Abstract
Background
Aflatoxins are a group of mycotoxins produced by Aspergillus flavus and Aspergillus parasiticus and are potent inducers of hepatotoxicity.
Objective
The present study was carried out to investigate the effect of black tea infusion on aflatoxin—induced hepatotoxicity in male mice.
Methods
A 2% black tea infusion in drinking water was prepared and orally administered along with aflatoxin (750 and 1500 μg/kg body weight) for 30 days. Morphological investigation, body weight and organ weight calculations and histopathological analysis were carried out. Serum hepatic marker enzymes namely alanine aminotransferase and aspartate aminotransferase were estimated.
Results
The results clearly indicated that aflatoxin treatment for 30 days caused significant dose-dependent reduction in body weight and increase in liver weight. The activities of ALT and AST were found to be elevated while protein content was found to be decreased in aflatoxin-treated mice as compared to vehicle control. Histopathological analysis showed hepatocellular necrosis and cytoplasmic vacuolization along with fatty infiltration in toxin-treated animals. Results revealed significant (p < 0.05) restoration of aflatoxin-induced damages in body weight, organ weight, serum chemistry and histopathological features in aflatoxin plus black tea infusion administered mice in a dose dependant manner.
Conclusion
It is concluded from the present study that supplementation of black tea infusion can be beneficial in positively modulating aflatoxin-induced alterations in liver.
Keywords: aflatoxin, black tea, liver, ALT, histopathology
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFB1, aflatoxins B1; IARC, International Agency for Research on Cancer; MFO, mixed function oxidase; TLC, thin-layer chromatography; LD, low dose; HD, high dose; ANOVA, Analysis of Variance; WHO-ART, World Health Organization for Adverse Reaction Terminology
Aflatoxins are a group of well-known mycotoxins (secondary toxic fungal metabolites) produced by Aspergillus flavus and Aspergillus parasiticus which are known to contaminate a wide variety of food stuffs. The term Aflatoxins is used to collectively represent the four major naturally occurring secondary compounds namely B1, B2, G1 and G2. Aflatoxins B1 (AFB1) is considered as the most potent of these toxins and is attributed with hepatotoxic and hepatocarcinogenic properties.1 The International Agency for Research on Cancer (IARC) has classified AFB1 and the mixtures of aflatoxins as Group 1 carcinogens. The liver is known to be the main target organ for aflatoxin and chronic exposure to low levels of these through intake of contaminated food stuffs can cause liver fibrosis and also primary liver cancer.2
Aflatoxins enter the dietary systems of human or animals through direct or indirect routes and once inside they are acted upon by microsomal mixed function oxidase (MFO) primarily in the liver and to some extent in the lungs, kidneys and other organs. AFB1 is first metabolized (Phase 1) by the Cytochrome P450 enzyme (CYP450) system found in the microsomes, producing a variety of intermediary metabolites such as AFB1 epoxide and other hydroxylated metabolites like AFM1, AFP1, AFQ1 and aflatoxicol. AFB1 epoxide is highly reactive and relatively unstable with inbuilt capacity to bind to cellular macromolecules like DNA, RNA, lipids and proteins, initiating the vicious cycle of lipid peroxidation and culminating in cellular injury.3 Aflatoxins are thus responsible for a wide range of pathological abnormalities in humans and animals. Aflatoxin-albumin adduct, a biomarker for aflatoxin exposure was found to be higher in the serum of individuals at risk for hepatocellular carcinoma thus indicating its importance in human health and diseases.4
Tea intake either as an herbal medicine or as a rejuvenating drink is a common practice among people belonging to different parts of the world. Tea infusions are estimated to be consumed by two thirds of the world's population. Black tea represents approximately 72% of total consumed tea in the world, whereas green tea accounts for approximately 26%.5 Tea infusions are used as therapeutic agent keeping in mind its ready availability and cost effectiveness. Tea is known to contain very high levels of total flavonoids and polyphenols.6 Tea polyphenols have been reported to inhibit DNA synthesis of leukemia and lung carcinoma cells.7 Tea flavonoids have been attributed with enormous antioxidative activity by a number of previous studies.8,9
It is widely believed that green tea consumption gives more beneficial health effects as compared to black tea due to the presence of catechins which are converted to theaflavins and thearubingins by fermentation during the transformation process.10 However many study reports have confirmed positive modulatory effect of black tea on a number of carcinogens and toxicants. Black tea has been reported to significantly protect against oxidative damage induced by a number of agents like hydrogen peroxide, primaquine and phenylhydrazine in red blood cells.11 Administration of 2% black tea extract along with sodium fluoride was found to produce a profound neuroprotective effect on fluorotic rats with improved motor functions and coordination performance.10 In one another study 2% black tea infusion was used to effectively combat sodium fluoride-induced behavioral and reproductive toxicity.12 Chung et al13 have reported the advantage of using 2% black tea over a 1% or 0.5% infusion on lung tumorigenesis induced by the nicotine-derived carcinogen 4-(methylnitrosamino)-l-(3-pyridyl)-l-butanone (NNK) in rats. Similarly 2% black tea preparation was found to prevent cigarette smoke-induced apoptosis and lung damage in guinea pigs.14 The protective effect of black tea against many potent toxins has been extensively studied in our lab and 2% infusion was found to be the most effective and further increase in tea concentration produced insignificant results.15,16 This prompted us to study the effect of 2% black tea infusion against aflatoxin which is one of the most potent hepatotoxin known thus far.
Materials and methods
Chemicals
Black tea (Lipton Yellow Label) was purchased from Hindustan lever Ltd., Mumbai, India. Olive oil was procured from Figaro, Madrid, Spain. Chemicals used in the present study were purchased from standard agencies and were of analytical grade. Aflatoxin standard was obtained as a gift from the International Agency for Research on Cancer, Lyon, France.
Aflatoxin Production
A. parasiticus var. globusus strain (MTCC 411) was procured from Institute of Microbial Technology, Chandigarh, India and was grown on sucrose-magnesium sulfate-potassium nitrate-yeast extract (SMKY) liquid medium at 28 ± 2 °C for 10 days. Briefly 50 ml of SMKY liquid medium was taken in a 500 mL Erlenmeyer flask and sterilized at 15 lb pressure for 20 min. The sterilized medium was inoculated with 0.5 ml spore suspension of A. parasiticus having 108 conidia/mL under strict aseptic condition. On the 11th day the contents were autoclaved and filtered and the pooled filtrate was extracted in chloroform and passed through anhydrous sodium sulfate, evaporated and was re-suspended in chloroform and qualitatively analyzed using thin-layer chromatography (TLC) technique. Briefly 100 μL of extracted aflatoxin was spotted along with standard aflatoxin on silica gel G coated activated TLC plates, developed using toluene: iso-amyl alcohol: methanol (90:32:2, v/v) and observed at 360 nm under UV light.11 Aflatoxin B1 and B2 were seen as blue colored fluorescent spots, while aflatoxin G1 and G2 were identified by their characteristic bluish-green fluorescent color. The spots were also chemically analyzed by spraying trifluoroacetic acid or 25% acid. Subsequently the individual spots were eluted, dissolved in chilled methanol and quantified and stored until use.
Preparation of Black Tea Infusion
The effective dose of black tea infusion was based on our previous study report.11 Briefly 2% black tea infusion was made using 2 g of black tea solids in 100 ml of deionized water and used.
Experimental Animals
Healthy male mice of Swiss strain weighing between 37–40 g of equivalent age groups were acclimatized for 15 days in polypropylene cages in the Animal House of Zoology Department, Gujarat University, Ahmedabad, India under controlled conditions of temperature (25 ± 2 °C) and relative humidity (50–55%) and12 h light/dark cycle. Animals were maintained on certified pelleted rodent feed supplied by Amrut Feeds, Pranav Agro Industries Limited, Pune, India and given water ad libitum. The experimental procedures were approved by “The Committee for the Purpose of Control and Supervision of Experiment on Animals” (Reg—167/1999/CPCSEA), New Delhi, India.
Experimental Design and Treatment Schedule
Seventy animals were randomized into seven groups and caged separately. Group 1 animals were marked as untreated control group and maintained without any treatment. Animals of Group 2 received olive oil as vehicle (0.2 mL/animal/day) and group 3 animals were administered with black tea infusion. Animals of Group 4 and 5 were orally administered with aflatoxin in olive oil (containing B1, B2, G1 and G2 in the ratio of 8:3:2:1 respectively) using a feeding needle attached to a hypodermic syringe with a dosage regimen of 750 μg/kg body weight (low dose i.e. LD) and 1500 μg/kg body weight (high dose i.e. HD) in 0.2 mL olive oil/animal/day respectively.17 The selected dose of aflatoxin was based on its LD50 value in male mice (9 mg/kg body wt).18 The experiments were carried out with mixed aflatoxins similar to the form they are normally distributed in food stuff. The group 6 animals were orally treated with LD aflatoxin and also given 2% black tea extract instead of drinking water, while animals of group 7 also received aflatoxin like group 5 animals (HD) and were given 2% black tea. The treatment in all groups continued for 30 days and animals were sacrificed on the 31st day.
Clinical Observation, Body Weight and Organ Weight
The behavioral and clinical changes throughout the experiment period of 30 days were recorded. The body weight of each group of mice was recorded individually and mean weights were calculated. Percent change in body weight for different treatment groups was calculated by the formula:
Where
W1 = Mean body weight of toxin or toxin plus black tea extract-treated mice on 30th day (groups 4–7).
W0 = Mean body weight of vehicle control mice on 30th day.
On completion of the 30 days treatment period blood was collected by cardiac puncture and the animals were sacrificed by cervical dislocation. Serum was separated by centrifugation at 1000 × g for 10 min and liver was isolated and blotted free of blood. The dissected liver from different groups of mice was weighed to the nearest mg on a balance. Relative organ weights were calculated.
Serum Biochemical Parameters
Protein Content
Protein content was estimated in serum by the method of Lowry et al19 by using bovine serum albumin as a standard. When protein reacts with phenol reagent of Folin Ciocalteau a deep blue color develops. The colour development is due to two reactions occurring simultaneously i.e., the reaction of alkaline copper sulfate solution with peptide bonds and reduction of phosphomolybdic and phosphotungstic acids by aromatic amino acids present in the protein. The blue colour that develops is quantitatively proportional to the total protein, which was measured at 540 nm. The protein contents were expressed as mg/100 mL for serum.
Alanine Transaminase (EC 2.6.1.2) Activity
The alanine transaminase (ALT) activity was determined by the method of Reitman and Frankel.20 Buffered solution of α-ketoglutarate and l-alanine were made to react with the serum. The quantity of pyruvate formed by the action of 2, 4-dinitrophenyl hydrazine in alkaline medium was measured at 540 nm. The enzyme activity was expressed as mU/mL.
Aspartate Transaminase (EC 2.6.1.1) Activity
The aspartate transaminase (AST) activity was assayed by the method of Reitman and Frankel.20 The procedure was same as ALT. However, AST assay consisted of l-aspartate instead of l-alanine and the tubes were incubated for exactly 1 h after addition of serum. The enzyme activity was expressed as mU/mL. The percent change (as compared to vehicle control) for all parameters were also calculated.
Histopathological Analysis
Histopathological studies were carried out using the standard technique of hematoxylin and eosin staining. Liver of controls and all treated groups of animals were dissected out, blotted free of blood and fixed (for 18 h) in alcoholic Bouin's fixative. The fixed tissues were dehydrated by passing through ascending grades of alcohol, cleared in xylene and embedded in paraffin wax (58–60 °C mp). Transverse sections of 5 μm thickness were cut on a rotary microtome. These sections were stained with Ehrlich's hematoxylin and eosin, dehydrated in alcohol, cleared in xylene, mounted in DPX and examined microscopically.
Statistical Analysis
The data was expressed as mean ± SEM. The data were statistically analyzed using one way Analysis of Variance (ANOVA) followed by Tukey Test. The levels of significance was accepted with p < 0.05. Comparisons of p-values between different groups were carried out.
Result
Clinical Observations
No treatment related clinical signs were observed in control animals (groups 1–3). Aflatoxin treatment for 30 days caused dullness and lethargy with signs of staggering (group 4 and 5). However aflatoxin plus black tea infusion-treated mice displayed absolutely normal liver which resembled control mice (group 6 and 7).
Body Weight
Table 1 depicts the results of aflatoxin-induced changes in body weight of different groups of animals. There was no significant difference in the average initial weight of different groups of animals. Results indicate no significant change in body weight between different control groups at the end of 30 days treatment period (Group 1, 2, 3). However treatment with aflatoxin for 30 days caused a dose-dependent significant reduction in body weight of mice as compared to vehicle control (LD: −8.05%; HD: −15.86%) and the change was more pronounced in aflatoxin HD-treated animals. The body weight gain at the end of 30 day treatment period for LD and HD aflatoxin-treated mice was 26.5% and 16.5% respectively which was 13.3% and 23.3% less as compared to vehicle control mice. Administration of black tea infusion along with aflatoxin (Group 6, 7) caused significant amelioration in aflatoxin-induced reduction in body weight. Amelioration was comparatively higher in HD aflatoxin-treated mice (12.18%) than that of LD (4.7%).
Table 1.
Effect of black tea on aflatoxin-induced changes in the body weight (gm) of mice.
| S. no | Experimental groups | Days of treatment | |
|---|---|---|---|
| 0 | 30 | ||
| 1 | Untreated control | 39.68 ± 0.32 | 53.26 ± 0.75 |
| 2 | Vehicle control | 38.11 ± 0.25 | 53.29 ± 0.81 |
| 3 | Black tea control | 39.60 ± 0.21 | 53.50 ± 1.07 |
| 4 | LD aflatoxin | 38.72 ± 0.23 | 49.00 ± 0.55a |
| 5 | HD aflatoxin | 38.48 ± 0.25 | 44.84 ± 0.70a |
| 6 | LD aflatoxin + black tea | 38.63 ± 0.24 | 51.50 ± 0.74b |
| 7 | HD aflatoxin + black tea | 38.94 ± 0.24 | 51.33 ± 0.62c |
Results are expressed as mean ± SEM., n = 10.
No significance difference was noted between groups 1, 2 and 3.
Level of significance p < 0.05.
As compared between vehicle control (group 2) and aflatoxin-treated mice (groups 4, 5).
As compared between LD aflatoxin-treated (group 4) and LD aflatoxin + black tea-treated mice (group 6).
As compared between HD aflatoxin-treated (group 5) and HD aflatoxin + black tea-treated mice (groups 7).
Organ Weight
No significant changes were observed among different control groups (Group 1, 2, 3). However, as compared to the controls, oral administration of aflatoxin for 30 days in groups 4 and 5 caused significant dose-dependent increase (LD: 17.58%; HD: 26.31%) in absolute liver weight of mice (Table 2). Black tea infusion treatment (Group 6, 7) significantly mitigated the aflatoxin-induced change in the absolute liver weight. Amelioration was more in HD aflatoxin along with black tea-treated mice (24.45%) than that of LD aflatoxin plus antidote treated mice (18.42%).
Table 2.
Effect of black tea on aflatoxin-induced changes in the liver weight.
| S. no | Experimental groups | Liver weight | |
|---|---|---|---|
| Absolute (g) | Relative (g/100 g b. wt) | ||
| 1 | Untreated control | 2.69 ± 0.085 | 5.06 ± 0.185 |
| 2 | Vehicle control | 2.74 ± 0.123 | 5.13 ± 0.201 |
| 3 | Black tea control | 2.62 ± 0.087 | 4.89 ± 0.151 |
| 4 | LD aflatoxin | 3.22 ± 0.116a | 6.57 ± 0.259a |
| 5 | HD aflatoxin | 3.46 ± 0.195a | 7.71 ± 0.356a |
| 6 | LD aflatoxin + black tea | 2.71 ± 0.144b | 5.27 ± 0.213b |
| 7 | HD aflatoxin + black tea | 2.79 ± 0.101c | 5.43 ± 0.213c |
Results are expressed as mean ± SEM., n = 10.
No significance difference was noted between groups 1, 2 and 3.
Level of significance p < 0.05.
As compared between vehicle control (group 2) and aflatoxin-treated mice (groups 4, 5).
As compared between LD aflatoxin-treated (group 4) and LD aflatoxin + black tea-treated mice (group 6).
As compared between HD aflatoxin-treated (group 5) and HD aflatoxin + black tea-treated mice (groups 7).
Similarly oral administration of aflatoxin (group 4, 5) for 30 days caused significant dose-dependent increase (LD: 27.87%; HD: 50.12%) in relative liver weight of mice (Table 2). Administration of black tea extract (group 6, 7) caused significant mitigation in aflatoxin-induced increase as compared to aflatoxin alone treated groups, in relative liver weight and it was comparatively higher in HD aflatoxin plus black tea extract treated mice (44.37%) than that of LD aflatoxin plus black tea extract treated animals (25.26%) as evident from Table 2.
Serum Parameters
Table 3 shows the effect of aflatoxin and aflatoxin plus black tea extract treatment on the serum biochemical parameters in the mice. No significant changes were observed among different control groups (Group 1, 2, 3). However, a significant decrease in protein content was seen in aflatoxin-treated animals and the effect was dose-dependent (Table 3; Figure 1).
Table 3.
Effect of black tea on aflatoxin-induced changes in the serum parameters.
| Parameters | Experimental groups | ||||||
|---|---|---|---|---|---|---|---|
| Untreated control | Vehicle control | Black tea control | LD aflatoxin | HD aflatoxin | LD Aflatoxin + black tea |
HD Aflatoxin + black tea |
|
| Protein (mg/100 mL) | 59.99 ± 1.07 | 60.15 ± 1.18 | 59.27 ± 1.08 | 54.18 ± 2.16a | 48.51 ± 1.92a | 60.81 ± 1.89b | 60.00 ± 1.85c |
| Alanine aminotransferase (mU/mL) | 18.60 ± 2.74 | 17.65 ± 2.61 | 18.12 ± 0.53 | 21.51 ± 0.66a | 27.15 ± 1.89a | 18.80 ± 0.47b | 24.29 ± 1.27c |
| Aspartate amino transferase (mU/mL) | 60.90 ± 1.92 | 61.21 ± 6.02 | 58.73 ± 2.79 | 93.40 ± 2.90a | 116.00 ± 4.46a | 66.09 ± 1.55b | 64.24 ± 1.37c |
Results are expressed as mean ± SEM., n = 10.
No significance difference was noted between groups 1, 2 and 3.
Level of significance p < 0.05.
As compared between vehicle control (group 2) and aflatoxin-treated mice (groups 4, 5).
As compared between LD aflatoxin-treated (group 4) and LD aflatoxin + black tea-treated mice (group 6).
As compared between HD aflatoxin-treated (group 5) and HD aflatoxin + black tea-treated mice (groups 7).
Figure 1.
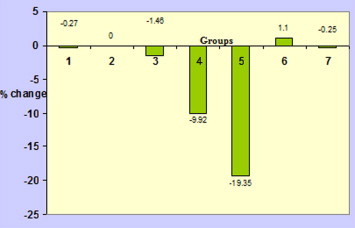
Percent change (as compared to vehicle control) in serum protein of different treatment groups of mice (group 1 = Untreated control; group 2 = Vehicle control; group 3 = Black tea control; group 4 = Vehicle + LD aflatoxin; group 5 = Vehicle + HD aflatoxin; group 6 = LD aflatoxin + black tea; group 7 = HD aflatoxin + black tea).
Results revealed a significant increase in the activities of ALT (Table 3; Figure 2) and AST (Table 3; Figure 3) in aflatoxin-treated mice for 30 days, as compared to the vehicle control. A 1.2 fold increase in ALT activity was observed in LD aflatoxin-treated mice; whereas HD aflatoxin treatment for 30 days resulted in 1.5 fold increase in the activity of this predominant liver marker enzyme. Similarly activities of AST were also found to be significantly higher in toxin-treated animals (1.5 and 1.9 fold increase in LD and HD aflatoxin-treated mice respectively). The simultaneous administration of black tea infusion along with aflatoxin caused significant mitigation in aflatoxin-induced effects in the protein content and ALT and AST activities as compared to the aflatoxin alone treated mice.
Figure 2.

Percent change (as compared to vehicle control) in alanine aminotransferase observed between different treatment groups (group 1 = Untreated control; group 2 = Vehicle control; group 3 = Black tea control; group 4 = Vehicle + LD aflatoxin; group 5 = Vehicle + HD aflatoxin; group 6 = LD aflatoxin + black tea; group 7 = HD aflatoxin + black tea).
Figure 3.

Percent change (as compared to vehicle control) in aspartate aminotransferase observed between different treatment groups (group 1 = Untreated control; group 2 = Vehicle control; group 3 = Black tea control; group 4 = Vehicle + LD aflatoxin; group 5 = Vehicle + HD aflatoxin; group 6 = LD aflatoxin + black tea; group 7 = HD aflatoxin + black tea).
Histopathological Studies
The transverse section of liver of vehicle control mice showed normal hepatocytes and sinusoids, a central vein with normal size and structure (Figure 4A) and it resembled untreated control mice. There was no apparent histopathological changes observed in the liver of black tea infusion alone treated mice (Figure 4B). Treatment with LD of aflatoxin induced histopathological alterations and caused mild to moderate hepatocellular necrosis (Figure 4C). On the other hand, oral administration of HD of aflatoxin for 30 days caused hepatocellular necrosis, pyknotic nuclei, fatty degeneration, cytoplasmic vacuolization (Figure 4D). Treatment of black tea infusion for 30 days along with aflatoxin caused almost complete amelioration in aflatoxin-induced effects in both LD (Figure 4E) and HD groups (Figure 4F) of mice as hepatocellular necrosis, fatty infiltration and cytoplasmic vacuolization were almost absent.
Figure 4.

Light micrograph (hematoxylin and Eosin staining) of liver. (A) Vehicle control mice showing normal architecture (100x). (B) Black tea infusion treated mice showing normal architecture and compactness. Vacuolization and necrosis are absent (100x). (C) LD aflatoxin-treated mice—indicating hepatocellular necrosis (100x). (D) HD aflatoxin treated mice—showing hepatocellular necrosis, pyknotic nuclei, fatty degeneration, cytoplasmic vacuolization (100x). (E) Black tea infusion plus LD aflatoxin-treated mice showing normal architecture and complete recovery (100x). (F) Black tea infusion plus HD aflatoxin-treated mice—Vacuolization, hepatocellular necrosis are absent (100x).
Discussion
Aflatoxins are group of related mycotoxins with enormous potential to induce hepatotoxicity, carcinogenicity, teratogenicity and mutagenicity.21 The extent of damage however differs and depends on the dose, duration of exposure and route of administration of the toxin and may range from acute to sub chronic, eliciting a carcinogenic response with long term chronic exposure. The present study is an attempt to evaluate the ability of time-tested black tea infusion to reverse aflatoxin-induced sub-acute liver damage in male mice.
A study conducted earlier on the toxicological aspects of aflatoxin has reported much higher elevation in ALT and AST levels in mice exposed to 25 μg of aflatoxin per day for 30 days over a 21 day exposure period.22 Daily exposure to aflatoxin for 30 days in rats resulted in hematological and biochemical changes typical to aflatoxicosis which was nullified by co-treatment with Nigella sativa and Syzygium aromaticum oils.23 Similarly treatment with whey-protein concentrates and Korean ginseng extract in rats fed with aflatoxin contaminated diet for 30 days was reported to successfully counteract aflatoxin-induced oxidative stress and cytotoxicity in bone marrow and lipid peroxidation in liver and testis.21
Results of the present study revealed that aflatoxin treatment for 30 days caused significant reduction in body weight gain of mice. The decrease in body weight gain is because of the reduced feed intake and anorexia which was seen throughout the treatment period with LD and HD of aflatoxin. The body performance data in the present study greatly agreed with the data reports of many other investigators. Weight loss as compared to control animals owing to aflatoxin treatment has been reported in rabbits,24 mice25 and rats.26 Adedara et al27 have reported a 12% body weight loss in mice treated with aflatoxin. A study conducted by Sharma et al28 has reported a 6.18% decrease in body weight in aflatoxin-treated mice which was significantly reversed by co-administration with Curcuma longa extract. Oral administration of aflatoxin was found to decrease the body weight of mice by 10.14% in one another study.29
Aflatoxin treatment for 30 days in the present study caused an increase in absolute and relative weights of liver of mice due to accumulation of lipid in the liver, which produces characteristic enlarged and fragile fatty liver. Previous studies conducted by other investigators have reported similar increase in absolute and relative liver weight during aflatoxicosis.30
Black tea extract alone treatment (Group 3) did not have any significant effect on morphological changes, change in body weight as well as absolute and relative weights of liver as compared to other controls (Group 1, 2). However, administration of black tea infusion along with either LD or HD of aflatoxin caused significant amelioration in morphological alterations, body weight gain as well as absolute and relative weights of liver as compared to aflatoxin alone treated animals which may be due to the antioxidative effect of tea-extract.17 Tea-infusions are well known for their characteristic antioxidative properties which offer beneficial health effects to its consumers.31
Aflatoxins are reported to impair protein biosynthesis by forming adducts with DNA, RNA and proteins,32 by down-regulating RNA synthesis and by inhibiting DNA-dependent RNA polymerase activity and causes degranulation of endoplasmic reticulum.33,34 Thus reduction in protein biosynthesis as well as increased necrosis could be responsible for decrease in protein. It is known that liver is the pivotal organ which is responsible for the secretion of majority of serum proteins and decrease in protein biosynthesis and also reduced secretion due to the ability of aflatoxin to for abducts with DNA and RNA will decrease the concentration of serum proteins. Aflatoxins have previously been shown to lower the total protein concentration in serum of pigs,35 rabbits36 and broilers.37
It is well known that leakage of hepatic housekeeping enzymes namely ALT (normal range in mice: 17–77 U/L) and AST (normal range in mice: 54–298 U/L)38 serve as biochemical indices of hepatocellular damage and recovery offered by therapeutic agents indicates hepatoprotection.39 Lenaerts et al40 have effectively used the human hepatotoxicity grading criteria devised by World Health Organization for Adverse Reaction Terminology (WHO-ART) on mice model of anti-tuberculosis drug-induced liver injury. The same if applied to the present study indicates a grade 1 hepatotoxicity with a 1.2 and 1.5 fold increase in the ALT activities and a 1.5 and 1.9 fold increase in AST activities in LD and HD aflatoxin-treated mice respectively as compared to vehicle control. However ALT and AST showed significantly lower values in the black tea with aflatoxin-treated groups which were almost comparable to the control groups. Thus the ability of the black tea infusion to reduce the serum levels of ALT and AST suggests that that the extract possesses enormous hepatoprotective potential.
A variety of physical and chemical agents have been reported to reduce aflatoxin-induced hepatotoxicity through varied mechanism of action. While some agents interfere with their absorption, biotransformation and covalent binding to cellular macromolecules, others enhance the detoxification of the toxin through conjugation or by curtailing lipid peroxidation17,41 due to their antioxidative ability.
Our results indicate that black tea infusion has a protective role in animal model of aflatoxin induced hepatotoxicity. Further research on detailed mechanism of action of the compound at macro and molecular level can throw more light on the hepatoprotective ability of black tea.
Conflicts of interest
All authors have none to declare.
References
- 1.Cullen J.M., Newberne P.M. Acute hepatotoxicity of aflatoxins. In: Eaton D.L., Groopman J.D., editors. The Toxicology of Aflatoxins: Human Health, Veterinary and Agricultural Significance. Academic Press; London: 1993. [Google Scholar]
- 2.Roberts T.A., Baird-Parker A.C., Tompkin R.B. Aspergillus. Microorganisms in Food. Microbiological Specification of Food Pathogens. ICMSF. Blackie Academic and Professional; London: 1996. Toxigenic fungi; pp. 347–438. 19. [Google Scholar]
- 3.Stresser D.M., Bailey G.S., Williams D.E. Indol-3-carbinol and beta-naphthoflavone induction of aflatoxin B1 metabolism and cytochromes P-450 associated with bioactivation and detoxication of aflatoxin B1 in the rat. Drug Metab Dispos. 1994;22:383–391. [PubMed] [Google Scholar]
- 4.Sun C.A., Wu D.M., Wang L.Y., Chen C.J., You S.L., Santella R.M. Determinants of formation of aflatoxin-albumin adducts: a seven township study in Taiwan. Br J Cancer. 2002;87:966–970. doi: 10.1038/sj.bjc.6600584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katiyar S., Mukhtar H. Tea in chemoprevention of cancer: epidemiological and experimental studies. Int J Oncol. 1996;8:221–238. doi: 10.3892/ijo.8.2.221. [DOI] [PubMed] [Google Scholar]
- 6.Lakenbrink C., Lapczynski S., Maiwald B., Engelhardt U.H. Flavonoids and other polyphenols in consumer brews of tea and other caffeinated beverages. J Agric Food Chem. 2000;48:2848–2852. doi: 10.1021/jf9908042. [DOI] [PubMed] [Google Scholar]
- 7.Yang G.Y., Liao J., Kim K., Yurkow E.J., Yang C.S. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis. 1998;19:611–616. doi: 10.1093/carcin/19.4.611. [DOI] [PubMed] [Google Scholar]
- 8.Karakaya El S.N., Tas A.A. Antioxidant activity of some foods containing phenolic compounds. Int J Food Sci Nutr. 2001;52:501–508. [PubMed] [Google Scholar]
- 9.Leung L.K., Su Y., Chen R., Zhang Z., Huang Y., Chen Z.Y. Theaflavins in black tea and catechins in green tea are equally effective antioxidants. J Nutr. 2001;131:2248–2251. doi: 10.1093/jn/131.9.2248. [DOI] [PubMed] [Google Scholar]
- 10.El-lethey H.S., Kamel M.M. Effects of black tea in mitigation of sodium fluoride potency to suppress motor activity and coordination in laboratory rats. J Am Sci. 2011;7:243–254. [Google Scholar]
- 11.Halder J., Bhaduri A.N. Protective role of black tea against oxidative damage of human red blood cells. Biochem Biophys Res Comm. 1998;244:903–907. doi: 10.1006/bbrc.1998.8366. [DOI] [PubMed] [Google Scholar]
- 12.El-lethey H.S., Shaheed I.B. Potential health impact of black tea against Na-F-Induced alterations in territorial aggression, sexual behaviour and fertility of male rats. Life Sci J. 2011;8:828–839. [Google Scholar]
- 13.Chung F.L., Wang M., Rivenson A. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: caffeine as an important constituent. Cancer Res. 1998;58:4096–4101. [PubMed] [Google Scholar]
- 14.Banerjee S., Maity P., Mukherjee S. Black tea prevents cigarette smoke-induced apoptosis and lung damage. J Inflamm. 2007:4. doi: 10.1186/1476-9255-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trivedi M.H., Verma R.J., Chinoy N.J. Amelioration by black tea of sodium fluoride-induced effects on DNA, RNA, and protein contents of liver and kidney and on serum transaminase activities in Swiss albino mice. Fluoride. 2008;41:61–66. [Google Scholar]
- 16.Verma R.J., Dave M., Mathuria N. A study on toxicity of gasoline and GM-10 on liver of mice and its amelioration by black tea extract. Acta Pol Pharm. 2008;65:601–605. [PubMed] [Google Scholar]
- 17.Choudhary A., Verma R.J. Ameliorative effects of black tea extract on aflatoxin-induced lipid peroxidation in liver of mice. Food Chem Toxicol. 2005;43:99–104. doi: 10.1016/j.fct.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Smith J.E., Moss M.O. John Wiley and Sons Ltd; New York: 1985. Mytotoxins—Formation, Analysis and Significance. [Google Scholar]
- 19.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurements with folin-phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 20.Reitman S., Frankel S. A colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvate transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 21.Abdel-Aziem S.H., Hassan A.M., Abdel-Wahhab M.A. Dietary supplementation with whey protein and ginseng extract counteracts oxidative stress and DNA damage in rats fed an aflatoxin-contaminated diet. Mutat Res. 2011;723:65–71. doi: 10.1016/j.mrgentox.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Yassein S.N., Zghair Z.R. Study of toxicity and pathogenicity of aflatoxin B1 and G1 in mice. Al Anbar J Vet Sci. 2012;5:23–31. [Google Scholar]
- 23.Abdel-Wahhab M.A., Aly S.E. Antioxidant property of Nigella sativa (black cumin) and Syzygium aromaticum (clove) in rats during aflatoxicosis. J Appl Toxicol. 2005;25:218–223. doi: 10.1002/jat.1057. [DOI] [PubMed] [Google Scholar]
- 24.Salem M.H., Kamel K.I., Yousef M.I., Hassan G.A., Nouty F.D. Protective role of ascorbic acid to enhance semen quality of rabbits treated with sublethal doses of aflatoxin B1. Toxicology. 2001;162:209–218. doi: 10.1016/s0300-483x(01)00366-3. [DOI] [PubMed] [Google Scholar]
- 25.Kocabas C.N., Coskun T., Yurdakok M., Haziroglu R. The effects of aflatoxin B1 on the development of kwarshiorkor in mice. Hum Exp Toxicol. 2003;22:155–158. doi: 10.1191/0960327103ht342oa. [DOI] [PubMed] [Google Scholar]
- 26.Pozzi C.R., Correa B., Xavier J.G., Direito G.M., Orsi R.B., Metarazzo S.V. Effects of prolonged oral administration of fumonisin B1 and aflatoxin B1 in rats. Mycopathologia. 2001;151:21–27. doi: 10.1023/a:1010954119980. [DOI] [PubMed] [Google Scholar]
- 27.Adedara I.A., Owumi S.E., Uwaifo A.O., Farombi E.O. Aflatoxin B1 and ethanol co-exposure induces hepatic oxidative damage in mice. Toxicol Ind Health. 2010;26:717–724. doi: 10.1177/0748233710377772. [DOI] [PubMed] [Google Scholar]
- 28.Sharma V., Sharma C., Pracheta Paliwal R., Sharma S. Protective effect of curcumin longa and curcumin on aflatoxin B1 induced hepatotoxicity in swiss albino mice. Asian J Pharm Hea Sci. 2011;1:116–122. [Google Scholar]
- 29.Darwish H.R., Omara E.A., Abdel-Aziz K.B., Farag I.M., Nada S.A., Tawfek N.S. Saccharomyces cerevisiae modulates aflatoxin-induced toxicity in male albino mice. Rep Opin. 2011;3:32–43. [Google Scholar]
- 30.Aravind K.L., Patil V.S., Devegowda G., Umakantha B., Ganpule S.P. Efficacy of esterified glucomannan to counteract mycotoxicosis in naturally contaminated feed on performance and serum biochemical and hematological parameters in broilers. Poult Sci. 2003;82:571–576. doi: 10.1093/ps/82.4.571. [DOI] [PubMed] [Google Scholar]
- 31.Kurihara H., Fukami H., Toyoda Y. Inhibitory effect of oolong tea on the oxidative state of low density lipoprotein (LDL) Biol Pharm Bull. 2003;26:739–742. doi: 10.1248/bpb.26.739. [DOI] [PubMed] [Google Scholar]
- 32.Busby W.F., Wogan G.N. Aflatoxins. In: Searle S.E., editor. Chemical Carcinogens. vol. 182. American Chemical Society; Washington, D.C: 1984. pp. 945–1136. (ACS Monograph). [Google Scholar]
- 33.Verma R.J., Nair A. Vitamin E ameliorates aflatoxin induced hyperglycaemia in mice. J Tissue Res. 2003;45:131. [Google Scholar]
- 34.Jha A., Shah K., Verma R.J. Aflatoxin-induced biochemical changes in liver of mice and its mitigation by black tea extract. Acta Pol Pharm. 2012;69:851–857. [PubMed] [Google Scholar]
- 35.Harvey R.B., Huff W.E., Kubena L.F., Corrier D.E., Phillips T.D. Progression of aflatoxicosis in growing pigs. Am J Vet Res. 1988;49:482–487. [PubMed] [Google Scholar]
- 36.Yousef M.I., Salem M.H., Kamel K.I., Hassan G.A., EL-Nouty F.D. Influence of ascorbic acid supplementation on the haematological and clinical biochemistry parameters of male rabbits exposed to aflatoxin B1. J Environ Sci Health B. 2003;38:193–209. doi: 10.1081/PFC-120018449. [DOI] [PubMed] [Google Scholar]
- 37.Raju M.V., Devegowda G. Influence of esterified-glucomannan on performance and organ morphology, serum biochemistry and haematology in broilers exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin and T-2 toxin) Br Poult Sci. 2000;41:640–650. doi: 10.1080/713654986. [DOI] [PubMed] [Google Scholar]
- 38.Normal hematology values. Research Animal Resources, University of Minnesota, reference values for laboratory animals. http://www.ahc.umn.edu/rar/refvalues.
- 39.Klaassen C.D., Watkins J.B. Mechanisms of bile formation, hepatic uptake and biliary excretion. Pharmacol Rev. 1984;36:1–67. [PubMed] [Google Scholar]
- 40.Lenaerts A.J., Johnson C.M., Marrieta K.S., Gruppo V., Orme I.M. Significant increases in the levels of liver enzymes in mice treated with anti-tuberculosis drugs. Int J Antimicrob Agents. 2005;26:152–158. doi: 10.1016/j.ijantimicag.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Choudhary A., Verma R.J. Black tea ameliorates aflatoxin-induced lipid peroxidation in the kidney of mice. Acta Pol Pharm Drug Res. 2006;63:307–310. [PubMed] [Google Scholar]


