Abstract
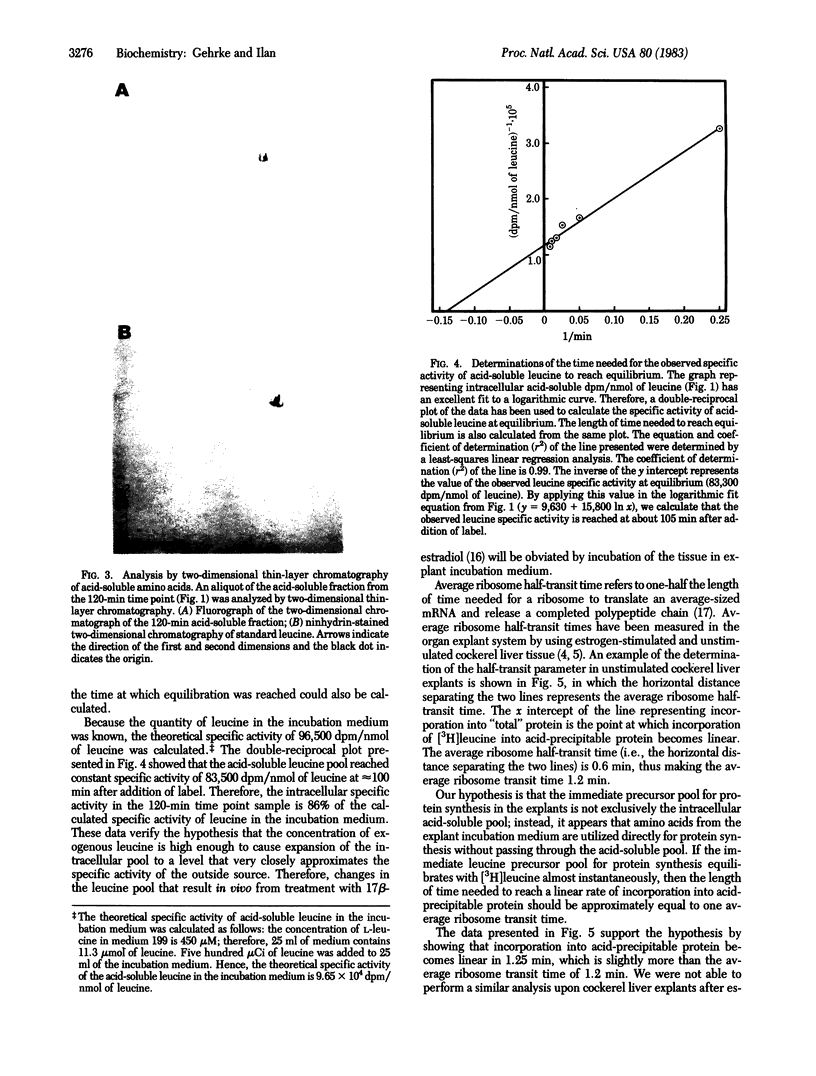
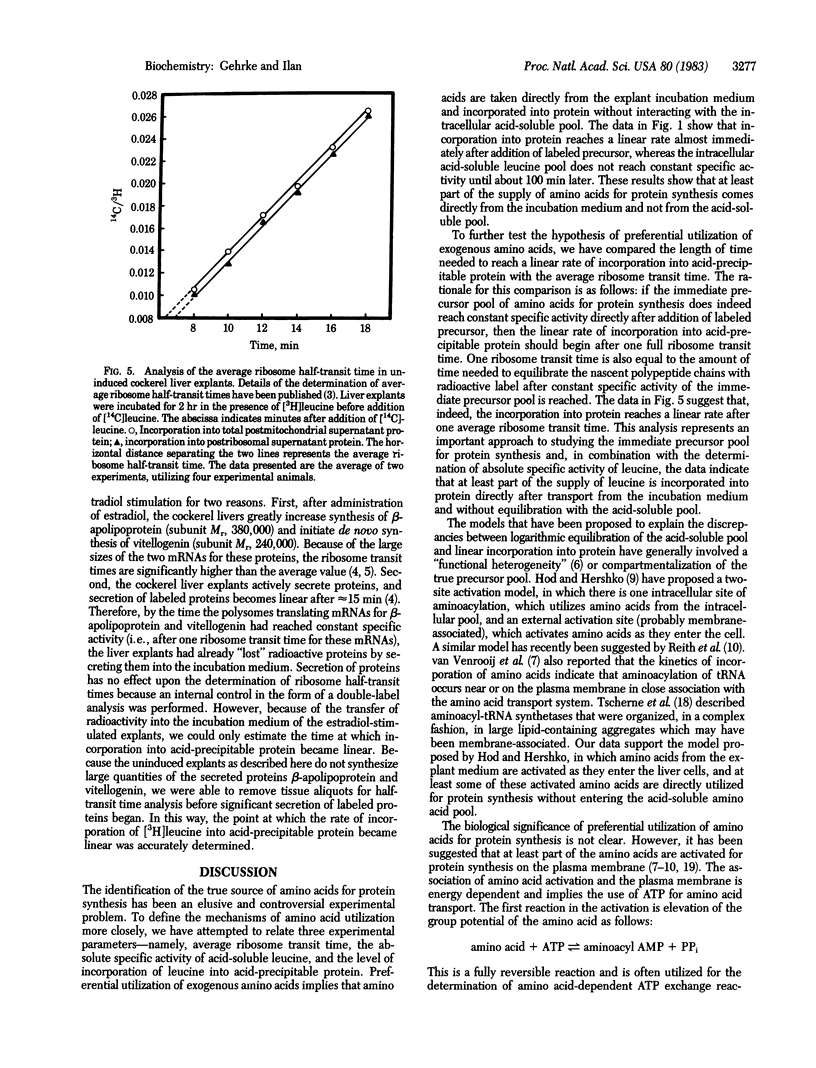
A cockerel liver explant system has been used to study protein synthesis and ribosome transit times. After a 2-hr preincubation of explant tissue in the presence of a large concentration of nonradioactive leucine, a small quantity of [3H]leucine was added and the kinetics of uptake of [3H]leucine into the intracellular acid-soluble leucine pool was compared to the incorporation of [3H]leucine into protein. Incorporation of [3H]leucine into protein reaches a linear rate almost immediately after addition of label, whereas the acid-soluble pool does not reach constant specific activity until much later. The length of time needed to reach a linear rate of incorporation of [3H]leucine into protein is approximately equal to the length of time needed to equilibrate nascent polypeptide chains with labeled precursor--that is, one average ribosome transit time. Therefore, it seems that the immediate precursor pool for protein synthesis reaches constant specific activity almost instantly after addition of [3H]leucine. The results indicate that at least part of the supply of leucine for protein synthesis is derived directly from the exogenous incubation medium and not from the intracellular acid-soluble amino acid pool.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhlen P., Stein S., Dairman W., Udenfriend S. Fluorometric assay of proteins in the nanogram range. Arch Biochem Biophys. 1973 Mar;155(1):213–220. doi: 10.1016/s0003-9861(73)80023-2. [DOI] [PubMed] [Google Scholar]
- CERIOTTI G. A microchemical determination of desoxyribonucleic acid. J Biol Chem. 1952 Sep;198(1):297–303. [PubMed] [Google Scholar]
- Dierks-Ventling C., Jost J. P. Effect of 17 beta-estradiol on the synthesis of non-histone nuclear proteins in chick liver. Eur J Biochem. 1974 Dec 16;50(1):33–40. doi: 10.1111/j.1432-1033.1974.tb03870.x. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Fry B. J., Gross P. R. Patterns and rates of protein synthesis in sea urchin embryos. II. The calculation of absolute rates. Dev Biol. 1970 Feb;21(1):125–146. doi: 10.1016/0012-1606(70)90065-5. [DOI] [PubMed] [Google Scholar]
- Gehrke L., Bast R. E., Ilan J. An analysis of rates of polypeptide chain elongation in avian liver explants following in vivo estrogen treatment. I. Determination of average rates of polypeptide chain elongation. J Biol Chem. 1981 Mar 10;256(5):2514–2521. [PubMed] [Google Scholar]
- Gehrke L., Bast R. E., Ilan J. An analysis of rates of polypeptide chain elongation in avian liver explants following in vivo estrogen treatment. II. Determination of the specific rates of elongation of serum albumin and vitellogenin nascent chains. J Biol Chem. 1981 Mar 10;256(5):2522–2530. [PubMed] [Google Scholar]
- Hod Y., Hershko A. Relationship of the pool of intracellular valine to protein synthesis and degradation in cultured cells. J Biol Chem. 1976 Jul 25;251(14):4458–4457. [PubMed] [Google Scholar]
- Ilan J., Ilan J. Preferential channeling of exogenously supplied methionine into protein by sea urchin embryos. J Biol Chem. 1981 Mar 25;256(6):2830–2834. [PubMed] [Google Scholar]
- Ilan J., Singer M. Sampling of the leucine pool from the growing peptide chain: difference in leucine specific activity of peptidyl-transfer RNA from free and membrane-bound polysomes. J Mol Biol. 1975 Jan 5;91(1):39–51. doi: 10.1016/0022-2836(75)90370-8. [DOI] [PubMed] [Google Scholar]
- KIPNIS D. M., REISS E., HELMREICH E. Functional heterogeneity of the intracellular amino acid pool in mammalian cells. Biochim Biophys Acta. 1961 Aug 19;51:519–524. doi: 10.1016/0006-3002(61)90608-4. [DOI] [PubMed] [Google Scholar]
- Martin A. F., Rabinowitz M., Blough R., Prior G., Zak R. Measurements of half-life of rat cardiac myosin heavy chain with leucyl-tRNA used as precursor pool. J Biol Chem. 1977 May 25;252(10):3422–3429. [PubMed] [Google Scholar]
- Reith M. E., Schotman P., van Zwieten B. J., Gispen W. H. The nature of the amino acid pool used for protein synthesis in rat brain slices. J Neurochem. 1979 Feb;32(2):413–420. doi: 10.1111/j.1471-4159.1979.tb00365.x. [DOI] [PubMed] [Google Scholar]
- Singer M., Ilan J. Nerve-dependent regulation of absolute rates of protein synthesis in newt limb regenerates. Measurement of methionine specific activity in peptidyl-tRNA of the growing polypeptide chain. Dev Biol. 1977 May;57(1):174–187. doi: 10.1016/0012-1606(77)90363-3. [DOI] [PubMed] [Google Scholar]
- Storti R. V., Rich A. Chick cytoplasmic actin and muscle actin have different structural genes. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2346–2350. doi: 10.1073/pnas.73.7.2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tscherne J. S., Weinstein I. B., Lanks K. W., Gersten N. B., Cantor C. R. Phenylalanyl transfer ribonucleic acid synthetase activity associated with rat liver ribosomes and microsomes. Biochemistry. 1973 Sep 25;12(20):3859–3865. doi: 10.1021/bi00744a010. [DOI] [PubMed] [Google Scholar]
- Udenfriend S., Stein S., Böhlen P., Dairman W., Leimgruber W., Weigele M. Fluorescamine: a reagent for assay of amino acids, peptides, proteins, and primary amines in the picomole range. Science. 1972 Nov 24;178(4063):871–872. doi: 10.1126/science.178.4063.871. [DOI] [PubMed] [Google Scholar]
- Van Venrooij W. J., Moonen H., Van Loon-Klaassen L. Source of amino acids used for protein synthesis in HeLa cells. Eur J Biochem. 1974 Dec 16;50(1):297–304. doi: 10.1111/j.1432-1033.1974.tb03898.x. [DOI] [PubMed] [Google Scholar]



