Abstract
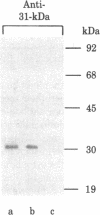
The complexed (ribosome-bearing) membrane fraction of Bacillus subtilis contains several proteins (CM-proteins) that are virtually absent from the ribosome-free fraction and hence might be components of the apparatus of protein secretion. We have determined, by trypsin digestion and by labeling with a nonpenetrating reagent (diazoiodosulfanilic acid), the accessibility of four of these proteins on the two surfaces of the membrane, as exposed either in protoplasts or in inverted membrane vesicles. The 68-kilodalton protein is a transmembrane protein and the 45-kilodalton protein faces only the external surface, whereas the 31-kilodalton protein is inaccessible from either side. Of particular interest is the 64-kilodalton protein: it can be digested by trypsin, and can bind antibody, on the cytoplasmic surface, but only after the ribosomes have been released. This protein is thus evidently a component of the apparatus of protein secretion, closely covered by secreting ribosomes. Whether the other CM-proteins are also involved in protein secretion is uncertain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emr S. D., Hanley-Way S., Silhavy T. J. Suppressor mutations that restore export of a protein with a defective signal sequence. Cell. 1981 Jan;23(1):79–88. doi: 10.1016/0092-8674(81)90272-5. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore R., Blobel G., Walter P. Protein translocation across the endoplasmic reticulum. I. Detection in the microsomal membrane of a receptor for the signal recognition particle. J Cell Biol. 1982 Nov;95(2 Pt 1):463–469. doi: 10.1083/jcb.95.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi S., Inoue M., Morino Y. Latent active site in rat-kidney gamma-glutamyl transpeptidase. The refolding process of the large subunit and characterization of the renatured enzyme. Eur J Biochem. 1980 Mar;105(1):93–102. doi: 10.1111/j.1432-1033.1980.tb04478.x. [DOI] [PubMed] [Google Scholar]
- Kreibich G., Czakó-Graham M., Grebenau R., Mok W., Rodriguez-Boulan E., Sabatini D. D. Characterization of the ribosomal binding site in rat liver rough microsomes: ribophorins I and II, two integral membrane proteins related to ribosome binding. J Supramol Struct. 1978;8(3):279–302. doi: 10.1002/jss.400080307. [DOI] [PubMed] [Google Scholar]
- Kreibich G., Freienstein C. M., Pereyra B. N., Ulrich B. L., Sabatini D. D. Proteins of rough microsomal membranes related to ribosome binding. II. Cross-linking of bound ribosomes to specific membrane proteins exposed at the binding sites. J Cell Biol. 1978 May;77(2):488–506. doi: 10.1083/jcb.77.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreibich G., Ulrich B. L., Sabatini D. D. Proteins of rough microsomal membranes related to ribosome binding. I. Identification of ribophorins I and II, membrane proteins characteristics of rough microsomes. J Cell Biol. 1978 May;77(2):464–487. doi: 10.1083/jcb.77.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D. I., Krause E., Dobberstein B. Secretory protein translocation across membranes-the role of the "docking protein'. Nature. 1982 Jun 24;297(5868):647–650. doi: 10.1038/297647a0. [DOI] [PubMed] [Google Scholar]
- Müller M., Ibrahimi I., Chang C. N., Walter P., Blobel G. A bacterial secretory protein requires signal recognition particle for translocation across mammalian endoplasmic reticulum. J Biol Chem. 1982 Oct 25;257(20):11860–11863. [PubMed] [Google Scholar]
- Oliver D. B., Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982 Aug;30(1):311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P. Cotranslational secretion of diphtheria toxin and alkaline phosphatase in vitro: involvement of membrane protein(s). J Bacteriol. 1980 Mar;141(3):1142–1147. doi: 10.1128/jb.141.3.1142-1147.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Davis B. D. Nascent peptide as sole attachment of polysomes to membranes in bacteria. Proc Natl Acad Sci U S A. 1978 Feb;75(2):814–817. doi: 10.1073/pnas.75.2.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai P. C., Davis B. D. Triphasic concentration effects of gentamicin on activity and misreading in protein synthesis. Biochemistry. 1979 Jan 9;18(1):193–198. doi: 10.1021/bi00568a029. [DOI] [PubMed] [Google Scholar]
- Talmadge K., Stahl S., Gilbert W. Eukaryotic signal sequence transports insulin antigen in Escherichia coli. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3369–3373. doi: 10.1073/pnas.77.6.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Purification of a membrane-associated protein complex required for protein translocation across the endoplasmic reticulum. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7112–7116. doi: 10.1073/pnas.77.12.7112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982 Oct 21;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- Walter P., Ibrahimi I., Blobel G. Translocation of proteins across the endoplasmic reticulum. I. Signal recognition protein (SRP) binds to in-vitro-assembled polysomes synthesizing secretory protein. J Cell Biol. 1981 Nov;91(2 Pt 1):545–550. doi: 10.1083/jcb.91.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]