Abstract
Protein tyrosine kinase 6 (PTK6) is a non-receptor tyrosine kinase expressed in epithelial cancers. Disruption of Ptk6 decreases AOM-induced colon tumorigenesis in mice by preventing STAT3 activation. Relocalization of PTK6 in prostate cancers contributes to increased growth. Although not expressed in normal breast or ovary, PTK6 promotes anchorage-independent survival of breast and ovarian tumor cells. We identified several potential PTK6 substrates in the human SW620 colon cancer cell line using mass spectrometry, including FAK (focal adhesion kinase). We show that FAK is a direct substrate of PTK6 in vitro and in vivo. Expression of membrane targeted active PTK6 (Palm-PTK6-YF) induces constitutive activation of FAK and cell morphology changes, which are independent of SRC family kinases in Src−/−, Yes−/−, Fyn−/− (SYF) mouse embryonic fibroblasts (MEFs). Palm-PTK6-YF expressing SYF cells are transformed and overcome contact inhibition, form colonies in transformation assays, proliferate in suspension, and form tumors in a xenograft model. Expression of FAK and Palm-PTK6-YF in Fak−/− MEFs synergistically activates AKT and protects cells against anoikis. However, expression of Palm-PTK6-YF in Akt1/2−/− MEFs fails to protect cells from anoikis, indicating AKT is critical in PTK6 and FAK mediated survival signaling. In a conditional Pten knockout murine prostate cancer model, we identify prostate epithelial cells with enhanced activation of endogenous PTK6 and FAK at the plasma membrane. Knockdown of PTK6 in the PC3 human prostate cancer cell line disrupts FAK and AKT activation and promotes anoikis, which can be rescued by exogenous expression of FAK. Our data reveal important roles for a PTK6-FAK-AKT signaling axis in promoting anchorage-independent cell survival.
Keywords: BRK, Sik, PTK6, FAK, AKT, anoikis, transformation
Introduction
Protein tyrosine kinase 6 (PTK6), (also called BRK for BReast tumor Kinase or Sik for SRC-related Intestinal Kinase), is an intracellular non-receptor tyrosine kinase (1, 2). Lack of an amino-terminal myristoylation sequence leads to increased flexibility in intracellular localization, and PTK6 can be found at the membrane, the cytoplasm and the nucleus (3–5). A variety of proteins have been identified as PTK6 substrates, including the RNA binding proteins Sam68 (3), SLM-1, SLM-2 (6), the transcription factors STAT3 (7), STAT5b (8), β-catenin (9), scaffold proteins BKS/STAP2 (10), paxillin (11), p130CAS (5), EGFR (12), the serine/threonine kinase AKT (13), and a GTPase activating protein p190RhoGAP (14).
Although PTK6 is not expressed in the normal mammary gland, it is frequently overexpressed in human breast tumors (15, 16). Increased expression or relocalization of PTK6 is also detected in other types of human cancer including colon (17), prostate (4), head and neck cancer (18), as well as serous carcinoma of ovary (19). Several recent studies revealed a role for PTK6 in cell proliferation and survival in vivo. In an AOM/DSS (azoxymethane/dextran sodium sulfate) murine colon cancer model, disruption of Ptk6 prevents activation of STAT3 and decreases AOM-induced colon tumorigenesis in mice (20). In a PTK6 transgenic mouse model, activation of a p38 MAPK pro-survival signaling pathway delays involution of mouse mammary gland (16). In addition, PTK6 is a critical regulator of IGF-1 mediated anchorage-independent survival of breast and ovarian tumor cells, but the downstream molecular mechanisms are still not clear (21).
Focal adhesion kinase (FAK) was identified as a highly phosphorylated protein localized in focal adhesion contacts of normal cells [reviewed in (22)]. Its activity is regulated by integrin mediated cell adhesion as well as activation of growth factor receptor and G-protein linked receptor signaling [reviewed in (23)]. FAK is involved in various cell functions including proliferation, survival, motility and invasion [reviewed in (23, 24)]. It regulates cell survival and apoptosis through several different mechanisms. FAK is able to activate PI3K/AKT signaling to promote survival of fibroblasts (25). Depending upon the cellular context, FAK forms signaling complexes with Paxillin or p130CAS to transmit distinct survival signals to fibroblasts and epithelial cells (26). Recently, nuclear FAK was reported to promote cell survival by facilitating p53 turnover via enhanced Mdm2-dependent p53 ubiquitination (27).
AKT, a serine threonine protein kinase that plays critical roles in cell proliferation, survival, angiogenesis and metabolism, is frequently activated in different types of human cancers [reviewed in (28)]. It is downstream of growth factor receptors and phosphatidylinositol 3-kinase (PI3K), and regulates cell survival through multiple mechanisms including inhibition of pro-apoptotic proteins such as Bad and FoxO transcription factors and activation of pro-survival genes through the IKK/NF-κB signaling pathway (28, 29). AKT regulates cell cycle and proliferation through direct phosphorylation of p21WAF1/CIP1 and p27KIP1 and indirect transcriptional regulation by inhibition of FoxO transcription factors (30, 31). AKT itself is sufficient to suppress anoikis, a form of programmed cell death induced by the loss of cell-matrix interactions (32). Anoikis resistance and anchorage independence are critical for a tumor cell to invade adjacent tissue, giving rise to metastases [reviewed in (33)].
We demonstrate FAK is a direct substrate of PTK6, and PTK6 mediated tyrosine phosphorylation of FAK and subsequent AKT activation promotes resistance to anoikis. We show that membrane associated active PTK6 transforms SYF cells in the absence of SRC family kinases. Knockdown of PTK6 in prostate cancer cells impairs their ability to maintain FAK and AKT activation under nonadherent growth condition, resulting in apoptosis. These studies delineate a role for a PTK6-FAK-AKT signaling axis in anchorage-independent cell survival, and enhance our understanding of the contributions of PTK6 to cancer cell signaling.
Results
Mass spectrometry analysis revealed several candidate PTK6 substrates
Mice lacking Ptk6 are resistant to AOM-induced colon tumorigenesis due probably to reduced activation of the PTK6 substrate STAT3 (20), but other mechanisms may also exist (7). Therefore we tried to identify potential PTK6 substrates using tandem mass spectrometry (LC/MS/MS) of differentially tyrosine phosphorylated proteins in the presence of active PTK6. SW620 cells were transfected with vector or PTK6-YF for 24 hours, serum starved for 48 hours to reduce basal protein tyrosine phosphorylation and then cells were serum stimulated for 1, 3 and 5 hours. Mutation of the regulatory tyrosine 447 at the carboxy-terminus of PTK6 to phenylalanine (YF) prevents autoinhibition and promotes constitutive activation (3, 36). Cells that were serum stimulated for five hours displayed the least basal tyrosine phosphorylation in vector control cells, and a distinct set of proteins were tyrosine phosphorylated in the presence of active PTK6 (Figure S1A). A large-scale anti-phospho-tyrosine (PY) immunoprecipitation was performed with lysates harvested at the five hour time point, and three distinct bands at approximately 120 kDa, 80 kDa and 68 kDa were enriched from cells expressing active PTK6, which were then excised from SDS-PAGE gels and digested with trypsin for LC/MS/MS sequencing (Figure S1B). Several novel candidate PTK6 substrates including FAK, CUL4, the dead box proteins DDX5 and DDX17, and Hsp70 along with confirmed substrates of PTK6 such as Sam68, Paxillin, PSF and p130CAS (3, 5, 11, 37) were identified.
PTK6 directly phosphorylates FAK independent of SRC family kinases
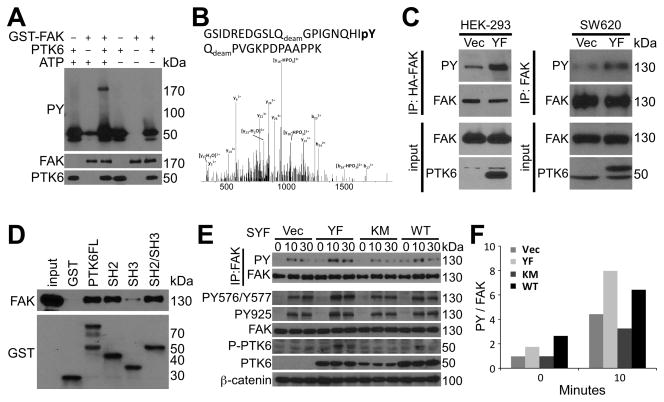
Focal adhesion kinase (FAK) was identified as a potential PTK6 substrate by LC/MS/MS sequencing, and it plays crucial roles in suppressing anoikis and promoting anchorage-independent survival in a variety of cell types (3, 25–27, 38, 39). FAK is tyrosine phosphorylated by SRC at several tyrosine residues including 576/577, 861 and 925. Phosphorylation of FAK tyrosine residues 576/577 is important for maximum activation, while phosphorylation of tyrosine residue 925 serves as a high affinity Grb2 binding site [reviewed in (24)]. Since phosphorylation of FAK at several tyrosine residues is important for its activation and downstream signaling, we investigated if FAK is a direct substrate of PTK6. In an in vitro kinase assay, tyrosine phosphorylation of FAK is increased in the presence of PTK6 and ATP (Figure 1A). We detected phosphorylation of FAK tyrosine residue 861 in vitro using LC/MS/MS (Figure 1B). Expression of active PTK6 in HEK-293 and SW620 cells induces the tyrosine phosphorylation of FAK, as evident by FAK immunoprecipitation followed by immunoblotting analysis with anti-phosphotyrosine antibodies (Figure 1C). In addition, GST pull down assays using PTK6 GST-fusion proteins containing full length PTK6, or its SH2, SH3, or SH2/SH3 domains showed that PTK6 interacts with FAK primarily through its SH2 domain, which recognizes phosphorylated tyrosine residues on FAK (Figure 1D).
Figure 1. PTK6 phosphorylates FAK independent of SRC family kinase.
A. FAK is a direct PTK6 substrate. Tyrosine phosphorylation of FAK is induced by active PTK6 in the presence of ATP in an in vitro kinase assay. Immunoblotting analysis of kinase reactions was performed using anti-PY, FAK and PTK6 antibodies. B. PTK6 phosphorylates FAK at tyrosine residue 861. The LC/MS/MS tandem mass spectrum of the triply charged tryptic peptide GSIDREDGSLQdeamGPIGNQHIpYQdeamPVGKPDPAAPPK acquired via CID using a hybrid linear ion trap-orbitrap mass spectrometer show the phosphorylation site Y861. C. PTK6 phosphorylates FAK in cells. HEK293 and SW620 cells were transiently transfected with PCDNA3-PTK6-YF or empty vector, and total cell lysates were harvested 24 hour after transfection. HA-tag-IP or FAK-IP was performed and tyrosine phosphorylation of FAK was detected by immunoblotting with anti-PY antibodies. Immunoblotting analysis of total cell lysates was performed using anti-FAK and PTK6 antibodies as input. D. PTK6 interacts with FAK through its SH2 domain. Glutathione-Sepharose CL-4B beads binding with GST fusion proteins were used to pull down FAK from HEK-293 cell lysates. Bound FAK was analyzed by immunoblotting with anti-FAK antibodies. 10% of the lysates added to the pulldown reaction served as input. E. PTK6 induces tyrosine phosphorylation of FAK independent of SRC family kinases. SYF cells stably expressing vector, PTK6-WT, PTK6-KM or PTK6-YF were serum starved for 48 hours and stimulated by 20% FBS for 10 or 30 minutes. FAK IP was performed and tyrosine phosphorylation of FAK was detected by immunoblotting with anti-PY antibodies. Immunoblotting analysis of total cell lysates was performed using anti-FAK, P-FAK (Tyr576/Tyr577), P-FAK (Tyr925), PTK6, P-PTK6 (Tyr-342), and β-catenin antibodies. F. PTK6 induced tyrosine phosphorylation of FAK is kinase dependent. The relative band density of PY blot in the FAK IP was normalized by the density of FAK using NIH ImageJ software (50).
Since FAK can be phosphorylated by SRC kinase (40), the SYF mouse embryonic fibroblast cell line (Src−/−, Yes−/−, Fyn−/−) was used to exclude interference by SRC family kinases. Following serum stimulation, higher tyrosine phosphorylation of FAK was observed in SYF cells stably expressing PTK6-YF or PTK6-WT, compared with cells expressing kinase dead PTK6-KM or vector controls (Figure 1E, FAK IP; 1F). This was also observed using phosphorylation-specific FAK antibodies PY925 or PY576/Y577 (Figure 1E). Interestingly, FAK reaches the peak of tyrosine phosphorylation at the 10-minute time point in PTK6-WT or PTK6-YF expressing cells, correlating with the activation of PTK6, monitored by phosphorylation of PTK6 tyrosine residue 342 (Figure 1E, P-PTK6). These data suggest that PTK6 induced tyrosine phosphorylation on FAK is dependent on the kinase activity of PTK6, and independent of SRC family kinases.
Membrane targeted active PTK6 induces constitutive FAK activation and transformation of SYF fibroblasts
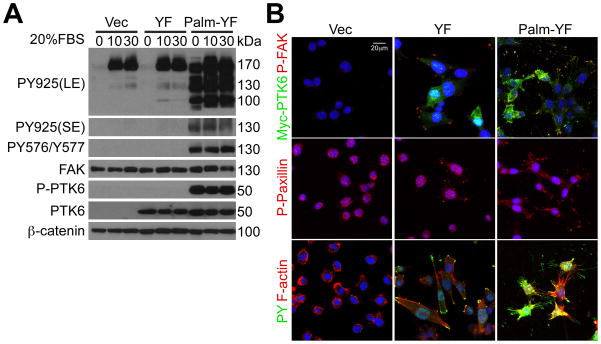
Although PTK6 is primarily cytoplasmic in the PC3 prostate cancer cell line, the active pool of PTK6 that is phosphorylated on tyrosine residue 342 is associated with membrane compartments (5). SYF cells stably expressing membrane targeted PTK6 with mutation of the carboxy-terminal regulatory tyrosine (Palm-PTK6-YF) have constitutively active PTK6, even in cells that are serum starved for 48 hours (Figure 2A, 0 timepoint, P-PTK6, Palm-YF). FAK is constitutively phosphorylated at tyrosine residues 576, 577 and 925 in Palm-YF cells (Figure 2A). A long exposure (LE) of the immunoblot for FAK phosphorylated at tyrosine residue 925 shows that Y925 phosphorylation increases upon serum stimulation in vector control cells and cells expressing untargeted PTK6-YF (130 kDa band). Immunostaining of SYF cells expressing untargeted PTK6-YF shows that it is capable of inducing formation of focal adhesions containing increased phospho-FAK and phospho-Paxillin (Figure 2B, YF). However, expression of membrane targeted Palm-PTK6-YF dramatically changed cell morphology, and led to formation of numerous thin long actin-bundle protrusions around plasma membrane, with tyrosine phosphorylated FAK and Paxillin at the tip of each protrusion (Figure 2B, Palm-PTK6-YF).
Figure 2. Membrane associated active PTK6 induces constitutive activation of FAK in SYF cells.
A. Membrane targeted PTK6 (Palm-PTK6-YF) is constitutively active. SYF cells stably expressing vector, PTK6-YF or Palm-PTK6-YF were serum starved for 48 hours and stimulated by 20% FBS for 10 or 30 minutes. Immunoblotting analysis of total cell lysates was performed using anti-FAK, P-FAK (Tyr576/Tyr577), P-FAK (Tyr925), PTK6, P-PTK6 (Tyr342), and β-catenin antibodies. Both long (LE) and short exposures (SE) of P-FAK (Tyr925) blots are shown; the specific P-FAK band migrates at 130 kDa. B. Membrane targeted PTK6 dramatically changes the morphology of SYF cells. SYF cells expressing vector, PTK6-YF or Palm-PTK6-YF were grown at 8-well chamber slides, and indirect immunofluorescence was performed using anti-Myc-tag, P-FAK (Tyr576/Tyr577), PY and P-Paxillin (Tyr118) antibodies. Rhodamine-conjugated phalloidin was used to selectively stain F-actin. Cells were counterstained with DAPI (blue). The size bar denotes 20 μm.
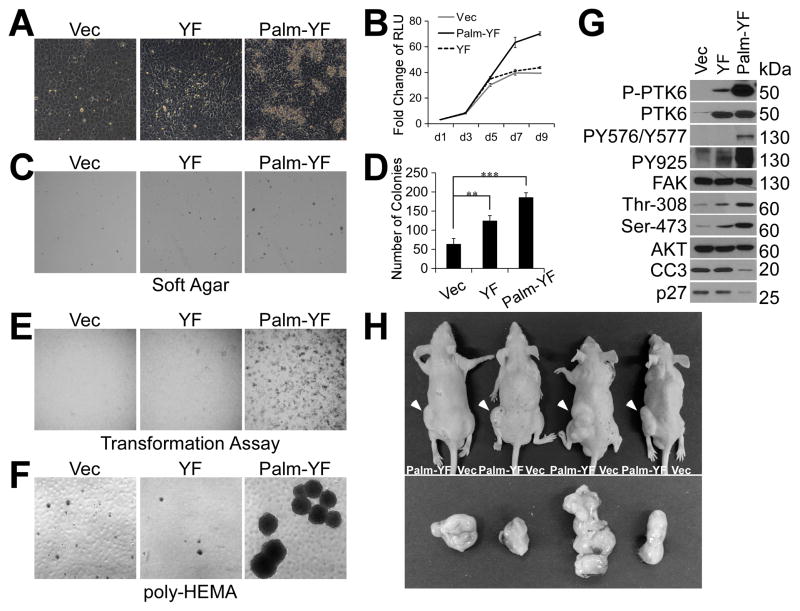
Next, we investigated the behavior of SYF cells with constitutively active PTK6 and FAK. While wild type SYF cells form a single cell layer due to contact inhibition after confluence, cells with Palm-PTK6-YF expression overcome contact inhibition and continue to grow after confluence. Cells expressing nontargeted PTK6-YF are more densely packed than vector control cells, but they do not lose contact inhibition (Figure 3A). This observation is consistent with the growth curve data, showing that Palm-PTK6-YF expressing cells continue to proliferate after cells reach confluence at day 5, while preconfluent growth rates are similar for the three SYF cell lines (Figure 3B). Anchorage independent growth of SYF cells in soft agar assays is augmented when Palm-PTK6-YF is expressed and to a lesser extent when PTK6-YF is present (Figure 3C; D). Foci-forming ability was also measured by performing transformation assays. Only Palm-PTK6-YF expressing SYF cells acquired the capacity to form foci on plates when mixed with the non-tumorigenic parental SYF cells (Figure 3E). Resistance to anoikis is another trait of transformed cells. SYF cells expressing Palm-PTK6-YF overcome anoikis and continue to proliferate in suspension. Significantly larger and more cell spheres were observed in Palm-PTK6-YF expressing cells at 6 days after seeding on poly-HEMA coated plates (Figure 3F). Immunoblotting of lysates prepared from cells following 72 hours of growth in suspension showed highly activated PTK6 (P-PTK6) and FAK (PY567/Y577, PY925) in Palm-PTK6-YF expressing cells, accompanied by increased AKT activation (Thr-308, Ser-473) and decreased levels of cleaved caspase 3 (CC3) and p27, which are markers of apoptosis and cell cycle arrest respectively (Figure 3G). To confirm that Palm-PTK6-YF is capable of transforming SYF MEFs, SYF cells expressing Palm-PTK-YF or vector alone (control) were injected subcutaneously into the left or right flanks, respectively, of male nude mice. Palm-PTK6-YF expressing cells produced large tumors after 4 weeks, while no tumors were formed by vector control cells (Figure 3H). Taken together, our data indicate that SYF cells can be transformed by Palm-PTK6-YF, and this may be due to the constitutive activation of a signaling cascade including PTK6, FAK and AKT.
Figure 3. Membrane associated active PTK6 transforms SYF MEFs.
A. SYF cells stably expressing Palm-PTK6-YF are able to overcome contact inhibition. SYF cells stably expressing vector, PTK6-YF or Palm-PTK6-YF were seeded at complete growth medium, and images were taken 5 days after confluence. B. Growth curve of SYF cells as measured by CellTiter-Glo luminescent cell viability assay demonstrates the ability of Palm-PTK6-YF expressing cells to continue growing after confluence. Y axis is the fold change of relative luminescence unit (RLU), with day 1 set to 1. C. SYF cells expressing PTK6-YF or Palm-PTK6-YF show increased anchorage independent growth in soft agar assay. D. A bar graph illustrates the number of colonies formed inn soft agar assay in C (**, p < 0.01; ***, p < 0.001). E. Transformation (foci-forming) assays show increased foci-forming ability of SYF cells expressing Palm-PTK6-YF. F. Palm-PTK6-YF expressing SYF cells are able to proliferate under suspended growth conditions. Anoikis assays were performed on poly-HEMA coated plates for 6 days. G. Palm-PTK6-YF activates FAK and AKT survival signaling pathways. Palm-PTK6-YF expressing SYF cells were seeded on poly-HEMA coated plates for 72 hours. Immunoblotting analysis was performed with anti-P-PTK6 (Tyr342), PTK6, P-FAK (Tyr576/Tyr577), P-FAK (Tyr925), FAK, P-AKT (Thr308), P-AKT (Ser473), AKT, cleaved caspase 3 (CC3) and p27 antibodies. H. SYF cells expressing Palm-PTK6-YF form tumors in xenograft mice. Palm-PTK6-YF expressing cells were injected subcutaneously in left flanks of nude mice, and control cells were injected in right flanks. Tumors were indicated by white arrows. Mice were sacrificed 4 weeks after injection, and tumors were taken out for further analysis.
PTK6 and FAK provide synergistic protection against anoikis
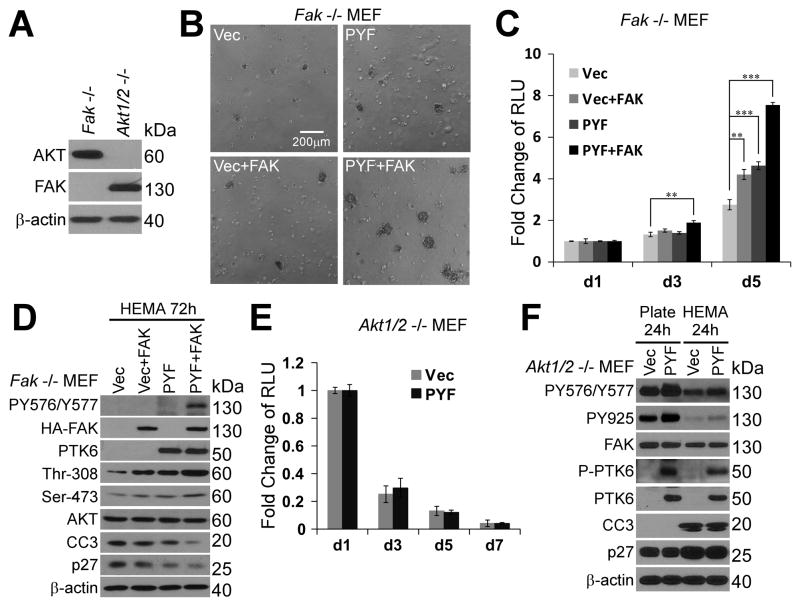
To further characterize the roles of FAK and AKT in PTK6 mediated resistance to anoikis, Fak−/− and Akt1/2−/− MEFs were utilized. The absence of endogenous FAK or AKT was shown by immunoblotting analysis (Figure 4A). While expression of Palm-PTK6-YF (PYF) or FAK alone protects Fak−/− MEFs against anoikis, co-expression of Palm-PTK6-YF and FAK exerts synergistic effects in protecting cells from anoikis, leading to increased viability and formation of larger spheres after 6 days of growth on poly-HEMA coated plates (Figure 4B; C). Indeed, Fak−/− MEFs stably expressing Palm-PTK6-YF and FAK acquire resistance to anoikis and continue proliferating under suspended conditions over a 14-day period (data not shown). Immunoblotting of lysates prepared from cells following 72 hours of growth in suspension showed that co-expression of Palm-PTK6-YF and FAK induces higher FAK activation (PY576/Y577) even under suspended conditions, resulting in substantially higher levels of AKT activation (phospho-Thr-308, phospho-Ser-473) and lower levels of cleaved caspase 3 (CC3) and p27 (Figure 4D).
Figure 4. Membrane associated active PTK6 mediates protection against anoikis through activation of FAK and AKT.
A. Immunoblotting analysis of lysates of Fak−/− MEFs and Akt1/2−/− MEFs demonstrates the absence of FAK in Fak−/− MEFs, and no expression of AKT in Akt1/2−/− MEFs. B. Expression of FAK and Palm-PTK6-YF (PYF) in Fak−/− MEFs shows synergistic protection against anoikis. Fak−/− MEFs stably expressing vector, FAK, Palm-PTK6-YF, or both FAK and Palm-PTK6-YF were seeded on poly-HEMA plates for 6 days. The size bar denotes 200 μm. C. Anoikis assays of Fak−/− MEFs were performed for 5 days and the cell viability was measured by CellTiter-Glo luminescent assay (**, p < 0.01; ***, p < 0.001). D. Expression of FAK and Palm-PTK6-YF synergistically activate AKT survival signaling in Fak−/− MEFs. Fak−/− MEFs stably expressing vector, FAK, Palm-PTK6-YF, or both FAK and Palm-PTK6-YF were incubated on poly-HEMA coated plates for 72 hours. Immunoblotting analysis was performed with anti-P-FAK (Tyr576/Tyr577), HA-tag, PTK6, P-AKT (Thr308), P-AKT (Ser473), AKT, cleaved caspase 3 (CC3), p27 and β-actin antibodies. E. Expression of Palm-PTK6-YF (PYF) in Akt1/2−/− MEFs fails to protect cells against anoikis. The viability of Akt1/2−/− MEFs was measured for 7 days by CellTiter-Glo luminescent assay. F. Palm-PTK6-YF induced FAK activation in Akt1/2−/− MEFs fails to protect cells against anoikis. Akt1/2−/− MEFs expressing vector or Palm-PTK6-YF were incubated on plastic plates or poly-HEMA coated plates for 24 hours. Immunoblotting analysis was performed with anti-P-FAK (Tyr576/Tyr577), P-FAK (Tyr925), FAK, P-PTK6 (Tyr342), PTK6, cleaved caspase 3 (CC3), p27 and β-actin antibodies.
AKT plays important roles in regulating cell survival and growth [reviewed in (41)]. Expression of Palm-PTK6-YF in Akt1/2−/− MEFs fails to protect them from anoikis (Figure 4E). Both control Akt1/2−/− MEFs and Palm-PTK6-YF expressing Akt1/2−/− MEFs undergo apoptosis when grown under nonadherent growth conditions, as measured by a cell viability assay (Figure 4E). This is consistent with immunoblotting analysis showing the same level of cleaved caspase 3 (CC3) induction after 24-hour suspension in both MEFs, although increased FAK phosphorylation (PY576/Y577, PY925) was observed in Palm-PTK6-YF expressing MEFs (Figure 4F). Our data suggest AKT is a critical downstream mediator of PTK6-FAK survival signaling that protects cells from anoikis.
Knockdown of PTK6 in prostate cancer cells promotes anoikis
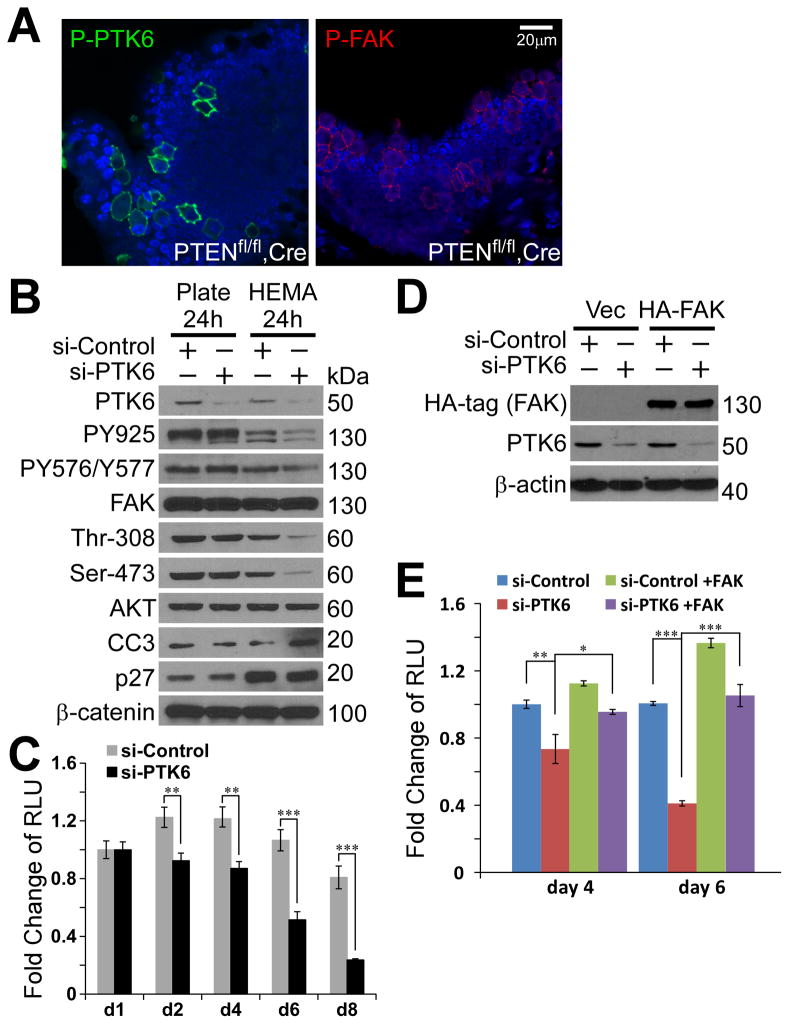
Active PTK6 is associated with membrane compartments in cultured tumor cells, but the status and localization of activated PTK6 in vivo in tumors are not clear. We utilized a PTENflox/flox, Probasin (PB)-Cre transgenic murine prostate cancer model, which forms metastatic prostate tumors at the age of 6 months (35). Immunohistochemistry showed highly induced PTK6 activation at the membrane in a group of Pten-null prostate cells, which possess enlarged nuclei and substantial cytoplasm (Figure 5A). Activated P-FAK signaling was also observed in the same group of cells, providing in vivo evidence of the tight relationship between PTK6 and FAK signaling in diseased prostate (Figure 5A). These cells, with activated PTK6 and P-FAK, were not observed in age-matched control prostates from PTENflox/flox mice lacking Cre (data not shown).
Figure 5. Knockdown of PTK6 in prostate cancer cells promotes anoikis.
A. A group of prostate tumor cells have highly activated PTK6 and FAK at plasma membrane in a murine prostate tumor model (PTENflox/flox, PB-Cre). Immunohistochemistry was performed with anti-P-PTK6 (Tyr342) and P-FAK (Tyr576/Tyr577) antibodies. The size bar denotes 20 μm. B. Knockdown of PTK6 in PC3 cells impairs FAK and AKT survival signaling under suspended condition. PC3 cells were transfected with siRNAs against PTK6 or control siRNAs for 24 hours and then seeded on plastic plates or poly-HEMA coated plates for 24 hours. Immunoblotting analysis was performed with anti-PTK6, P-FAK (Tyr576/Tyr577), P-FAK (Tyr925), FAK, P-AKT (Ser473), P-AKT (Thr308), AKT, cleaved caspase 3 (CC3), p27 and β-catenin antibodies. C. Knockdown of PTK6 promotes PC3 cells to undergo anoikis. PC3 cells transfected with siRNAs against PTK6 or control were seeded on poly-HEMA coated plates for indicated periods. Cell viability was measured by CellTiter-Glo luminescent assays. D–E. Expression of FAK is able to rescue PTK6-knockdown induced anoikis. PC3 cells were transfected with PCDNA3-HA-FAK or control plasmids for 24 hours, and then transfected with PTK6 siRNAs or control siRNAs for 24 hours. Cells were trypsinized and seeded on poly-HEMA coated plates (day 1). Protein lysates were harvested at day 2, and anoikis assays were performed at day 4 and day 6.
Next, we investigated the role of PTK6 in protecting human prostate cancer cells from anoikis. Endogenous PTK6 was knocked down in PC3 cells using siRNAs against PTK6 (Figure 5B, PTK6). Although knockdown of PTK6 did not affect FAK activation when cells were grown under adherent conditions (Plate), decreased FAK activation was observed in suspended PTK6 knockdown cells grown on poly-HEMA coated plates, indicating that a primary role of PTK6 is to maintain the level of FAK activation when cells are detached from the extracellular matrix (Figure 5B, PY576/Y577, PY925). Decreased FAK activation was accompanied by impaired AKT activation and increased expression of cleaved caspase 3 (Figure 5B, Thr-308, Ser-473, CC3). The population of PC3 cells treated with scrambled siRNAs (si-Control) reached a balance between proliferation, cell cycle arrest and cell death under suspended growth conditions, as measured by cell viability assays performed from day 1 to day 8 (Figure 5C). Knockdown of PTK6 triggered apoptosis, and only 20% of the cells were viable after 8 days of growth on poly-HEMA coated plates (Figure 5C). Importantly, overexpression of HA-FAK in PC3 cells rescued anoikis induced by PTK6-knockdown (Figure 5D, E). FAK promotes survival of control-siRNA treated cells grown in suspension on poly-HEMA (Figure 5E, si-Control +FAK), and restores survival of cells with PTK6 knockdown to the levels of control PC3 cells (Figure 5E, si-PTK6+FAK). These data demonstrate critical roles for PTK6/FAK signaling in regulating the anchorage independent survival of prostate cancer cells.
Two breast cancer cell lines MBA-MD-231 and T47D, which express PTK6, were also utilized to investigate whether PTK6 generally promotes resistance to anoikis in cancer cells. Anoikis assays demonstrated that knockdown of PTK6 led to increased anoikis in MBA-MD-231 cells grown under nonadherent conditions on poly-HEMA coated plates (Figure S2A). This was accompanied by decreased FAK/AKT survival signaling and increased levels of cleaved caspase 3, which was also observed in T47D cells (Figure S2B, C).
PTK6 is primarily responsible for resistance of PC3 cells to anoikis
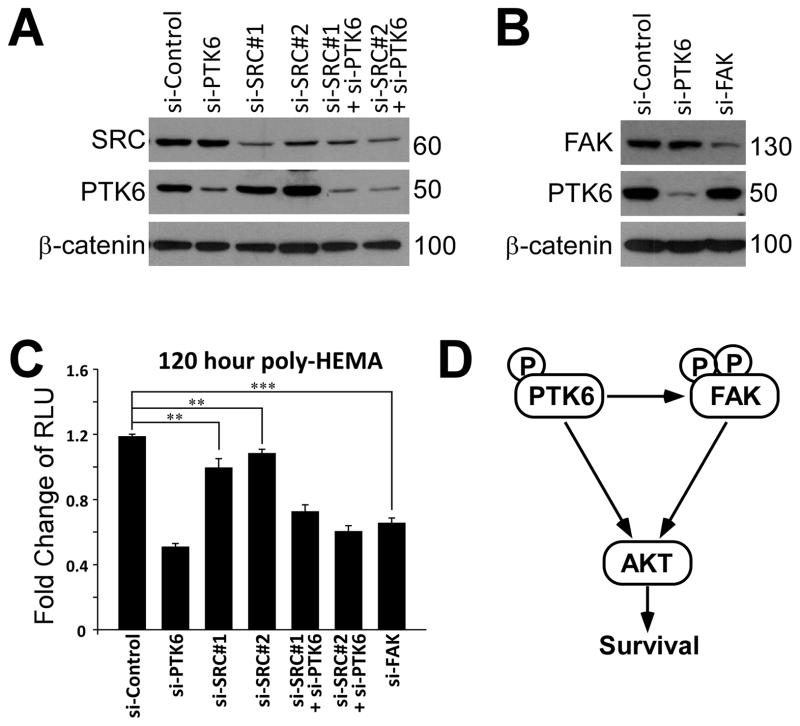
Since FAK can be phosphorylated by SRC kinase (40), and FAK/SRC signaling is involved in regulating survival and anoikis of human intestinal epithelial cells (42), we investigated whether PTK6 or SRC is more relevant for anoikis resistance in prostate cancer cells. Two different siRNAs against SRC were used to knockdown endogenous SRC in PC3 cells (Figure 6A). Knockdown of SRC does cause a slight decrease of the percentage of live cells compared with control siRNAs treated cells, (Figure 6C). However, survival of PTK6-siRNA treated cells is much more reduced after 120 hours of growth on poly-HEMA coated plates, indicating that PTK6 is primarily responsible for protecting prostate cancer cells against anoikis (Figure 6C). In addition, knockdown of PTK6 and SRC together does not show a synergistic effect in inducing anoikis, and is comparable to knockdown of PTK6 alone (Figure 6C). To demonstrate the role of FAK in regulating anoikis, siRNAs against FAK were used to knockdown endogenous FAK in PC3 cells (Figure 6B). Knockdown of FAK efficiently induces anoikis, and the level is close to that of PTK6 knockdown cells (Figure 6C). This is consistent with our hypotheses that FAK is a critical downstream target of PTK6 in protecting cells from anoikis.
Fig. 6. PTK6/FAK signaling is important in regulating anoikis resistance of PC3 cells.
A–B. SRC and FAK are efficiently knocked down by siRNAs in PC3 cells. PC3 cells were transfected with siRNAs against PTK6, SRC, FAK or control siRNAs for 24 hours and then seeded on poly-HEMA coated plates (day 1). Protein lysates were harvested at day 2, and immunoblotting was performed with anti-PTK6, SRC, FAK and β-catenin antibodies. Expression of β-catenin serves as a loading control. C. PTK6 is primarily responsible for anoikis resistance of PC3 cells. Anoikis assays were performed using various knockdown cells described in A-B after 120 hours of suspended growth on poly-HEMA coated plates (day 6). D. A proposed model shows how PTK6 regulates cell survival. When cells detached from extracellular matrix (ECM), membrane associated active PTK6 is able to phosphorylate and activate FAK and following AKT survival signaling. PTK6 is also able to protect cells from anoikis independent of FAK, probably through direct phosphorylation and activation of AKT (13).
Discussion
PTK6 shares structural similarity with SRC kinases (43). PTK6 tyrosine residue 342 is conserved between PTK6 and SRC, and its autophosphorylation causes the activation loop to move out of the substrate-binding site, therefore allowing access to substrates (36). Phosphorylation of PTK6 tyrosine residue 342 serves as a marker for its activation. Phosphorylation of the conserved carboxy-terminal tyrosine residue 447 results in autoinhibition due to the binding to its own SH2 domain (36). Mutation of tyrosine residue 447 to phenylalanine (PTK6-YF) increases kinase activity, since there is no autoinhibition. However, the activity of nontargeted PTK6-YF can still be down-regulated by serum starvation (Figure 1E). Interestingly, membrane targeted PTK6 (Palm-PTK6-YF) is not down-regulated by serum starvation (Figure 2A), and represents an authentic constitutively active form of PTK6. This could contribute to involvement of PTK6 in multiple receptor-signaling pathways including EGFR, HER2, IGF-1R, MET and integrin (5, 12, 21, 44, 45).
Our data show that constitutive activation of PTK6 transforms SYF MEFs, as manifested by their ability to overcome contact inhibition and anchorage dependence, resistance to anoikis and tumor forming ability in vivo (Figure 3). AKT plays a critical role downstream of PTK6 in survival signaling, since Palm-PTK6-YF fails to protect Akt1/2−/− MEFs against anoikis, which show greater sensitivity to apoptosis under suspended growth conditions compared with SYF and Fak−/− MEFs (Figure 4C; 4E; data not shown). In Fak−/− MEFs, overexpression of FAK is able to provide protection for cells lacking signals from extracellular matrix, while co-expression of FAK and Palm-PTK6-YF induces stronger FAK and AKT activation and synergistically promotes cell survival (Figure 4B; C). Expression of Palm-PTK6-YF alone also shows some protective effect, indicating that the constitutively active PTK6 is able to activate survival signaling independent of FAK (Figure 4B; C). This is probably through directly phosphorylating AKT at tyrosine residues and thereby lowering sensitivity to growth receptor signaling (Figure 6D) (13).
Metastatic prostate cancer is the second leading cause of cancer-related deaths in American men. Increased PTK6 expression is detected in human primary and metastatic prostate cancers, and this is accompanied by the loss of its nuclear localization (4, 5). Cytoplasmic retention of PTK6 promotes proliferation and migration of prostate tumor cells, while nuclear-targeted PTK6 negatively regulates cell growth (5, 46). Relocalization of PTK6 from the nucleus to the cytoplasm might facilitate the activation of PTK6 by integrin and growth factor receptor signaling, as well as its access to cytoplasmic substrates such as AKT, p130CAS, Paxillin, p190RhoGAP, EGFR and FAK, therefore promoting cancer cell proliferation, migration and survival (5, 11–14). Here we showed increased activation of both PTK6 and FAK at plasma membrane in Pten null prostate tumor cells (Figure 5A), which may positively regulate tumorigenesis and metastasis. A more detailed study addressing the significance of PTK6 relocalization and activation at the membrane in prostate tumor cell metastasis is currently underway.
Resistance to anoikis is a characteristic of metastatic cancer cells, allowing them to escape death under adverse conditions. Cancer cells escape anoikis primarily through regulating the extrinsic death receptor pathway and the ECM-integrin mediated cell survival pathway [reviewed in (47)]. Different strategies that target various players in integrin/SRC/FAK/AKT survival signaling have shown promise in inhibiting tumor growth in vitro and in vivo [reviewed in (47, 48)]. In our study, metastatic PC3 prostate cancer cells display the ability to escape from anoikis, and have a 90% viability rate after eight days of growth on poly-HEMA coated plates (Figure 5C). Apoptosis is not induced, but levels of p27 are substantially increased upon detachment (Figure 5B), contributing to cell cycle arrest and promoting cell survival (49). Knockdown of PTK6 induces apoptosis under suspended growth conditions, and cell viability decreases to about 20% (Figure 5B, CC3; 5C).
PTK6 shares several direct substrates with SRC, including AKT, p130CAS, p190RhoGAP, Paxillin and FAK, indicating they may have redundant functions (5, 11, 13, 14). Importantly, here we demonstrated that it is PTK6, but not SRC, that plays crucial roles in protecting prostate cancer cells from anoikis (Figure 6A, C). PTK6 induced FAK phosphorylation and subsequent AKT activation appears critical for the survival of cancer cells in the absence of correct extracellular stimuli, and highlights the potential for combinational cancer therapeutics that target integrin signaling and PTK6 in human prostate cancer.
Materials and Methods
Antibodies
Anti-human PTK6 (C-18), anti-mouse PTK6 (C-17), anti-FAK (C-20), and anti-phosphotyrosine (PY20) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phosphotyrosine (4G10), anti-SRC (GD11) and anti-P-PTK6 (Tyr342) antibodies were purchased from Millipore (Bedford, MA). Antibodies directed against AKT, P-AKT (Thr308), P-AKT (Ser473), P-FAK (Tyr925), P-FAK (Tyr576/Tyr577), Cleaved Caspase-3 (Asp175), HA-tag (6E2) and Myc-tag (9B11) were obtained from Cell Signaling Technology (Danvers, MA). Antibodies directed against p27 and β-catenin were obtained from BD Pharmingen (San Diego, CA). Anti-P-paxillin (Tyr118) antibodies were obtained from Invitrogen (Carlsbad, CA). Anti-β-actin (AC-15) antibodies were purchased from Sigma-Aldrich (St. Louis, MO). Anti-glutathione S-transferase (GST) tag antibody was purchased from Covance (Cumberland, VA). Donkey anti-rabbit or sheep anti-mouse antibodies conjugated to horseradish peroxidase were used as secondary antibodies (Amersham Biosciences, Piscataway, NJ) and detected by chemiluminescence with SuperSignal West Dura extended duration substrate from Pierce (Rockford, IL).
Plasmids and siRNAs
Myc-tagged full-length human active PTK6 (YF) in the pcDNA3 vector and the Myc-tagged human PTK6-YF and Palm-PTK6-YF constructs in pBABE-puro vector have been described (5, 13). Coding sequences of Palm-PTK6-YF were subcloned into the pBABE-hygro vector (Cell Biolabs, Inc., San Diego, CA). The GST-PTK6 (mouse) constructs were previously described (13). A pcDNA3-HA-FAK (mouse) plasmid was a gift from Dr. David Schlaepfer (UCSD, San Diego, CA). Coding sequences of mouse FAK were amplified by PCR and subcloned into the pBABE-puro vector. Dicer-substrate siRNAs against PTK6 were purchased from IDT predesigned DsiRNAs library (Coralville, IA). The sequence for Dsi-PTK6 is: 5′-AGGTTCACAAATGTGGAGTGTCTGC-3′. The sequence for Dsi-FAK is 5′-GCAATGGAGCGAGTATTAAAGGACT-3′. The sequences for Dsi-SRC are 5′-CCCUUCGAGAUCAUCACUUCCUUGC-3′, and 5′-AGAGAACCUGGUGUGCAAAGUGGCC-3′.
Cell culture and transfections
The human embryonic kidney cell line 293 (HEK-293) (ATCC CRL-1573), the mouse embryonic fibroblast cell line SYF (ATCC CRL-2459), the Fak−/− mouse embryonic fibroblast cell line (ATCC CRL-2644), the human colon cancer cell line SW620 (ATCC CCL-227), the human prostate cancer cell line PC3 (ATCC CRL-1435) and the human breast cancer cell lines T47D (ATCC HTB-133) and MDA-MB-231 (ATCC HTB-26) were cultured in recommended medium according to ATCC guidelines. Akt1/2−/− mouse embryonic fibroblast cells immortalized by DN-p53 (kindly provided by Dr. Nissim Hay, University of Illinois at Chicago, Chicago, IL) were cultured in DMEM containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin (34). Transfections were performed using the Lipofectamine 2000 transfection reagent (Invitrogen) as per manufacturer’s instructions.
Retrovirus production and transduction
pBABE-puro or pBABE-hygro plasmids were transfected into Phoenix-Eco cells using Lipofectamine 2000 (Invitrogen). Retrovirus was collected 48 and 72 hours later. Mouse embryonic fibroblast cells were infected with retrovirus at a multiplicity of infection (MOI) of 100 for 24 hours. Stable cell pools were selected in growth medium containing 2 μg/ml puromycin or 100 μg/ml hygromycin B for a week.
Protein lysates, Immunoprecipitation (IP) and GST pull down assay
Transfected cells were lysed in 1% Triton X-100 lysis buffer (1% Triton X-100, 20 mM HEPES, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 10 mM Na-pyrophosphate, 100 mM NaF, 5 mM iodoacetic acid, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), protease inhibitor cocktail (Roche, Indianapolis, IN)) 18 to 24 h after transfection. Procedures for IPs and GST pull down assays were described previously (5). Samples were subjected to SDS-PAGE and transferred onto Immobilon-P membranes for immunoblotting. For large scale IP, tyrosine phosphorylated proteins were immunoprecipitated from 10mg soluble SW620 cell lysates in Triton X-100 buffer. Samples were subjected to SDS-PAGE gels and stained with Coomassie Brilliant Blue R-250 (Bio-Rad). Three distinct bands at approximately 120 kDa, 80 kDa and 68 kDa were excised for tandem mass spectrometry sequencing.
In vitro kinase assays
Kinase assays were performed with 50 ng recombinant human PTK6 (Invitrogen) and 250 ng recombinant human FAK (Novus Biologicals, Littleton, CO) in 30 μl kinase buffer (10 mM HEPES, pH 7.5, 150 mM NaCl, 2.5 mM dithiothreitol (DTT), 0.01% Triton X-100, 10 mM MnCl2) with or without 200 μM ATP for 10 min at 30°C. The reaction was stopped by adding 30 μl of 2× reducing Laemmli sample buffer and boiling. Samples were subjected to SDS-PAGE and transferred onto Immobilon-P membranes for immunoblotting.
Tandem mass spectrometry (LC/MS/MS)
Samples were subjected to in-gel digestion using sequencing grade trypsin followed by C18 reversed-phase microcapillary liquid chromatography-tandem mass spectrometry using a hybrid linear ion trap – Orbitrap XL mass spectrometer (Thermo Scientific) in positive-ion data-dependent acquisition mode. MS/MS spectra were searched against the reversed and concatenated Swiss-Prot protein database using the Sequest algorithm (Proteomics browser; Thermo Scientific) with differential modifications for STY phosphorylation (79.97) and methionine oxidation (15.99). Phosphorylation sites were identified if they initially passed the following Sequest scoring thresholds: 2 ions, Xcorr ≥ 1.90, Sf ≥ 0.4, P > 0; 3 ions, Xcorr ≥ 2.55, Sf ≥ 0.5, P > 0 against the forward database. Peptides with gas phase charges of 1 and 4 were generally not accepted due to difficulty of interpretation. Passing MS/MS spectra were then manually validated to ensure that all b- and y- fragment ions aligned with the assigned modified protein sequence. Determination of the exact sites of phosphorylation was aided using GraphMod software (Proteomics Browser Software, Thermo Scientific), resulting in a false-positive identification rate of less than 1.2%.
Proliferation, soft agar, and transformation assays
For proliferation assays, subconfluent cells were seeded in triplicate for each time point at a density of 2 × 103 per well in 48-well plates. The fold increase of cell number was measured by the CellTiter-Glo luminescent cell viability assay (Promega). For soft agar assays, 5 × 103 cells were seeded in triplicate on the top layer of six-well plates, which contained 0.35% agar in DMEM containing 10% FBS. The bottom layer of soft agar contained 0.7% agar in DMEM containing 10% FBS. Cells were fed twice a week, and colonies were counted at three weeks post-plating. For transformation assays, 5 × 103 cells of the line to be tested were mixed with 1 × 105 untransformed SYF cells, plated into six well dishes, maintained at confluence and scored for foci after 10 days.
Anoikis assays
MEFs were trypsinized and plated onto poly-HEMA-coated 10 cm plates in growth medium to prohibit attachment. Cells were transfected with siRNAs for 24 hours, and then seeded at poly-HEMA-coated plates in growth medium. Viability of the cells was measured every other day by the CellTiter-Glo luminescent cell viability assay (Promega). The cells were harvested with Triton X-100 lysis buffer at indicated time.
Immunostaining
Cells were washed with PBS and fixed in Carnoy’s solution (60% ethanol, 30% chloroform, 10% acetic acid), and then blocked with 3% BSA for one hour and incubated with primary antibodies overnight. After washing, samples were incubated with fluorescein isothiocyanate (FITC)-conjugated anti-mouse secondary antibodies or rhodamine-conjugated anti-rabbit secondary antibodies (Sigma-Aldrich). Rhodamine-conjugated phalloidin was used to selectively stain F-actin as per manufacturer’s instructions (Invitrogen). Slides were mounted in Vectashield fluorescent mount media containing 4′, 6-diamidino-2-phenylindole (DAPI) (Vector Laboratories).
Murine prostate tumor model and immunohistochemistry
C57BL/6J PTENflox/flox PB-Cre fixed mouse tissues were provided by Dr. Nissim Hay (35). Mice were sacrificed at 6 months and prostates were formalin-fixed and paraffin-embedded. Antigen retrieval was performed in 10 mM sodium citrate buffer on heat plate with a temperature above 90°C for 20 minutes. Immunohistochemistry was performed using the VECTASTAIN Elite ABC Kit (rabbit IgG) as per manufacturer’s instructions. Reactions were visualized with FITC or rhodamine-conjugated avidin. Slides were mounted in Vectashield fluorescent mount media containing DAPI (Vector Laboratories). Tissues were then observed using standard UV, rhodamine, or FITC filters under 40 X water-immersion objectives by the Carl Zeiss LSM 700 laser scanning microscope. Images were obtained with Axiocam HRc Color Digital Camera and LSM 700’s ZEN software (Zeiss, Jena, Germany).
Xenograft model
SYF cells stably expressing vector or Palm-PTK6-YF were trypsinized and resuspended in 4 × 107 cells/ml in PBS. Male nude Mice (CrTac:NCr-Foxn1nu, Taconic, Germantown, NY), 10 weeks of age, were anesthetized with a ketamine/xylazine mix. 3 × 106 Palm-PTK6-YF expressing SYF cells in a volume of 150 μl (1:1 mixed with Matrigel (BD Biosciences)) were injected subcutaneously in the left flank. The same number of vector expressing SYF cells were injected in the right flank as controls. Mice were sacrificed 4 weeks after injection.
Statistics
For all the cell studies, data represent the mean of at least three independent experiments ± SD. P-values were determined using the two tailed Student’s T test (Microsoft Excel 2010). A difference was considered statistically significant if the P-value was equal to or less than 0.05.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants 5R01DK044525 (A.L.T.), 5P01CA120964 (J.M.A.) and NIH DF/HCC Cancer Center Support Grant 5P30CA006516 (J.M.A.). The authors thank Wenjun Bie and Min Yuan for technical assistance, Dr. Nissim Hay (University of Illinois at Chicago, Chicago, IL) for his gifts of Akt1/2−/− MEFs and PTENfl/fl, PB-Cre and control mouse tissues, and Dr. David D. Schlaepfer (University of California, San Diego) for providing FAK expression constructs.
Footnotes
Conflict of interest.
The authors declare no conflict of interest.
References
- 1.Mitchell PJ, Barker KT, Martindale JE, Kamalati T, Lowe PN, Page MJ, et al. Cloning and characterisation of cDNAs encoding a novel non-receptor tyrosine kinase, brk, expressed in human breast tumours. Oncogene. 1994;9:2383–90. [PubMed] [Google Scholar]
- 2.Siyanova EY, Serfas MS, Mazo IA, Tyner AL. Tyrosine kinase gene expression in the mouse small intestine. Oncogene. 1994;9(7):2053–7. [PubMed] [Google Scholar]
- 3.Derry JJ, Richard S, Valderrama Carvajal H, Ye X, Vasioukhin V, Cochrane AW, et al. Sik (BRK) phosphorylates Sam68 in the nucleus and negatively regulates its RNA binding ability. Mol Cell Biol. 2000 Aug;20(16):6114–26. doi: 10.1128/mcb.20.16.6114-6126.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derry JJ, Prins GS, Ray V, Tyner AL. Altered localization and activity of the intracellular tyrosine kinase BRK/Sik in prostate tumor cells. Oncogene. 2003 Jul 3;22(27):4212–20. doi: 10.1038/sj.onc.1206465. [DOI] [PubMed] [Google Scholar]
- 5.Zheng Y, Asara JM, Tyner AL. Protein-tyrosine kinase 6 promotes peripheral adhesion complex formation and cell migration by phosphorylating p130 CRK-associated substrate. J Biol Chem. 2012 Jan 2;287(1):148–58. doi: 10.1074/jbc.M111.298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haegebarth A, Heap D, Bie W, Derry JJ, Richard S, Tyner AL. The nuclear tyrosine kinase BRK/Sik phosphorylates and inhibits the RNA-binding activities of the Sam68-like mammalian proteins SLM-1 and SLM-2. J Biol Chem. 2004 Dec 24;279(52):54398–404. doi: 10.1074/jbc.M409579200. [DOI] [PubMed] [Google Scholar]
- 7.Liu L, Gao Y, Qiu H, Miller WT, Poli V, Reich NC. Identification of STAT3 as a specific substrate of breast tumor kinase. Oncogene. 2006 Aug 10;25(35):4904–12. doi: 10.1038/sj.onc.1209501. [DOI] [PubMed] [Google Scholar]
- 8.Weaver AM, Silva CM. Signal transducer and activator of transcription 5b: a new target of breast tumor kinase/protein tyrosine kinase 6. Breast Cancer Res. 2007;9(6):R79. doi: 10.1186/bcr1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palka-Hamblin HL, Gierut JJ, Bie W, Brauer PM, Zheng Y, Asara JM, et al. Identification of {beta}-catenin as a target of the intracellular tyrosine kinase PTK6. J Cell Sci. 2010 Dec 21; doi: 10.1242/jcs.053264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mitchell PJ, Sara EA, Crompton MR. A novel adaptor-like protein which is a substrate for the non-receptor tyrosine kinase, BRK. Oncogene. 2000 Aug 31;19(37):4273–82. doi: 10.1038/sj.onc.1203775. [DOI] [PubMed] [Google Scholar]
- 11.Chen HY, Shen CH, Tsai YT, Lin FC, Huang YP, Chen RH. Brk activates rac1 and promotes cell migration and invasion by phosphorylating paxillin. Mol Cell Biol. 2004 Dec;24(24):10558–72. doi: 10.1128/MCB.24.24.10558-10572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Lu Y, Liang K, Hsu JM, Albarracin C, Mills GB, et al. Brk/PTK6 sustains activated EGFR signaling through inhibiting EGFR degradation and transactivating EGFR. Oncogene. 2012 Jan 9; doi: 10.1038/onc.2011.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zheng Y, Peng M, Wang Z, Asara JM, Tyner AL. Protein tyrosine kinase 6 directly phosphorylates AKT and promotes AKT activation in response to epidermal growth factor. Mol Cell Biol. 2010 Sep;30(17):4280–92. doi: 10.1128/MCB.00024-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen CH, Chen HY, Lin MS, Li FY, Chang CC, Kuo ML, et al. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration, and invasion. Cancer Res. 2008 Oct 1;68(19):7779–87. doi: 10.1158/0008-5472.CAN-08-0997. [DOI] [PubMed] [Google Scholar]
- 15.Barker KT, Jackson LE, Crompton MR. BRK tyrosine kinase expression in a high proportion of human breast carcinomas. Oncogene. 1997;15(7):799–805. doi: 10.1038/sj.onc.1201241. [DOI] [PubMed] [Google Scholar]
- 16.Lofgren KA, Ostrander JH, Housa D, Hubbard GK, Locatelli A, Bliss RL, et al. Mammary gland specific expression of Brk/PTK6 promotes delayed involution and tumor formation associated with activation of p38 MAPK. Breast Cancer Res. 2011 Sep 17;13(5):R89. doi: 10.1186/bcr2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llor X, Serfas MS, Bie W, Vasioukhin V, Polonskaia M, Derry J, et al. BRK/Sik expression in the gastrointestinal tract and in colon tumors. Clin Cancer Res. 1999 Jul;5(7):1767–77. [PubMed] [Google Scholar]
- 18.Lin HS, Berry GJ, Fee WE, Jr, Terris DJ, Sun Z. Identification of tyrosine kinases overexpressed in head and neck cancer. Arch Otolaryngol Head Neck Surg. 2004 Mar;130(3):311–6. doi: 10.1001/archotol.130.3.311. [DOI] [PubMed] [Google Scholar]
- 19.Schmandt RE, Bennett M, Clifford S, Thornton A, Jiang F, Broaddus RR, et al. The BRK Tyrosine Kinase is Expressed in High-Grade Serous Carcinoma of the Ovary. Cancer Biol Ther. 2006 Sep 28;5(9) doi: 10.4161/cbt.5.9.2953. [DOI] [PubMed] [Google Scholar]
- 20.Gierut J, Zheng Y, Bie W, Carroll RE, Ball-Kell S, Haegebarth A, et al. Disruption of the mouse protein tyrosine kinase 6 gene prevents STAT3 activation and confers resistance to azoxymethane. Gastroenterology. 2011 Oct;141(4):1371–80. 80, e1–2. doi: 10.1053/j.gastro.2011.06.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irie HY, Shrestha Y, Selfors LM, Frye F, Iida N, Wang Z, et al. PTK6 regulates IGF-1-induced anchorage-independent survival. PLoS One. 2010;5(7):e11729. doi: 10.1371/journal.pone.0011729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003 Apr 15;116(Pt 8):1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 23.Schaller MD. Biochemical signals and biological responses elicited by the focal adhesion kinase. Biochim Biophys Acta. 2001 Jul 25;1540(1):1–21. doi: 10.1016/s0167-4889(01)00123-9. [DOI] [PubMed] [Google Scholar]
- 24.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004 Feb;14(1):92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Xia H, Nho RS, Kahm J, Kleidon J, Henke CA. Focal adhesion kinase is upstream of phosphatidylinositol 3-kinase/Akt in regulating fibroblast survival in response to contraction of type I collagen matrices via a beta 1 integrin viability signaling pathway. J Biol Chem. 2004 Jul 30;279(31):33024–34. doi: 10.1074/jbc.M313265200. [DOI] [PubMed] [Google Scholar]
- 26.Zouq NK, Keeble JA, Lindsay J, Valentijn AJ, Zhang L, Mills D, et al. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia: differential roles for paxillin and p130Cas. J Cell Sci. 2009 Feb 1;122(Pt 3):357–67. doi: 10.1242/jcs.030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim ST, Chen XL, Lim Y, Hanson DA, Vo TT, Howerton K, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008 Jan 18;29(1):9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007 Jun 29;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Tang N, Hadden TJ, Rishi AK. Akt, FoxO and regulation of apoptosis. Biochim Biophys Acta. 2011 Nov;1813(11):1978–86. doi: 10.1016/j.bbamcr.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Liang J, Zubovitz J, Petrocelli T, Kotchetkov R, Connor MK, Han K, et al. PKB/Akt phosphorylates p27, impairs nuclear import of p27 and opposes p27-mediated G1 arrest. Nat Med. 2002 Oct;8(10):1153–60. doi: 10.1038/nm761. [DOI] [PubMed] [Google Scholar]
- 31.Rossig L, Jadidi AS, Urbich C, Badorff C, Zeiher AM, Dimmeler S. Akt-dependent phosphorylation of p21(Cip1) regulates PCNA binding and proliferation of endothelial cells. Mol Cell Biol. 2001;21(16):5644–57. doi: 10.1128/MCB.21.16.5644-5657.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne PH, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. Embo J. 1997 May 15;16(10):2783–93. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guadamillas MC, Cerezo A, Del Pozo MA. Overcoming anoikis--pathways to anchorage-independent growth in cancer. J Cell Sci. 2011 Oct 1;124(Pt 19):3189–97. doi: 10.1242/jcs.072165. [DOI] [PubMed] [Google Scholar]
- 34.Nogueira V, Park Y, Chen CC, Xu PZ, Chen ML, Tonic I, et al. Akt determines replicative senescence and oxidative or oncogenic premature senescence and sensitizes cells to oxidative apoptosis. Cancer Cell. 2008 Dec 9;14(6):458–70. doi: 10.1016/j.ccr.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trotman LC, Niki M, Dotan ZA, Koutcher JA, Di Cristofano A, Xiao A, et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 2003 Dec;1(3):E59. doi: 10.1371/journal.pbio.0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu H, Miller WT. Regulation of the nonreceptor tyrosine kinase Brk by autophosphorylation and by autoinhibition. J Biol Chem. 2002 Sep 13;277(37):34634–41. doi: 10.1074/jbc.M203877200. [DOI] [PubMed] [Google Scholar]
- 37.Lukong KE, Huot ME, Richard S. BRK phosphorylates PSF promoting its cytoplasmic localization and cell cycle arrest. Cell Signal. 2009 Sep;21(9):1415–22. doi: 10.1016/j.cellsig.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996 Aug;134(3):793–9. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu LH, Yang X, Bradham CA, Brenner DA, Baldwin AS, Jr, Craven RJ, et al. The focal adhesion kinase suppresses transformation-associated, anchorage-independent apoptosis in human breast cancer cells. Involvement of death receptor-related signaling pathways. J Biol Chem. 2000 Sep 29;275(39):30597–604. doi: 10.1074/jbc.M910027199. [DOI] [PubMed] [Google Scholar]
- 40.Calalb MB, Polte TR, Hanks SK. Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol. 1995 Feb;15(2):954–63. doi: 10.1128/mcb.15.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005 Jan-Mar;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bouchard V, Demers MJ, Thibodeau S, Laquerre V, Fujita N, Tsuruo T, et al. Fak/Src signaling in human intestinal epithelial cell survival and anoikis: differentiation state-specific uncoupling with the PI3-K/Akt-1 and MEK/Erk pathways. J Cell Physiol. 2007 Sep;212(3):717–28. doi: 10.1002/jcp.21096. [DOI] [PubMed] [Google Scholar]
- 43.Serfas MS, Tyner AL. Brk, Srm, Frk, and Src42A form a distinct family of intracellular Src-like tyrosine kinases. Oncol Res. 2003;13(6–10):409–19. doi: 10.3727/096504003108748438. [DOI] [PubMed] [Google Scholar]
- 44.Xiang B, Chatti K, Qiu H, Lakshmi B, Krasnitz A, Hicks J, et al. Brk is coamplified with ErbB2 to promote proliferation in breast cancer. Proc Natl Acad Sci U S A. 2008 Aug 26;105(34):12463–8. doi: 10.1073/pnas.0805009105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castro NE, Lange CA. Breast tumor kinase and extracellular signal-regulated kinase 5 mediate Met receptor signaling to cell migration in breast cancer cells. Breast Cancer Res. 2010;12(4):R60. doi: 10.1186/bcr2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brauer PM, Zheng Y, Wang L, Tyner AL. Cytoplasmic retention of protein tyrosine kinase 6 promotes growth of prostate tumor cells. Cell Cycle. 2010 Oct 15;9(20):4190–9. doi: 10.4161/cc.9.20.13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakamoto S, Kyprianou N. Targeting anoikis resistance in prostate cancer metastasis. Mol Aspects Med. 2010 Apr;31(2):205–14. doi: 10.1016/j.mam.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010 Jan;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu FY, Wang SE, Sanders ME, Shin I, Rojo F, Baselga J, et al. Reduction of cytosolic p27(Kip1) inhibits cancer cell motility, survival, and tumorigenicity. Cancer Res. 2006 Feb 15;66(4):2162–72. doi: 10.1158/0008-5472.CAN-05-3304. [DOI] [PubMed] [Google Scholar]
- 50.Rasband WS. ImageJ. U S National Institutes of Health; Bethesda, Maryland, USA: 1997–2011. http://imagej.nih.gov/ij/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.