at birth.
Our primary analysis established that most associations between HIV-1 severity and birth outcomes were meaningfully different by malaria status. Our final sensitivity analysis assessed whether our findings were robust to the method of malaria diagnosis; in other words, was the same pattern observed for women with malaria diagnosed by peripheral blood smear, placental blood smear, and placental histology? For both LBW and PTD, the patterns observed in the primary analysis were repeated in the sensitivity analysis, with no meaningful differences by method of malaria diagnosis (Supplemental Tables 4,5).
DISCUSSION
In this secondary analysis of a large cohort of HIV-infected pregnant women who delivered a live, singleton infant in Malawi between 2000 and 2004, we tested the hypothesis that severity of HIV-1 infection is associated with risk of LBW or PTD. This cohort is unique because all women were infected with HIV-1 subtype C, were antiretroviral treatment naïve, were rigorously tested for malaria, and were normotensive. This study was not designed to comprehensively determine significant predictors of LBW or PTD in HIV-infected women; rather, it was designed to characterize the independent associations between three specific measures of HIV-1 disease severity, LBW and PTD, adjusting for factors that confounded or modified those associations. Although our malaria-positive sample size was small, HIV-1 severity in this group appeared not to be associated with adverse birth outcomes. However in malaria-negative women, maternal HIV-1 disease severity was significantly associated with increased prevalence of LBW and PTD. This association persisted in multivariate models adjusted for residence, primigravid status, maternal education, and maternal anemia, and the result was also robust to several sensitivity analyses, including exclusion of PTD, exclusion of infants who became HIV-infected in utero, and stratification by mode of malaria diagnosis.
The average peripheral viral load of participants in this study, all of whom were antiretroviral treatment naïve, was approximately 32,000 copies/mL. The adjusted PR for LBW corresponding to a three-log increase in viral load among women without malaria is 2.82 (95% CI: 1.32 to 6.07), meaning that women with average viral loads have three times the prevalence of LBW as women with an undetectable viral load. Administration of antiretroviral therapy (ART) that reduces viral load to an undetectable limit could lead to extremely significant relative reductions in LBW among malaria-negative Malawian women.
An association between maternal HIV-1 infection and LBW has been previously observed1, 2, including several studies in Malawi3, 18. In a meta analysis of six cohorts of HIV-infected Malawian women, 13% of infants born to HIV-infected women had LBW, although malaria status was not reported3. In this study, 21% of the infants born to HIV-infected women were LBW, more than double the 8% prevalence of LBW among HIV-negative women in the MHP cohort (unpublished observation). Our findings are consistent with the results of a study from Lusaka, Zambia, which quantified the association between maternal viral load and infant birth outcomes, and found that greater HIV-1 disease severity, characterized as peripheral viral load above 100,000 copies/mL, was associated with decreased infant weight and increased infant mortality and morbidity25. Several US-based studies have also quantified the association between HIV-1 disease severity and LBW (or IUGR). Lambert et al. (2000) found neither HIV-1 RNA copy number nor CD4+ T-cell counts were associated with LBW, but increasing HIV-1 culture titer was associated with LBW26; these authors also observed that timing of ART initiation – before or during pregnancy – was not associated with LBW. Tuomala et al (2002) compared women who received ART to ART-naïve women and found no difference in the prevalence of LBW between the treated and untreated women27. Although the authors did not report viral loads, presumably the women on ART had lower viral loads than the untreated women, and thus it can be inferred that viral load was not associated with LBW.
The relationship between severity of HIV-1 infection and PTD differed slightly from the LBW results. Among women without malaria, decreasing CD4 T-cell count and increasing placental viral load, but not increasing peripheral viral load, was associated with increased prevalence of preterm delivery. Unexpectedly, among women with malaria, increasing peripheral viral load was protective against PTD. While the basis for this observed association is currently unknown, one study suggests that HIV-1 infection can suppress the innate immune response to P. falciparum 28, which has the potential to modulate inflammation and onset of delivery 29 30. Additional analyses are required to determine if this observation is biologically relevant or if it reflects an uncharacterized bias.
There are several limitations to our findings. We cannot determine if the observed associations between severity of HIV-1 infection and LBW are a direct effect of HIV-1, or if the association is secondary to HIV-associated nutritional deficiency; the sample size for the malaria-positive stratum was smaller than the sample size for the malaria-negative stratum; neither prepregnancy body mass index nor other measures of nutritional status were available for analysis; gestational ages were self-reported, and no other measures of gestational age (e.g. e.g., ultrasonographic fetal measurements31) were available; the results are generalizable only to ART-naïve HIV-infected women, with normal blood pressure, who delivered in a hospital. Finally, cigarette smoking, a known LBW and PTD risk factor, was not assessed; however, estimates suggest that less than 10% of the adult population in Malawi smoke cigarettes32.
Normal fetal growth represents a finely tuned immune state requiring a temporary immune tolerance to the fetal-placental unit, while still enabling fetal protection from pathogens. Placental inflammation, which could result from a co-infection, can disrupt this tenuous immunological balance and cause LBW. However, clinical data from the MHP cohort suggest that co-infection with malaria or syphilis is not driving HIV-associated LBW. Further, although chorioamnionitis was associated with PTD and LBW, it was not a confounder of the HIV-1 severity-associations: to be a confounder, a variable must be associated with both the exposure (HIV-1 severity) and the outcome (LBW or PTD). The mean placental viral load among women with chorioamnionitis was 3.84 log10 copies/mL compared to 3.73 log10 copies/mL among women without chorioamnionitis (p=0.5); the mean log-transformed peripheral viral load among women with chorioamnionitis was 4.43 log10 copies/mL compared to 4.39 log10 copies/mL among women without chorioamnionitis (p=0.6), and the mean CD4+ T-cell count among women with chorioamnionitis was 380 cells/µL compared to 386 cells/µL among women without chorioamnionitis (p=0.9). Additional studies to determine the prevalence of other pathogenic microbes in this cohort would address the possibility that these results are confounded by a heretofore undetected co-infection.
Following the paradigm of HIV-associated disruption of the blood-brain barrier33 and HIV-mediated impairment of the mucosal epithelial barrier34, a working hypothesis posits that HIV-1 replication impairs placental function and causes LBW. HIV-1 can replicate in the placenta and it has been detected in placental cells in vivo35, and data from several groups indicate that HIV-1 can infect ex vivo placental explant cultures36, 37. Our previous work has shown that the placenta represents a unique and hospitable niche for HIV-1 replication; specifically, we observed that the concentration of HIV-1 in the placenta was different than the concentration of HIV-1 in peripheral blood13. In addition to the pathogenic effects on HIV-infected cells, it is also plausible that cellular exposure to HIV-1 could be pathogenic and disrupt placental function.
In summary, indicators of severe maternal HIV-1 infection, defined as high placental or peripheral viral load or a low CD4+ T-cell count, were associated in a dose-dependent manner with increased prevalence of LBW. This association was only observed in malaria-negative women, and it was not a secondary effect of PTD or in utero HIV-1 infection. The mechanism of HIV-associated LBW remains unknown.
Supplementary Material
ACKNOWLEDGMENTS
Funding. NIH: KL2RR025754 (ANT); Wellcome Trust (SR); R01-AI49084 (SRM); R00- HD056586 (JJK).
We are grateful for the participation of the Malawian women and their newborns and the Malaria and HIV-1 in Pregnancy Cohort staff. The MHP cohort was funded by the NIH (R01-AI49084). This research was supported in part by NIH grants R00HD056586 to JJK; ANT was supported by KL2RR025754, through the Ohio State University Center for Clinical and Translational Science (OSU CCTS); the OSU CCTS is supported by the National Center for Advancing Translational Sciences, Grant 8UL1TR000090-05; SJR was supported by a Senior Fellowship from the Wellcome Trust. The content of this article is solely the responsibility of the authors and it does not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest. The authors declare no conflicts of interest.
Author contributions. ANT and JJK designed the study, performed the data analyses, and wrote the manuscript. WEA and ST interpreted the data and edited the manuscript. SR, SM, VM conducted the parent MHP cohort, interpreted the data, and edited the manuscript.
REFERENCES
- 1.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105(8):836–848. doi: 10.1111/j.1471-0528.1998.tb10227.x. [DOI] [PubMed] [Google Scholar]
- 2.Schulte J, Dominguez K, Sukalac T, Bohannon B, Fowler MG. Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: Pediatric Spectrum of HIV Disease, 1989–2004. Pediatrics. 2007;119(4):e900–e906. doi: 10.1542/peds.2006-1123. [DOI] [PubMed] [Google Scholar]
- 3.Taha TE, Dadabhai SS, Rahman MH, Sun J, Kumwenda J, Kumwenda NI. Trends in birth weight and gestational age for infants born to HIV-infected, antiretroviral treatment-naive women in Malawi. Pediatr Infect Dis J. 2012;31(5):481–486. doi: 10.1097/INF.0b013e31824d9bd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steketee RW, Nahlen BL, Parise ME, Menendez C. The burden of malaria in pregnancy in malaria-endemic areas. Am J Trop Med Hyg. 2001;64(1–2 Suppl):28–35. doi: 10.4269/ajtmh.2001.64.28. [DOI] [PubMed] [Google Scholar]
- 5.McCormick MC. The contribution of low birth weight to infant mortality and childhood morbidity. N Engl J Med. 1985;312(2):82–90. doi: 10.1056/NEJM198501103120204. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379(9832):2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 7.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379(9832):2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 8.Jauniaux E, Van Oppenraaij RH, Burton GJ. Obstetric outcome after early placental complications. Curr Opin Obstet Gynecol. 2010;22(6):452–457. doi: 10.1097/GCO.0b013e3283404e44. [DOI] [PubMed] [Google Scholar]
- 9.Benirschke K, Kaufmann P, Baergen RN. Pathology of the Human Placenta. Fifth Edition. Springer; 2006. [Google Scholar]
- 10.Guyatt HL, Snow RW. Impact of malaria during pregnancy on low birth weight in sub-Saharan Africa. Clin Microbiol Rev. 2004;17(4):760–769. doi: 10.1128/CMR.17.4.760-769.2004. , table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogerson SJ, Mwapasa V, Meshnick SR. Malaria in pregnancy: linking immunity and pathogenesis to prevention. Am J Trop Med Hyg. 2007;77(6 Suppl):14–22. [PubMed] [Google Scholar]
- 12.Mwapasa V, Rogerson SJ, Molyneux ME, et al. The effect of Plasmodium falciparum malaria on peripheral and placental HIV-1 RNA concentrations in pregnant Malawian women. AIDS. 2004;18(7):1051–1059. doi: 10.1097/00002030-200404300-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kumar SB, Handelman SK, Voronkin I, et al. Different regions of HIV-1 subtype C env are associated with placental localization and in utero mother-to-child transmission. J Virol. 2011;85(14):7142–7152. doi: 10.1128/JVI.01955-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menu E, Mbopi-Keou FX, Lagaye S, et al. Selection of maternal human immunodeficiency virus type 1 variants in human placenta. European Network for In Utero Transmission of HIV-1. J Infect Dis. 1999;179(1):44–51. doi: 10.1086/314542. [DOI] [PubMed] [Google Scholar]
- 15.Faye A, Pornprasert S, Mary JY, et al. Characterization of the main placental cytokine profiles from HIV-1-infected pregnant women treated with anti-retroviral drugs in France. Clin Exp Immunol. 2007;149(3):430–439. doi: 10.1111/j.1365-2249.2007.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar SB, Rice CE, Milner DAJ, et al. Elevated cytokine and chemokine levels in the placenta are associated with in-utero HIV-1 mother-to-child transmission. AIDS. 2012;26(6):685–694. doi: 10.1097/QAD.0b013e3283519b00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moussa M, Roques P, Fievet N, et al. Placental cytokine and chemokine production in HIV-1-infected women: trophoblast cells show a different pattern compared to cells from HIV-negative women. Clin Exp Immunol. 2001;125(3):455–464. doi: 10.1046/j.1365-2249.2001.01629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nkhoma ET, Kalilani-Phiri L, Mwapasa V, Rogerson SJ, Meshnick SR. Effect of HIV Infection and Plasmodium falciparum Parasitemia on Pregnancy Outcomes in Malawi. Am J Trop Med Hyg. 2012;87(1):29–34. doi: 10.4269/ajtmh.2012.11-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mwapasa V, Rogerson SJ, Kwiek JJ, et al. Maternal syphilis infection is associated with increased risk of mother-to-child transmission of HIV in Malawi. AIDS. 2006;20(14):1869–1877. doi: 10.1097/01.aids.0000244206.41500.27. [DOI] [PubMed] [Google Scholar]
- 20.Kwiek JJ, Mwapasa V, Alker AP, et al. Socio-demographic characteristics associated with HIV and syphilis seroreactivity among pregnant women in Blantyre, Malawi, 2000–2004. Malawi Med J. 2008;20(3):80–85. doi: 10.4314/mmj.v20i3.10965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abrams ET, Milner DA, Jr, Kwiek J, et al. Risk factors and mechanisms of preterm delivery in Malawi. Am J Reprod Immunol. 2004;52(2):174–183. doi: 10.1111/j.1600-0897.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 22.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157(10):940–943. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 23.Greenland S, Pearl J, Robins JM. Causal diagrams for epidemiologic research. Epidemiology. 1999;10(1):37–48. [PubMed] [Google Scholar]
- 24.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138(11):923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 25.Kuhn L, Kasonde P, Sinkala M, et al. Does severity of HIV disease in HIV-infected mothers affect mortality and morbidity among their uninfected infants? Clin Infect Dis. 2005;41(11):1654–1661. doi: 10.1086/498029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert JS, Watts DH, Mofenson L, et al. Risk factors for preterm birth, low birth weight, and intrauterine growth retardation in infants born to HIV-infected pregnant women receiving zidovudine. Pediatric AIDS Clinical Trials Group 185 Team. AIDS. 2000;14(10):1389–1399. doi: 10.1097/00002030-200007070-00012. [DOI] [PubMed] [Google Scholar]
- 27.Tuomala RE, Shapiro DE, Mofenson LM, et al. Antiretroviral therapy during pregnancy and the risk of an adverse outcome. N Engl J Med. 2002;346(24):1863–1870. doi: 10.1056/NEJMoa991159. [DOI] [PubMed] [Google Scholar]
- 28.Finney CA, Ayi K, Wasmuth JD, et al. HIV infection deregulates innate immunity to malaria despite combination antiretroviral therapy. AIDS. 2013;27(3):325–335. doi: 10.1097/QAD.0b013e32835b3dfa. [DOI] [PubMed] [Google Scholar]
- 29.Golightly E, Jabbour HN, Norman JE. Endocrine immune interactions in human parturition. Mol Cell Endocrinol. 2011;335(1):52–59. doi: 10.1016/j.mce.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 30.Green NS, Damus K, Simpson JL, et al. Research agenda for preterm birth: recommendations from the March of Dimes. Am J Obstet Gynecol. 2005;193(3 Pt 1):626–635. doi: 10.1016/j.ajog.2005.02.106. [DOI] [PubMed] [Google Scholar]
- 31.Kramer MS, Papageorghiou A, Culhane J, et al. Challenges in defining and classifying the preterm birth syndrome. Am J Obstet Gynecol. 2012;206(2):108–112. doi: 10.1016/j.ajog.2011.10.864. [DOI] [PubMed] [Google Scholar]
- 32.Muula AS, Siziya S, Rudatsikira E. Prevalence and correlates of cigarette smoking among adolescents in Malawi: results from the Global Youth Tobacco Survey 2005. Tanzan J Health Res. 2008;10(3):166–176. doi: 10.4314/thrb.v10i3.14357. [DOI] [PubMed] [Google Scholar]
- 33.Yang B, Singh S, Bressani R, Kanmogne GD. Cross-talk between STAT1 and PI3K/AKT signaling in HIV-1-induced blood-brain barrier dysfunction: role of CCR5 and implications for viral neuropathogenesis. J Neurosci Res. 2010;88(14):3090–3101. doi: 10.1002/jnr.22458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(4):e1000852. doi: 10.1371/journal.ppat.1000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Backe E, Jimenez E, Unger M, Schafer A, Jauniaux E, Vogel M. Demonstration of HIV-1 infected cells in human placenta by in situ hybridisation and immunostaining. J Clin Pathol. 1992;45(10):871–874. doi: 10.1136/jcp.45.10.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amirhessami-Aghili N, Spector SA. Human immunodeficiency virus type 1 infection of human placenta: potential route for fetal infection. J Virol. 1991;65(5):2231–2236. doi: 10.1128/jvi.65.5.2231-2236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Polliotti BM, Sheikh AU, Subbarao S, et al. HIV-1 infection of human placental villous tissue in vitro. Placenta. 1998;19:205–223. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.