Abstract
Background
An association of Coronary artery disease (CAD) with hepatitis C (HCV) has been suggested, but definitive data are still lacking.
Aim
Our study sought to estimate the prevalence and severity of CAD in HCV patients compared to with age-, sex-, and race-matched controls without HCV infection.
Subjects and methods
63 HCV-infected patients were compared with 63 age, race, and sex-matched controls without HCV infection undergoing coronary angiography for evaluation of CAD. CAD was defined as more than a 50% blockage in any of the proximal coronary arteries on angiogram. The severity of the stenosis was defined by the modified Reardon severity scoring system: <50% stenosis of the luminal diameter, 1 point; 50–74%, 2 points; 75–99%, 3 points; 100% or total obstruction, 4 points. The points for each lesion in the proximal coronary circulation were summed to give the score for severity.
Results
A significantly higher prevalence of CAD was noted in the HCV population (69.8% vs. 47.6%, = 0.01). The combined Reardon's severity score in the HCV group was significantly higher compared to the controls (6.26 ± 5.39 vs. 2.6 ± 3.03, P < 0.0005). Additionally, significant multivessel CAD (>50% stenosis and ≥2 vessels involved) was also noted significantly more commonly in the HCV group compared to controls (57.1% vs. 15.9%, P < 0.0005).
Conclusion
In this retrospective study the prevalence and severity of CAD was higher in HCV patients who were evaluated for CAD by angiogram compared with matched non-HCV patients. HCV-positive status is potentially a risk factor for CAD.
Keywords: hepatitis C, coronary artery disease, prevalence
Abbreviations: CAD, coronary artery disease; HCV, hepatitis C virus; DM, diabetes mellitus; HDL, high density lipoprotein; LDL, low density lipoprotein; IVDU, intravenous drug use; ACE, angiotensin converting enzyme; IR, insulin resistance
A number of recent studies have suggested an association between hepatitis C virus (HCV) infection and coronary artery disease (CAD), but there is no general consensus regarding such an association.1–3 Reports of a negative association between HCV infection and CAD have further compounded any meaningful inferences.4–6 Reports of an increased risk1–3 or an increase in measures of subclinical atherosclerosis have fueled continued interest to further explore any such associations.7–9 One of the major drawbacks of the earlier negative studies was the lack of well-designed controls and failure to control for the risk factors associated with CAD.
Further, persons with HCV infection are at an increased risk of developing hepatic steatosis, which shares many clinical features with the metabolic syndrome.10,11 Hepatic steatosis has also been associated with elevated serum levels of markers of inflammation and endothelial dysfunction.12 These factors suggest a biologically plausible mechanism of increased risk of CAD in at least a subset of HCV-infected persons.
We therefore set out to determine in a case control design the prevalence and severity of CAD in hepatitis C patients who underwent angiogram to evaluate CAD and compared to age-, sex-, and race-matched controls without HCV infection.
Subjects and Methods
This study was approved by the North Shore-Long Island Jewish Medical Center Institutional Review Board. All subjects with an established diagnosis of hepatitis C based on their ICD 9 code in the hospital registry and with a documented positive anti HCV antibody test irrespective of their HCV RNA status between May 2002 and December 2008 at the Long Island Jewish Medical Center were reviewed for inclusion into the study. Subjects with hepatitis C were than cross-referenced with the angiography database at our cardiac center for coronary angiography. Sixty-three of 934 hepatitis C patients who had coronary angiography to evaluate CAD during the research period were included in the study. Basic demographic characteristics such as age, ethnicity, gender, body mass index, and presence or absence of various coronary artery disease, as well as risk factors like diabetes mellitus (DM), hypertension, high cholesterol, family history, and smoking were recorded. For the purpose of this study, subjects were identified as having DM based on review of the medical chart for an established diagnosis and further confirmed with review of their medication list. An age-, race-, and sex-matched control (1:1) were then obtained from 6017 patients seen between October 2008 to September 2010 and who had cardiac angiography to evaluate CAD. Patients with known HCV infection were excluded from the controls. The clinician selecting controls was blinded to the clinical information of the patients except for the data on age, race, and sex.
Coronary artery disease was defined as more than a 50% blockage in any of the proximal coronary arteries. The severity of stenosis was defined by the Modified Reardon severity scoring system.13 Coronary circulation was divided into eight segments for analysis (Figure 1). The percentage narrowing of each lesion in the proximal coronary artery circulation was assessed according to the maximal narrowing of the diameter of the artery in all projections. The extent and severity of proximal coronary disease was assessed by assigning points to each lesion as follows: <50% stenosis of the luminal diameter, 1 point; 50–74%, 2 points; 75–99%, 3 points; 100% or total obstruction, 4 points. A score of 0 was assigned when there was no evidence of CAD. The points for each lesion in the proximal coronary circulation were summed to give the severity score.
Figure 1.
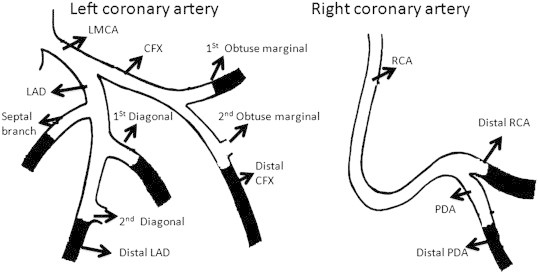
The figure depicts the proximal segments of the coronary circulation that was used in assessing the score for severity of coronary atherosclerosis (Readorn's score). LMCA, left main coronary artery; LAD, left anterior descending; CFX, circumflex; RCA, right coronary artery; PDA, posterior descending artery. Lesions in the darkened portions were not scored.
Statistics
Descriptive statistics were calculated for all of the key variables. Frequencies and percentages were measured for categorical variables; mean and standard deviation were calculated for continuous variables. Comparisons for continuous variables were made using the Mann–Whitney tests and Chi-Square tests as appropriate. Yates corrections were applied when appropriate. To analyze factors predictive of CAD, multiple demographic (age, gender, race, BMI), clinical (DM, hypertension, tobacco dependence, family history of CAD, alcohol abuse, IVDU) and laboratory parameters (serum LDL, HDL, TG, cholesterol) were used in a univariate and multivariate regression model. Statistical analyses were performed with the SPSS (Statistical Package of Services Solutions, SPSS Inc., Chicago, IL) software; version 17.0. The level of statistical significance for analyses were set at P < 0.05.
Results
Clinical, Demographic, and Laboratory Characteristics of Hepatitis C Virus and Control Groups
Baseline demographic data are presented in Table 1. There were no significant differences in terms of age, ethnicity, sex, or major risk factors attributable to atherosclerotic CAD such hypertension, diabetes mellitus, body mass index, history of smoking, or family history of CAD. The frequencies of the prevalent risk factors for CAD, however, were high in both the groups. A higher prevalence of hypercholesterolemia was noted in the control population, but it was not statistically significant. The mean fasting levels of serum low density lipoprotein (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, and total cholesterol were not significantly different between the groups, although a higher level of serum triglycerides (TG) was noted in the HCV group (125 ± 68 vs. 102 ± 78, P = 0.02). Frequency of factors such as alcohol abuse and intravenous drug use (IVDU) were comparable between the groups. Mean alanine aminotransferase level was significantly higher in the HCV group compared to control (51 ± 41 vs. 43 ± 63, = 0.01). The mean hemoglobin level was significantly lower in the HCV group (12.2 ± 1.9 vs. 13.1 ± 1.8, = 0.01).
Table 1.
Clinical and laboratory characteristics in cases and controls.
| Parameters | HCV cases (N = 63) | Controls (N = 63) | P |
|---|---|---|---|
| Age (year) | 60.9 ± 10.5 | 60.8 ± 10.5 | 0.93 |
| Gender (male) | 41 (65.1) | 41 (65.1) | 1 |
| Race | |||
| White | 35 (55.6) | 35 (55.6) | 1 |
| AA | 18 (28.6) | 17 (27) | 0.84 |
| Hispanic | 3 (4.8) | 4 (6.3) | 1 |
| Asian | 7 (11.1) | 7 (11.1) | 1 |
| BMI | 32.2 ± 13.5 | 29.8 ± 6.1 | 0.51 |
| DM | 28 (44.4) | 26 (41.3) | 0.72 |
| Hypertension | 52 (82.5) | 51 (81) | 0.81 |
| Hyperlipidemia | 33 (52.4) | 43 (68.4) | 0.06 |
| Tobacco use | 29 (46) | 26 (41.3) | 0.59 |
| Alcohol abuse | 8 (12.7) | 10 (15.9) | 0.61 |
| Family history of CAD | 21 (33.3) | 28 (44.4) | 0.20 |
| IVDU | 3 (4.8) | 3 (4.8) | 1 |
| SBP (mm Hg) | 138 ± 26 | 140 ± 23 | 0.52 |
| DBP (mm Hg) | 77 ± 13 | 81 ± 12 | 0.14 |
| LDL (mg/dL) | 91 ± 30 | 88 ± 35 | 0.46 |
| HDL (mg/dL) | 42 ± 21 | 42 ± 12 | 0.56 |
| Cholesterol (mg/dL) | 147 ± 40 | 144 ± 43 | 0.59 |
| Triglyceride (mg/dL) | 125 ± 68 | 102 ± 78 | 0.02 |
| ALT IU/L | 51 ± 41 | 43 ± 63 | 0.01 |
| Hb (%) | 12.2 ± 1.9 | 13.1 ± 1.8 | 0.01 |
| Aspirin | 31 (49.2) | 43 (68.3) | 0.03 |
| Beta blocker | 29 (46) | 37 (58.7) | 0.15 |
| ACE inhibitors | 30 (47.6) | 32 (50.8) | 0.72 |
| Ca++ channel blocker | 8 (12.7) | 10 (15.9) | 0.54 |
| Statin | 26 (41.3) | 44 (70) | 0.001 |
AA, African American; BMI, body mass index; DM, diabetes mellitus; CAD, coronary artery disease; IVDU, intravenous drug use; SBP, systolic blood pressure; DBP, diastolic blood pressure; LDL, low density lipoprotein cholesterol; HDL, high density lipoprotein cholesterol; ALT, alanine aminotransferases.
Among oral medications, a significantly lower use of aspirin (49.2% vs. 68.3%, = 0.03) and statin, a cholesterol lowering agent, (41.3% vs. 70%, = 0.001) was noted in the HCV group compared to controls. Both groups were similar in terms of the use of other oral medications such as beta blockers, calcium channel blockers, and angiotensin converting enzyme inhibitors (ACE) inhibitors.
Comparison of Angiographic Parameters in Hepatitis C Virus and Control Groups
A significantly higher prevalence of coronary artery disease (>50% stenosis) in the HCV group compared to controls (69.8% vs. 47.6%, = 0.01) was noted. On further analyses, the prevalence of significant, multivessel disease (defined as > 50% stenosis in ≥2 vessels) was significantly higher in the HCV group compared to controls (57.1% vs. 15.9%, = <0.0005). When the number of coronary vessels involved in the HCV and control groups was compared, a distinct pattern with increasing frequency of involvement was noted in the HCV group (Figure 2). The Reardon severity score was significantly higher in the HCV group compared to the control group (6.26 ± 5.39 vs. 2.6 ± 3.03, P < 0.0005; Table 2). In addition, analyses of the Reardon severity score of individual coronary vessels revealed a higher mean severity score in the majority of the coronary vessels (Table 2). Even in those vessels where the difference was not statistically different, a trend toward a higher score was noted in the HCV group.
Figure 2.
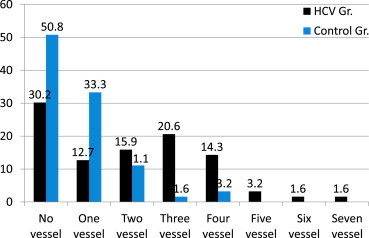
The figure depicts the comparative frequency of involvement coronary vessels in the HCV group and the control group.
Table 2.
Prevalence and severity of coronary artery disease.
| Parameters | HCV cases (N = 63) | Control (N = 63) | P |
|---|---|---|---|
| CAD (> 50% stenosis) | 44 (69.8%) | 30 (47.6%) | 0.01 |
| CAD (> 75% stenosis) | 42 (66.7%) | 29 (46%) | 0.02 |
| Multivessel disease | 36 (57.1%) | 10 (15.9%) | <0.0005 |
| Combined Reardon severity score | 6.26 ± 5.39 | 2.6 ± 3.03 | <0.0005 |
| LAD: junction of middle and distal 1/3rd | 1.54 ± 1.54 | 0.71 ± 1.22 | 0.004 |
| LAD: proximal 1/3rd of septal branch | 0.51 ± 1.13 | 0.00 ± 0.00 | 0.001 |
| LAD: proximal 1/3rd diagonal branch | 0.33 ± 0.91 | 0.19 ± 0.64 | 0.51 |
| Proximal 1/3rd of the obtuse marginal branch of the CFX | 0.73 ± 1.31 | 0.14 ± 0.59 | 0.003 |
| CFX | 0.89 ± 1.32 | 0.60 ± 1.22 | 0.18 |
| RCA: up to and the origin of the PDA | 1.38 ± 1.66 | 0.84 ± 1.34 | 0.06 |
| Origin of PDA | 0.59 ± 0.21 | 0.00 ± 0.00 | <0.0005 |
| Proximal 1/3rd of PDA | 0.30 ± 0.83 | 0.11 ± 0.54 | 0.11 |
Severity of CAD is described as an average Reardon's score of a specific coronary artery. CAD, coronary artery disease; LAD, left anterior descending; CFX, circumflex; RCA, right coronary artery; PDA, posterior descending artery.
Logistic Regression Analyses to Look for Factors Predictive of Coronary Artery Disease
Assuming both controls and the HCV groups has the same risk for developing CAD we performed logistic regression analyses including both the control and the HCV group in order to analyze the factors predictive of CAD (Table 3). Multiple demographic, clinical and laboratory variable were included in a univariate logistic regression analyses as reported in the Table. Age (P = 0.03, OR = 1.03, 95% C.I. 0.99–1.06), DM (P = 0.05, OR = 2.05, 85% C.I. 0.98–4.30), history (current and past smoker) of tobacco use (P = 0.006, OR = 2.90, 95% C.I. 1.36–6.17), family history of CAD (P = 0.054, OR = 2.09, 95% C.I. 0.98–4.45), and positive HCV status (P = 0.01, OR = 2.5, 95% C.I. 1.22–5.29) were found to be positively associated with CAD. Other factors such as; gender, race, BMI, hypertension, hyperlidemia, alcohol abuse, IVDU, serum levels of LDL, HDL, total cholesterol, TG and ALT were not predictive for coronary artery disease in the entire cohort. Further analyses with multivariate logistic regression analyses revealed only age (P = 0.03, OR = 1.04, 95% C.I. 1.00–1.08), tobacco dependence (P = 0.007, OR 3.26, 95% C.I. 1.39–7.63) and HCV-positive status (P = 0.007, OR 3.12, 95% C.I. 1.37–7.10) were significant factors that predicted coronary artery disease.
Table 3.
Univariate and multivariate logistic regression analyses to look for factors associated with CAD (>50% blockage).
| Parameters | Regression | P | OR | 95% C.I. | Regression | P | OR | 95% C.I. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Upper | Lower | |||||||||
| Age | 0.03 | 0.08 | 1.03 | 0.99 | 1.06 | 0.04 | 0.03 | 1.04 | 1.00 | 1.08 |
| Male gender | 0.40 | 0.28 | 1.50 | 0.71 | 3.15 | |||||
| White | 0.38 | 0.29 | 1.46 | 0.71 | 2.99 | |||||
| AA | −0.25 | 0.53 | 0.77 | 0.35 | 1.70 | |||||
| Hispanics | −0.06 | 0.93 | 0.93 | 0.20 | 4.35 | |||||
| Asians | −0.39 | 0.48 | 0.67 | 0.22 | 2.04 | |||||
| BMI | 0.02 | 0.39 | 1.02 | 0.97 | 1.07 | |||||
| DM | 0.72 | 0.05 | 2.05 | 0.98 | 4.30 | 0.56 | 0.18 | 1.74 | 0.77 | 3.94 |
| Hypertension | 0.11 | 0.81 | 1.11 | 0.44 | 2.78 | |||||
| Hyperlipidemia | 0.05 | 0.89 | 1.05 | 0.50 | 2.16 | |||||
| Tobacco use | 1.06 | 0.006 | 2.90 | 1.36 | 6.17 | 1.18 | 0.007 | 3.26 | 1.39 | 7.63 |
| Alcohol abuse | −0.15 | 0.76 | 0.86 | 0.31 | 2.35 | |||||
| Family History of CAD | 0.74 | 0.054 | 2.09 | 0.98 | 4.45 | 0.75 | 0.08 | 2.11 | 0.90 | 4.95 |
| IVDU | −0.37 | 0.65 | 0.69 | 0.13 | 3.56 | |||||
| LDL | 0.007 | 0.39 | 1.08 | 0.99 | 1.02 | |||||
| HDL | −0.002 | 0.90 | 0.99 | 0.96 | 1.03 | |||||
| TG | −0.003 | 0.43 | 0.99 | 0.99 | 1.00 | |||||
| Cholesterol | 0.005 | 0.48 | 1.00 | 0.99 | 1.01 | |||||
| ALT | −0.001 | 0.83 | 0.99 | 0.99 | 1.00 | |||||
| HCV + status | 0.93 | 0.01 | 2.54 | 1.22 | 5.29 | 1.13 | 0.007 | 3.12 | 1.37 | 7.10 |
Note: Multivariate regression analysis was performed on factors with P < 0.20.
Discussion
The results of this study indicate that CAD is significantly more prevalent in HCV-seropositive patients compared to age-, race-, and sex-matched controls undergoing evaluation by coronary angiogram for suspected CAD. We also noted a higher number of HCV-seropositive patients with more severe coronary disease (>75% stenosis) and significantly higher number of patients with significant multivessel coronary artery disease (≥2 vessels). The study further confirmed earlier reported results of more severe CAD in HCV-infected patients based on a visual scoring system adapted from Reardon et al.2,13 A large retrospective controlled study using the national Veterans Administration database found a significant association of HCV infection with CAD after adjusting for multiple traditional risk factors.3 Similar associations were reported in another earlier reported study.1 In the current study we were able to show that HCV-seropositive status is a strong predictor for CAD in addition to other traditional factors such as age, DM, tobacco dependence and family history of CAD. Although in the multivariate model DM and family history of CAD could not reach statistical significance likely a result of small sample size.
Based on earlier reported studies, we can only speculate on the mechanism responsible for the increased prevalence and severity of CAD in HCV-infected patients. It is widely recognized that chronic hepatitis C infection is strongly associated with type 2 diabetes mellitus and insulin resistance. Chronic hepatitis C virus infection promotes insulin resistance (IR) mainly through interfering with the insulin signaling pathway in hepatocytes and increasing the inflammatory response with production of cytokines such TNF-α and IL-6 and increasing oxidative stress.14 The resultant insulin resistance may potentially lead to deregulated glucose and lipid metabolism with resultant metabolic abnormalities. Several studies have found an increased rate of atherosclerosis in patients with HCV infection.2,7–9 In fact a recent metanalysis even concluded an increased risk of CAD in HCV-infected individuals.15
We also noted that a significantly lower number of patients in the HCV group were receiving antiplatelet agents like aspirin and lipid-lowering agents such as statins potentially exacerbating coronary atherosclerosis. Potential concern for gastrointestinal bleeding or hepatotoxicity may have influenced clinical decisions for such therapy. However, lack of data on the severity of liver disease limits our supposition about this.
The results of our study should be interpreted with caution. Many of the known inherent deficiencies in a retrospective study design apply to the current research. HCV infection was determined based on HCV antibody seropositivity; and data on HCV genotype, HCV viral load, and liver histopathology that would have more accurately characterized whether the patients included in the HCV group of patients have active HCV infection were unavailable. Information on the treatment of HCV infection, which in some studies has been shown to alter the lipid profile, was not available in our study. The small sample size may have led type I and type II errors; a larger sample size in future studies will be useful in making a firmer conclusion. Evaluation of the coronary angiogram was based on reported data from multiple observers, and thus a possibility of observation variation cannot be excluded. Indications for the coronary angiography was not recorded in the current study, rather all patients irrespective of their reason for angiography were included both in the cases and control groups. In fact, such blinded collection of the coronary angiographic data, we believe is the strength of the study, as there is no bias in selection of the patients with known CAD avoiding any spurious increase in the prevalence in either category.
In conclusion, CAD is significantly more common and severe in HCV-seropositive patients. It is not clear whether this association is related to the known metabolic complications related to insulin resistance in patients with chronic HCV infection or due to under treatment with antiplatelet and lipid-lowering agents because of concerns for gastrointestinal bleeding or hepatotoxicity and these findings needs to be extrapolated with caution considering the retrospective design of the study. Further prospective controlled studies with larger sample size are needed to clarify these issues. We recommend physicians to remain vigilant about the risk of CAD in HCV-infected patients and consider treating risk factors early on and refer to cardiologist if needed.
Conflicts of interest
All authors have none to declare.
References
- 1.Vassalle C., Masini S., Bianchi F., Zucchelli G.C. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565–566. doi: 10.1136/hrt.2003.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alyan O., Kacmaz F., Ozdemir O. Hepatitis C infection is associated with increased coronary artery atherosclerosis defined by modified Reardon severity score system. Circ J. 2008;72:1960–1965. doi: 10.1253/circj.cj-08-0459. [DOI] [PubMed] [Google Scholar]
- 3.Butt A.A., Xiaoqiang W., Budoff M., Leaf D., Kuller L.H., Justice A.C. Hepatitis C virus infection and the risk of coronary disease. Clin Infect Dis. 2009;15(49):225–232. doi: 10.1086/599371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arcari C.M., Nelson K.E., Netski D.M., Nieto F.J., Gaydos C.A. No association between hepatitis C virus seropositivity and acute myocardial infarction. Clin Infect Dis. 2006;43:e53–e56. doi: 10.1086/507031. [DOI] [PubMed] [Google Scholar]
- 5.Volzke H., Schwahn C., Wolff B. Hepatitis B and C virus infection and the risk of atherosclerosis in a general population. Atherosclerosis. 2004;174:99–103. doi: 10.1016/j.atherosclerosis.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Momiyama Y., Ohmori R., Kato R., Taniguchi H., Nakamura H., Ohsuzu F. Lack of any association between persistent hepatitis B or C virus infection and coronary artery disease. Atherosclerosis. 2005;181:211–213. doi: 10.1016/j.atherosclerosis.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 7.Ishizaka N., Ishizaka Y., Takahashi E. Association between hepatitis C virus seropositivity, carotidartery plaque, and intima-media thickening. Lancet. 2002;359:133–135. doi: 10.1016/s0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 8.Sawayama Y., Okada K., Maeda S., Ohnishi H., Furusyo N., Hayashi J. Both hepatitis C virus and Chlamydia pneumoniae infection are related to the progression of carotid atherosclerosis in patients undergoing lipid lowering therapy. Fukuoka Igaku Zasshi. 2006;97:245–255. [PubMed] [Google Scholar]
- 9.Fukui M., Kitagawa Y., Nakamura N., Yoshikawa T. Hepatitis C virus and atherosclerosis in patients with type 2 diabetes. JAMA. 2003;289:1245–1246. doi: 10.1001/jama.289.10.1245-b. [DOI] [PubMed] [Google Scholar]
- 10.Sanyal A.J., Contos M.J., Sterling R.K. Nonalcoholic fatty liver disease in patients with hepatitis C is associated with features of the metabolic syndrome. Am J Gastroenterol. 2003;98:2064–2071. doi: 10.1111/j.1572-0241.2003.07640.x. [DOI] [PubMed] [Google Scholar]
- 11.Sanyal A.J. Review article: non-alcoholic fatty liver disease and hepatitis C—risk factors and clinical implications. Aliment Pharmacol Ther. 2005;22:48–51. doi: 10.1111/j.1365-2036.2005.02596.x. [DOI] [PubMed] [Google Scholar]
- 12.Targher G., Bertolini L., Scala L., Zoppini G., Zenari L., Falezza G. Non-alcoholic hepatic steatosis and its relation to increased plasma biomarkers of inflammation and endothelial dysfunction in non-diabetic men: role of visceral adipose tissue. Diabet Med. 2005;22:1354–1358. doi: 10.1111/j.1464-5491.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- 13.Reardon M.F., Nestel P.J., Craig I.H., Harper R.W. Lipoproteins predictors of the severity of coronary artery disease in men and women. Circulation. 1985;71:881–888. doi: 10.1161/01.cir.71.5.881. [DOI] [PubMed] [Google Scholar]
- 14.Hung C.H., Lee C.M., Lu S.N. Hepatitis C virus-associated insulin resistance: pathogenic mechanisms and clinical implications. Expert Rev Anti Infect Ther. 2011;9:525–533. doi: 10.1586/eri.11.33. [DOI] [PubMed] [Google Scholar]
- 15.Roed T., Lebech A.M., Kjaer A., Weis N. Hepatitis C virus infection and risk of coronary artery disease: a systematic review of the literature. Clin Physiol Funct Imaging. 2012 Nov;32(6):421–430. doi: 10.1111/j.1475-097X.2012.01152.x. [DOI] [PubMed] [Google Scholar]


