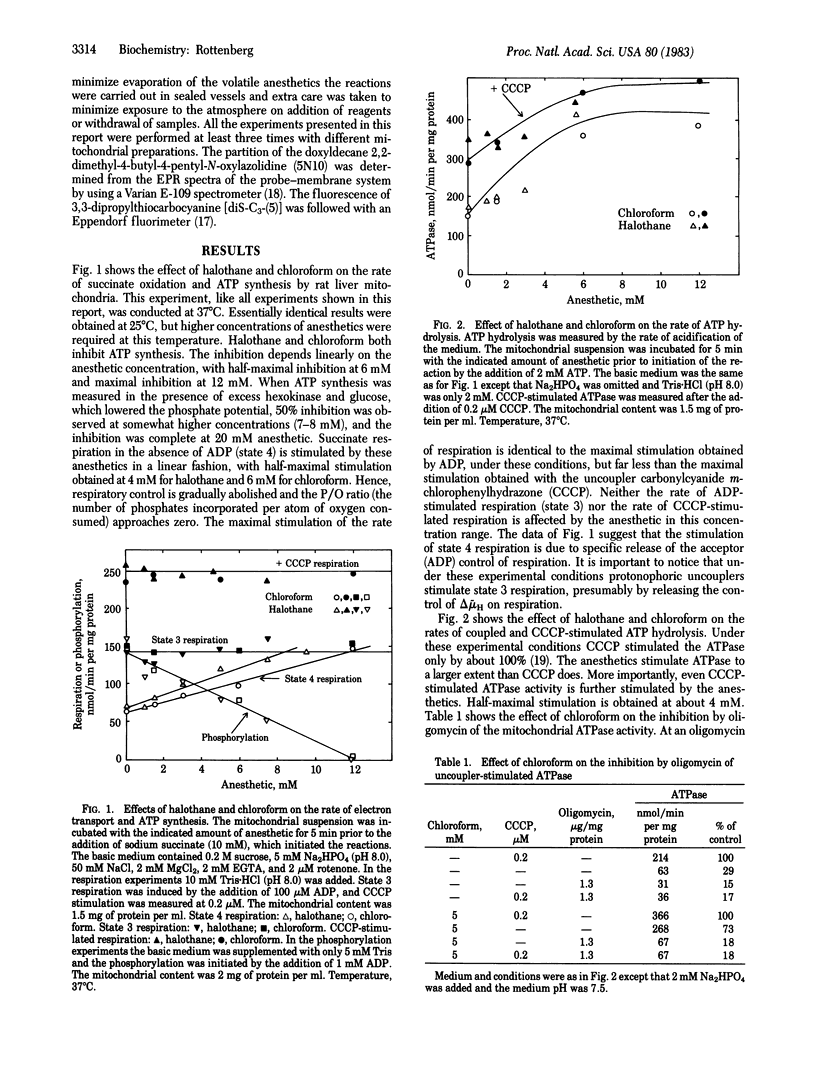
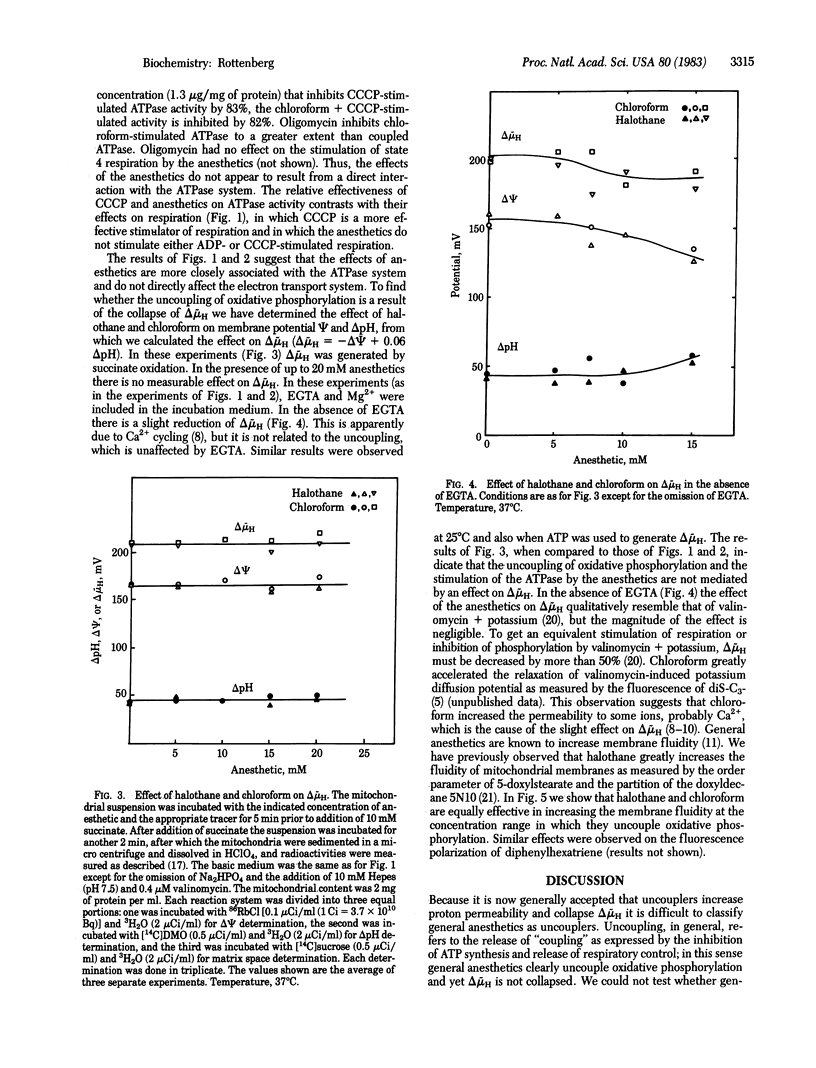
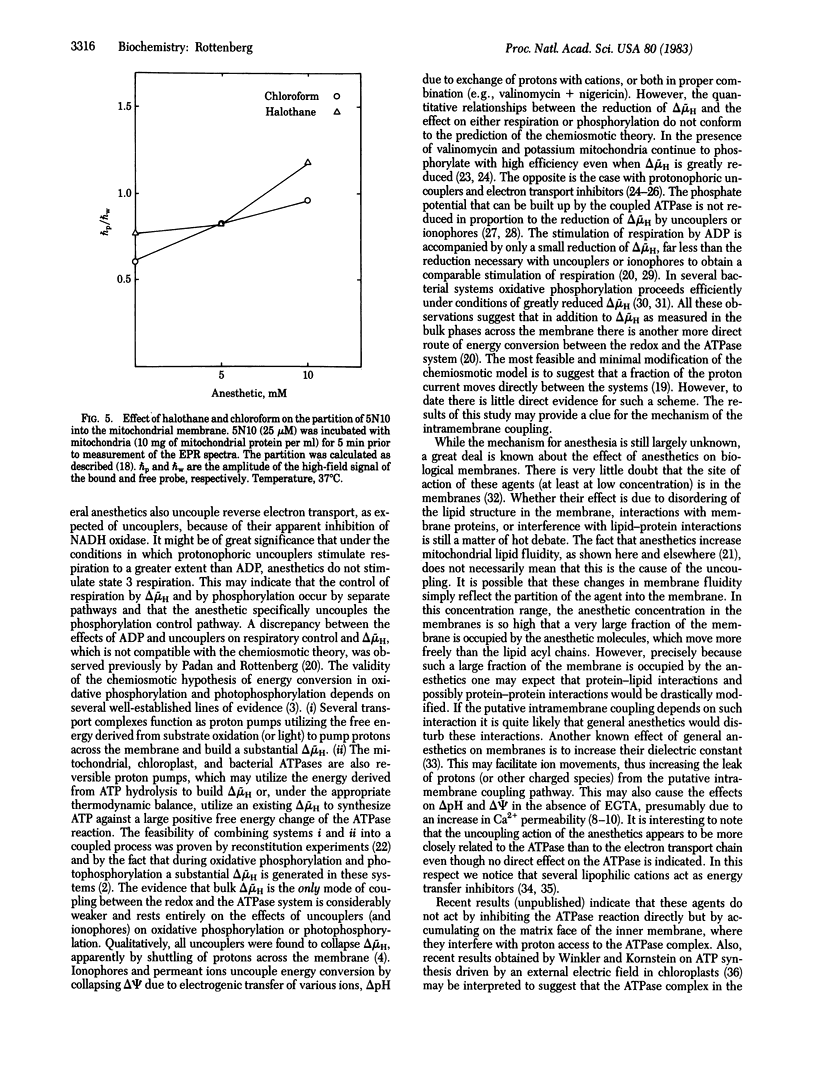
Abstract
The general anesthetics chloroform and halothane inhibit ATP synthesis in rat liver mitochondria, in the millimolar concentration range (1-12 mM), in parallel with a reduction of respiratory control and the ratio of ATP produced to oxygen consumed. In these effects, halothane and chloroform are similar to classical, protonophoric, uncouplers. The rate of ADP-stimulated respiration or the rate of uncoupler-stimulated respiration is not affected. Like classical uncouplers, halothane and chloroform also stimulate mitochondrial ATPase activity. However, the extent of stimulation by these agents is larger than by protonophoric uncouplers and, more significantly, ATPase activity stimulated by carbonylcyanide m-chlorophenylhydrazone is further stimulated by these agents. In the presence of the Ca2+ chelator EGTA, halothane and chloroform have no measurable effect on the magnitude of the proton electrochemical potential, delta mu H. In the absence of EGTA these anesthetics have a small effect on delta mu H, apparently due to stimulation of Ca2+ cycling. Under these conditions the membrane potential is decreased while delta pH is increased, but the total value of delta mu H is only slightly decreased. The uncoupling activity of the anesthetics is the same in the presence of absence of EGTA. Thus, in contrast to protonophoric uncouplers, the uncoupling effect of general anesthetics does not depend on the collapse of delta mu H. In the same concentration range in which anesthetics uncouple oxidative phosphorylation both halothane and chloroform increase membrane fluidity, as measured by the partitioning of the hydrophobic spin probe 5-doxyldecane. These findings suggest a role for intramembrane processes in energy conversion that is not dependent on the bulk delta mu H.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azzone G. F., Pozzan T., Massari S., Bragadin M. Proton electrochemical gradient and rate of controlled respiration in mitochondria. Biochim Biophys Acta. 1978 Feb 9;501(2):296–306. doi: 10.1016/0005-2728(78)90035-x. [DOI] [PubMed] [Google Scholar]
- Azzone G. F., Pozzan T., Massari S. Proton electrochemical gradient and phosphate potential in mitochondria. Biochim Biophys Acta. 1978 Feb 9;501(2):307–316. doi: 10.1016/0005-2728(78)90036-1. [DOI] [PubMed] [Google Scholar]
- Grist E. M., Baum H. Evidence for a halothane-dependent cyclic flux of calcium in rat-liver mitochondria. Eur J Biochem. 1975 Sep 15;57(2):617–620. doi: 10.1111/j.1432-1033.1975.tb02337.x. [DOI] [PubMed] [Google Scholar]
- Grist E. M., Baum H. The mechanism of the halothane-dependent efflux of calcium from rat-liver mitochondria. Eur J Biochem. 1975 Sep 15;57(2):621–626. doi: 10.1111/j.1432-1033.1975.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Guffanti A. A., Blumenfeld H., Krulwich T. A. ATP synthesis by an uncoupler-resistant mutant of Bacillus megaterium. J Biol Chem. 1981 Aug 25;256(16):8416–8421. [PubMed] [Google Scholar]
- Harris R. A., Munroe J., Farmer B., Kim K. C., Jenkins P. Action of halothane upon mitochondrial respiration. Arch Biochem Biophys. 1971 Feb;142(2):435–444. doi: 10.1016/0003-9861(71)90507-8. [DOI] [PubMed] [Google Scholar]
- Higuti T., Arakaki N., Niimi S., Nakasima S., Saito R., Tani I., Ota F. Anisotropic inhibition of energy transduction in oxidative phosphorylation in rat liver mitochondria by tetraphenylarsonium. J Biol Chem. 1980 Aug 25;255(16):7631–7636. [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. G., Dilger J. P. Transport of protons across membranes by weak acids. Physiol Rev. 1980 Jul;60(3):825–863. doi: 10.1152/physrev.1980.60.3.825. [DOI] [PubMed] [Google Scholar]
- Melandri B. A., Venturoli G., de Santis A., Baccarini-Melandri A. The induction kinetics of bacterial photophosphorylation. Threshold effects by the phosphate potential and correlation with the amplitude of the carotenoid absorption band shift. Biochim Biophys Acta. 1980 Aug 5;592(1):38–52. doi: 10.1016/0005-2728(80)90112-7. [DOI] [PubMed] [Google Scholar]
- Michel H., Oesterhelt D. Electrochemical proton gradient across the cell membrane of Halobacterium halobium: comparison of the light-induced increase with the increase of intracellular adenosine triphosphate under steady-state illumination. Biochemistry. 1980 Sep 30;19(20):4615–4619. doi: 10.1021/bi00561a012. [DOI] [PubMed] [Google Scholar]
- Miller R. N., Hunter F. E., Jr Is halothane a true uncoupler of oxidative phosphorylation? Anesthesiology. 1971 Sep;35(3):256–261. doi: 10.1097/00000542-197109000-00006. [DOI] [PubMed] [Google Scholar]
- NISHIMURA M., ITO T., CHANCE B. Studies on bacterial photophosphorylation. III. A sensitive and rapid method of determination of photophosphorylation. Biochim Biophys Acta. 1962 May 7;59:177–182. [PubMed] [Google Scholar]
- Padan E., Rottenberg H. Respiratory control and the proton electrochemical gradient in mitochondria. Eur J Biochem. 1973 Dec 17;40(2):431–437. doi: 10.1111/j.1432-1033.1973.tb03212.x. [DOI] [PubMed] [Google Scholar]
- Pang K. Y., Braswell L. M., Chang L., Sommer T. J., Miller K. W. The perturbation of lipid bilayers by general anesthetics: a quantitative test of the disordered lipid hypothesis. Mol Pharmacol. 1980 Jul;18(1):84–90. [PubMed] [Google Scholar]
- Pang K. Y., Chang T. L., Miller K. W. On the coupling between anesthetic induced membrane fluidization and cation permeability in lipid vesicles. Mol Pharmacol. 1979 May;15(3):729–738. [PubMed] [Google Scholar]
- Racker E., Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974 Jan 25;249(2):662–663. [PubMed] [Google Scholar]
- Reyes J., Latorre R. Effect of the anesthetics benzyl alcohol and chloroform on bilayers made from monolayers. Biophys J. 1979 Nov;28(2):259–279. doi: 10.1016/S0006-3495(79)85175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. ATP synthesis and electrical membrane potential in mitochondria. Eur J Biochem. 1970 Jul;15(1):22–28. doi: 10.1111/j.1432-1033.1970.tb00971.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. Phase transitions and coupling in energy transducing membranes. FEBS Lett. 1978 Oct 15;94(2):295–297. doi: 10.1016/0014-5793(78)80960-0. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Robertson D. E., Rubin E. The effect of ethanol on the temperature dependence of respiration and ATPase activities of rat liver mitochondria. Lab Invest. 1980 Mar;42(3):318–326. [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Waring A., Rubin E. Tolerance and cross-tolerance in chronic alcoholics: reduced membrane binding of ethanol and other drugs. Science. 1981 Jul 31;213(4507):583–585. doi: 10.1126/science.6264608. [DOI] [PubMed] [Google Scholar]
- Rzeczycki W., Valdivia E. Mitochondria uncoupling effect of halothane dependent on magnesium. Biochem Biophys Res Commun. 1973 May 1;52(1):270–275. doi: 10.1016/0006-291x(73)90983-2. [DOI] [PubMed] [Google Scholar]
- SZARKOWSKA L., KLINGENBERG M. ON THE ROLE OF UBIQUINONE IN MITOCHONDRIA. SPECTROPHOTOMETRIC AND CHEMICAL MEASUREMENTS OF ITS REDOX REACTIONS. Biochem Z. 1963;338:674–697. [PubMed] [Google Scholar]
- Schäfer G. Some new aspects on the interaction of hypoglycemia-producing biguanides with biological membranes. Biochem Pharmacol. 1976 Sep 15;25(18):2015–2024. doi: 10.1016/0006-2952(76)90424-x. [DOI] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]
- Slater E. C. Mechanism of oxidative phosphorylation. Annu Rev Biochem. 1977;46:1015–1026. doi: 10.1146/annurev.bi.46.070177.005055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorgato M. C., Branca D., Ferguson S. J. The rate of ATP synthesis by submitochondrial particles can be independent of the magnitude of the protonmotive force. Biochem J. 1980 Jun 15;188(3):945–948. doi: 10.1042/bj1880945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinkler C., Korenstein R. Characterization of external electric field-driven ATP synthesis in chloroplasts. Proc Natl Acad Sci U S A. 1982 May;79(10):3183–3187. doi: 10.1073/pnas.79.10.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring A. J., Rottenberg H., Ohnishi T., Rubin E. The effect of chronic ethanol consumption on temperature-dependent physical properties of liver mitochondrial membranes. Arch Biochem Biophys. 1982 Jun;216(1):51–61. doi: 10.1016/0003-9861(82)90187-4. [DOI] [PubMed] [Google Scholar]
- Zoratti M., Pietrobon D., Azzone G. F. On the relationship between rate of ATP synthesis and H+ electrochemical gradient in rat-liver mitochondria. Eur J Biochem. 1982 Sep 1;126(3):443–451. doi: 10.1111/j.1432-1033.1982.tb06800.x. [DOI] [PubMed] [Google Scholar]



