Abstract
Importance
Convergent biological, epidemiological and clinical data identified urate elevation as a candidate strategy for slowing disability progression in Parkinson disease (PD).
Objective
To determine the safety, tolerability and urate-elevating capability of the urate precursor inosine in early PD; and to assess its suitability and potential design features for a disease-modification trial.
Design
The Safety of URate Elevation in PD (SURE-PD) study, a randomized, double-blind, placebo-controlled, dose-ranging trial of inosine, enrolled participants from 2009–2011 and followed them for up to 25 months.
Setting
Outpatient visits to 17 credentialed clinical study sites of the Parkinson Study Group across the United States.
Participants
Seventy-five consenting adults (mean age 62; 55% women) with early PD not yet requiring symptomatic treatment and a serum urate concentration below 6 mg/dL (the approximate population median) were enrolled.
Intervention
Participants were randomized to one of three treatment arms: placebo or inosine titrated to produce mild (6.1–7.0 mg/dL) or moderate (7.1–8.0 mg/dL) serum urate elevation using 500 mg capsules taken orally up to two thrice daily. They were followed for up to 24 months (median 18) on study drug plus 1 washout month.
Main Outcome Measures
The pre-specified primary outcomes were absence of unacceptable serious adverse events (safety), continued treatment without adverse event requiring dose reduction (tolerability), and elevation of urate assessed serially in serum and once (at 3 months) in cerebrospinal fluid (CSF).
Results
Serious adverse events (17), including infrequent cardiovascular events, occurred at the same or lower rates in inosine groups relative to placebo. No participant developed gout and three receiving inosine developed symptomatic urolithiasis. Treatment was tolerated by 95% of participants at 6 months, and no participant withdrew due to an adverse event. Serum urate rose by 2.3 and 3.0 mg/dL in the two inosine groups (p<0.001 for each) vs placebo, and CSF urate was greater in both inosine groups (p=0.006 and <0.001, respectively). Secondary analyses demonstrated non-futility of inosine treatment for slowing disability.
Conclusion and Relevance
Inosine was generally safe, tolerable, and effective in raising serum and CSF urate levels in early PD. The findings support advancing to more definitive development of inosine as a potential disease-modifying therapy for PD.
Trial registration
ClinicalTrials.gov NCT00833690 (http://clinicaltrials.gov/ct2/show/NCT00833690)
Urate is the enzymatic end product of purine metabolism in humans, possesses potent antioxidant and metal chelator properties in vitro,1,2 and confers neuroprotection against oxidative stress-induced dopaminergic neuron death in rodent models of Parkinson disease (PD).3–5 Studies of prospectively followed cohorts identified blood urate in healthy individuals as an inverse risk factor for PD.6–8 Among 1,604 early PD patients enrolled in the PRECEPT9 and DATATOP10 trials, higher serum11,12 or CSF12 urate levels at baseline predicted slower rates of clinical (disability)11,12 or radiographic (dopamine transporter imaging)11 progression over two years. Thus higher urate is a predictor of both reduced risk and slower progression of PD.
The convergence of these biological, epidemiological and clinical data warrants consideration of urate elevation as a potential disease-modifying treatment for PD. Although urate appears to be rapidly degraded within the intestinal tract by bacterial flora, its precursor inosine when taken orally produces a rapid elevation of serum urate.13,14 Long-term inosine treatment in multiple sclerosis cohorts,14–17 comprising mostly women 30 to 50 years old, increased serum urate for one or more years with few side effects other than urolithiasis (which developed in as many as 25% of participants). The older and predominantly male PD population may be more susceptible to adverse effects (AEs) of urate elevation, including gout and uric acid urolithiasis (i.e., diseases of crystal formation), and possibly cardiovascular, renal and metabolic disorders.18 Accordingly, we undertook a phase 2 study of oral inosine in early PD with primary goals of determining its safety, tolerability and ability to elevate serum and CSF urate levels. Although therapeutic elevation of serum and CSF urate may seem a medical oxymoron, there are many precedents for rationally raising levels of an endogenous factor often viewed as pathogenic (and vice versa). Examples range from raising serum sodium levels to treat orthostatic hypotension (despite their pathogenic role in cardiovascular disease) to raising central nervous system dopamine levels in PD (despite their pathogenic role in various psychotic disorders). The Safety of URate Elevation in PD (SURE-PD) trial was designed19 with the broader purpose of determining whether and how inosine should be pursued as a urate-elevating strategy in any subsequent phase 3 trials of its disease-modifying potential in PD.
METHODS
Details of the trial design are posted20 and published19 separately, and are summarized below.
PARTICIPANTS AND SITES
Enrollment criteria were modeled after those of the PRECEPT9 and DATATOP10 trials, in which urate was linked to slower PD progression, except for the following differences: i) only those individuals whose serum urate fell below the predicted median of ~6 mg/dL (~360 μM) were included, as this subpopulation is at risk of faster disability progression and could more safely accommodate an increase in serum urate to the levels associated with slower disease progression; and ii) individuals at greatest risk of known effects of increased urate (i.e., those with a history of gout or urolithiasis, or with urine pH ≤5.0 – a major risk factor for uric acid urolithiasis21) were excluded from enrolling, as were those at high risk for possible effects of increased urate (e.g., cardiovascular or renal disease). Key inclusion criteria also specified early PD not yet requiring symptomatic antiparkinsonian treatment (except that a stable dose of a monoamine oxidase-B [MAO-B] inhibitor was permitted after a protocol amendment was implemented in late 2010 as a strategy to address initial slow enrollment).
From June 2009 through December 2011 participants were enrolled at 16 of the 17 participating sites selected from credentialed Parkinson Study Group clinical sites across the United States. The original and amended protocols19 were approved by institutional review boards of the administrative and coordination centers and all clinical sites. Goals for the total number enrolled and the duration of follow-up were based on a priori power analyses.19
INTERVENTION, DOSE TITRATION, AND FOLLOW-UP
Eligible participants were randomized at their baseline visits in a 1:1:1 distribution to three treatment groups: 1) placebo, 2) inosine titrated to mildly elevate serum urate (to 6.1 – 7.0 mg/dL), and 3) inosine titrated to moderately elevate serum urate (to 7.1 – 8.0 mg/dL). Treatments constituted oral administration of white opaque gelatin capsules containing 500 mg of study drug: inosine (active drug) or lactose (placebo).
Initiation, maintenance and discontinuation of dosing
Treatment was initiated gradually with 1 capsule taken two times daily for 2 weeks. After the 2- and 4-week visits participants received up to 2 capsules two and three times daily, respectively, as algorithmically determined by serum urate concentration and treatment group assignment. Scheduled discontinuation of study drug occurred after 24 months (or at the 9 to 21 month visit for those who had not reached 24 months of follow-up in the fall of 2012 at the time of administrative trial termination due to slower than expected enrollment as well as budgetary and statistical considerations).19 Participants returned 1 month later for a safety visit.
Dose titration to serum urate
Active study drug dosing was adjusted based on serum urate values obtained at study visits 2, 4, 6, 9 and 12 weeks, and then 6, 9, 12, 15, 18 and 21 months after randomization.19 In order to preserve the blind, serum urate levels (and other potentially discriminating assays) were centrally tested and were available only to an unblinded data manager who directed participant titrations and a Data and Safety Monitoring Committee (DSMC). Placebo dosing was determined by a titration algorithm intended to match daily capsule intake to that of active drug. Dosing ranged from one capsule daily (each morning) up to two capsules three times daily (i.e., for a maximum intake of 3 g of inosine or lactose per day).
RISK REDUCTION MEASURES
In addition to frequent monitoring of serum urate and gradual study drug initiation, we monitored urinary pH, a major determinant of uric acid urolithiasis. Because the effect of inosine on urine pH was unknown, all participants self-monitored their urine pH at least daily for the first 12 weeks. Any participant who developed persistently acidic (pH 5≤.0) urine implemented a urine alkalinization program with potassium citrate. Urolithiasis prophylaxis was also pursued by encouraging hydration for all participants.
OUTCOMES
Safety
Pre-specified primary outcomes were safety, tolerability and efficacy of urate elevation. Safety was defined as the absence of serious AEs (SAEs) that warranted terminating an inosine treatment arm or the trial, as determined by the trial’s DSMC.
Tolerability
Tolerability of study drug was defined as the extent to which assigned treatment could continue without prolonged dose reduction (> 48 consecutive days or > 73 cumulative days, which is 10% of total 2-year follow-up) due to AEs, and was assessed after 6 and 24 months on study drug.
Efficacy of urate elevation
An inosine treatment was considered effective in elevating urate if either CSF urate levels (measured at the 12-week visit 2.5 hours after the first study drug dose of the day) or serum urate (measured as change from baseline) was significantly greater than in the placebo group. A less stringent non-futility criterion was also specified but was superseded by tests of efficacy.
Secondary outcomes
Additional outcomes were intended to provide preliminary data to aid the design of a potential phase 3 clinical efficacy trial.19 These included clinical outcomes based on serial measurements of parkinsonism (UPDRS subscales,22 and determinations of the need for dopaminergic therapy), cognitive function (Montreal Cognitive Assessment [MoCA])23 and mood (Geriatric Depression Scale-short form [GDS-S])24.
STATISTICAL ANALYSES
Safety was assessed by comparing time to first SAE by log-rank test and by comparing overall SAE and AE event rates by Poisson and negative binomial regression, respectively. Proportions of participants tolerant to study drug at 6 months and 2 years were estimated as Kaplan-Meier product-limit estimates with complementary log-log confidence bounds. Censoring for assessment of tolerability was only due to administrative early stopping of study drug and thus was reasonably considered non-informative. Serum urate levels were compared using mixed model ANOVAs with random site-specific intercepts, random participant-specific intercepts and slopes, and treatment-dependent variance heterogeneity. CSF urate levels were log-transformed and analyzed in a linear model with terms for treatment group, gender, and their interaction. All analyses followed the intention-to-treat principle. Details of methods for secondary analyses are described elsewhere.19 Analyses were performed using SAS (version 9.3, SAS Institute, Cary NC), and inference was based on two-tailed tests at alpha = 0.05.
RESULTS
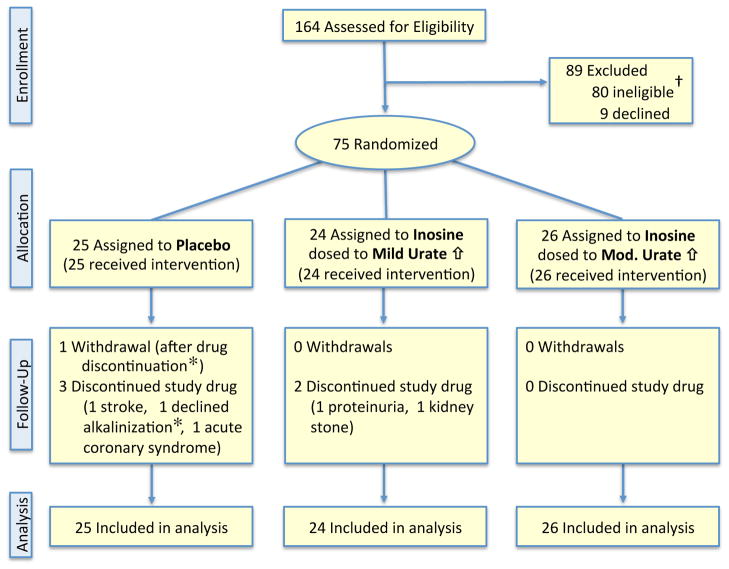
Of 164 participants who consented, 75 met eligibility criteria consistent with the expectation that half of those screened would be excluded due to a serum urate concentration above the approximate population median value of 6 mg/dL11,12 (Figure 1). Eligible participants were randomized to the three treatment groups, which showed similar baseline characteristics (Table 1).
Figure 1.
Consolidated Standards for Reporting Trials flow diagram for the SURE-PD trial.
† A majority of these 80 participants (89%) were determined ineligible based on screening serum urate values above the population median, a criterion that was expected to exclude approximately half of all consented individuals.
* The subject who withdrew did so after discontinuation of study drug, which was due to declining to receive alkalinization treatment for acidic urine.
Table 1.
Baseline Characteristics of the SURE-PD Cohort, by Treatment Group and Overall.
| Feature at baseline | Placebo | Ino → Mild | Ino → Mod | Overall |
|---|---|---|---|---|
| Number randomized | 25 | 24 | 26 | 75 |
| Age (years) | 61 ± 11 | 62 ± 10 | 62 ± 11 | 62 ± 10 |
| Women (proportion of total) | 52% | 58% | 54% | 55% |
| Serum urate (mg/dL) | 4.5 ± 0.7 | 4.3 ± 1.2 | 4.6 ± 0.9 | 4.5 ± 0.9 |
| Serum urate (mg/dL) in women | 4.4 ± 0.8 | 3.9 ± 1.3 | 4.4 ± 0.8 | 4.2 ± 1.0 |
| Serum urate (mg/dL) in men | 4.7 ± 0.5 | 5.0 ± 0.8 | 4.9 ± 0.9 | 4.8 ± 0.7 |
| Smoker (prior or current) | 32% | 38% | 27% | 32% |
| Caffeinated coffee (≥1/day) | 52% | 46% | 46% | 48% |
| Years of symptoms | 2.4 ± 1.3 | 2.8 ± 1.9 | 2.2 ± 2.0 | 2.4 ± 1.8 |
| Years since diagnosis | 1.1 ± 1.3 | 1.3 ± 1.0 | 0.6 ± 0.7 | 1.0 ± 1.1 |
| Resting tremor at diagnosis | 76% | 92% | 85% | 84% |
| UPDRS score total | 23 ± 10 | 20 ± 9 | 21 ± 10 | 22 ± 10 |
| Taking an MAO-B inhibitor | 28% | 33% | 19% | 27% |
| MoCA score | 28 ± 1.9 | 28 ± 1.8 | 28 ± 2.0 | 28 ± 1.9 |
| GDS-S score | 1.5 ± 1.9 | 1.4 ± 1.7 | 1.8 ± 2.6 | 1.5 ± 2.1 |
Abbreviations: Ino→Mild (Inosine dosed to mildly elevate urate), Ino→Mod (Inosine dosed to moderately elevate urate), MoCA (Montreal Cognitive Assessment), GDS-S (Geriatric Depression Scale-short), dx (diagnosis), UPDRS (Unified Parkinson’s Disease Rating Scale), MAO-B (monoamine oxidase B). Values presented as mean ± SD except where noted.
A third (24) of the participants completed 2 years of follow-up, one participant withdrew from the study after 9 months (see Figure 1), and the remaining 50 concluded follow-up early after 8 to 23 months in order to complete all follow-up visits by November 2012. All but the one subject who withdrew consent completed the Safety Visit one month after study drug discontinuation. Median pre-Safety Visit follow-up was 18 months.
SAFETY
Oral inosine dosed to elevate serum urate to the targeted levels appeared safe. A total of 17 SAEs were reported, all after randomization, among 15 participants (Table 2A); no participant died. Only musculoskeletal events differed substantially among treatment arms (comparison-wise p = 0.019), and they occurred only among placebo participants. Similarly, time to first SAE was shorter among placebo participants (eFigure 1, eTable 1).
Table 2.
Serious Adverse Events and Adverse Events of Special Concern in SURE-PD.
| Adverse Events (AEs)a | Placebod | Ino → Mildd | Ino → Modd | Overalld |
|---|---|---|---|---|
| A) Serious AEs | ||||
| Cardiac | 1 (4%) | 0 | 1 (4%) | 2 (3%) |
| Acute coronary syndrome | 1 (4%) | 0 | 0 | 1 (1%) |
| Coronary artery disease | 0 | 0 | 1 (4%) | 1 (1%) |
| Hepatobiliary / Cholecystitis | 0 | 0 | 1 (4%) | 1 (1%) |
| Infections and Infestations | 2 (8%) | 1 (4%) | 0 | 3 (4%) |
| Human ehrlichiosis | 0 | 1 (4%) | 0 | 1 (1%) |
| Pneumonia | 1 (4%) | 0 | 0 | 1 (1%) |
| Urosepsis | 1 (4%) | 0 | 0 | 1 (1%) |
| Injury / Cervical Fracture | 0 | 1 (4%) | 0 | 1 (1%) |
| Musculoskeletal | 4 (16%) | 0 | 0 | 4 (5%) |
| Arthritis | 3 (12%) | 0 | 0 | 3 (4%) |
| Synovial Cyst | 1 (4%) | 0 | 0 | 1 (1%) |
| Nervous System | 2 (8%) | 0 | 0 | 2 (3%) |
| Cerebrovascular accident | 1 (4%) | 0 | 0 | 1 (1%) |
| Radiculopathy | 1 (4%) | 0 | 0 | 1 (1%) |
| Psychiatric | 1 (4%) | 0 | 1 (4%) | 2 (3%) |
| Depression | 1 (4%) | 0 | 0 | 1 (1%) |
| Suicide ideation | 0 | 0 | 1 (4%) | 1 (1%) |
| Renal / Nephrolithiasis | 0 | 0 | 1 (4%) | 1 (1%) |
| Respiratory / Pulmon. fibrosis | 1 (4%) | 0 | 0 | 1 (1%) |
| Overall | 11 (36%) | 2 (8%) | 4 (15%) | 17 (20%) |
| B) AEs of Special Concern | ||||
| Cardiovascular | 5 (16%) | 0 | 1 (4%) | 6 (7%) |
| Acute coronary syndrome | 1 (4%) | 0 | 0 | 1 (1%) |
| Atrial fibrillation | 1 (4%) | 0 | 0 | 1 (1%) |
| Cerebrovascular accident | 1 (4%) | 0 | 0 | 1 (1%) |
| Coronary artery disease | 0 | 0 | 1 (4%) | 1 (1%) |
| Palpitations | 1 (4%) | 0 | 0 | 1 (1%) |
| Tachycardia | 1 (4%) | 0 | 0 | 1 (1%) |
| Gout-like Symptoms | 1 (4%) | 3 (8%) | 2 (7%) | 6 (7%) |
| Arthralgia of toe(s) b | 1 (4%) | 2 (4%) | 1 (4%) | 4 (5%) |
| Swelling of toe(s) c | 0 | 1 (4%) | 1 (4%) | 2 (3%) |
| Urolithiasis or its Symptoms | 0 | 2 (2%) | 2 (2%) | 4 (5%) |
| Hematuria | 0 | 1 (4%) | 0 | 1 (1%) |
| Nephrolithiasis | 0 | 1 (4%) | 2 (7%) | 3 (4%) |
| Overall | 6 (20%) | 5 (17%) | 5 (19%) | 16 (19%) |
MedDRA (Medical Dictionary for Regulatory Activities) system organ class and preferred terms.
Arthralgia of toes combines the following preferred terms in instances where the verbatim complaints mentioned feet or toes: arthralgia and pain in extremity.
Swelling of toes combines the following preferred terms in instances where the verbatim complaints mentioned feet or toes: joint swelling and local swelling.
Values show total number of events (% of participants).
Among 259 AEs (including the SAEs) of any type, most were judged mild or unrelated to study medication. Of the 38 AEs that were either moderate or severe in intensity and at least possibly related to study drug, none showed a statistically significant difference among treatment arms. Analyses of overall AE and SAE rates (eTable 1) also showed no evidence of general safety concerns after 27,876 person-days cumulative exposure to inosine at urate-elevating doses (see below).
Some AEs were of specific concern (Table 2B), including episodes of symptomatic urolithiasis in three subjects. These were only reported in women after more than 4 months on study drug and may have been dose-dependent (0, 1 and 2 events in placebo, mild and moderate groups, respectively [eTable 1]). Need for alkalinization was rare because urine pH was unaffected by inosine (eTables 2 and 3). Urine collected at each visit was also assessed for the presence of various crystals, and their potential use in monitoring inosine-induced urolithiasis risk was investigated (eTable 4). Although no crystal type was predictive of urolithiasis, uric acid crystals were observed in urine from ten participants with a dose-dependent distribution (0 placebo, 3 mild, 7 moderate). The one subject who developed a documented symptomatic uric acid stone (after 14 months of inosine in the moderate urate elevation arm) had tested positive for uric acid crystalluria and had relatively low urine pH hovering at 5.5 (just above the trigger for alkalinization). Stones in two other participants were likely not uric acid because the composition of one was documented as ‘65% calcium oxalate dihydrate + 35% carbonate apatite’, and the other though not analyzed was from a subject whose urine pH was around 6.5, which is usually incompatible with uric acid stone formation.
Secondary safety outcomes, including those associated with hyperuricemia,18 did not differ between treatment groups. For example, serial vital signs, serum assays and ECGs showed no effect of inosine on blood pressure (eTables 5 and 6), body mass index (BMI; eTable 7), serum glucose and cholesterol (eTables 8), or electrocardiographic parameters (eTable 9). Similarly, despite the increased frequency of urolithiasis on inosine, there were no other renal SAEs and renal function measures of glomerular filtration rate and serum creatinine remained unchanged from baseline in all groups (data not shown).
TOLERABILITY
Inosine as administered was well tolerated (Figure 2A). Five participants (3 randomized to placebo and 2 to mild elevation) permanently discontinued study drug (Figure 1) and 10 temporarily suspended study drug (2 on placebo, 3 on low inosine and 5 on high inosine), including one who ultimately discontinued permanently. Greater than 95% (73) of the 75 participants were tolerant of study drug at 6 months in all treatment groups, with lower confidence bounds well above the 30% threshold pre-specified19 for judging a treatment sufficiently tolerable to justify continued study of oral inosine. Kaplan-Meier estimates of two-year tolerance were greater than 90% with the lowest observed rate among placebo-treated participants.
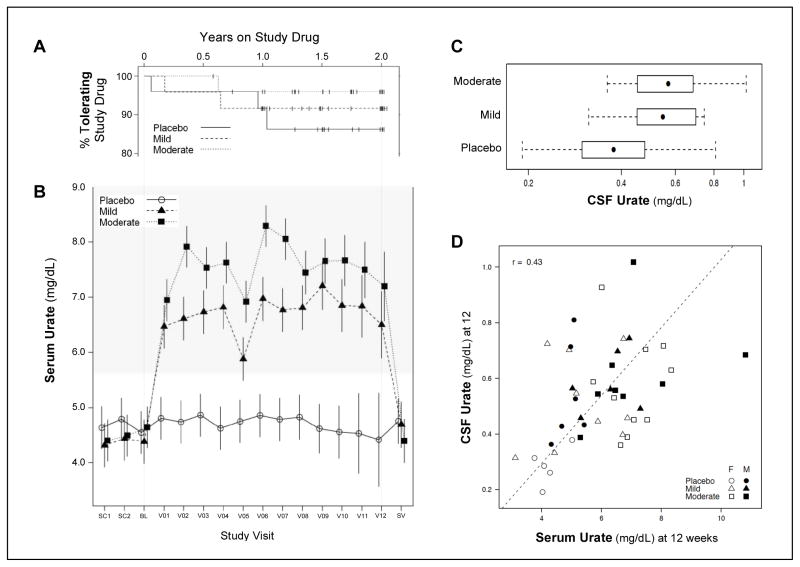
Figure 2. Tolerability of inosine and its effects on serum and CSF urate.
A, Tolerability of study drug from baseline to drug discontinuation displayed as Kaplan-Meier survival curves over the maximum two-year period for participants taking placebo, or inosine dosed to mildly or moderately raise serum urate. Tick marks indicate B, Estimated time course of serum urate levels across study visits with study drug initiated at the baseline (BL) visit and continued for as long as 24 months (V12) until one month before the final (safety) visit (SV). Means and 95% confidence intervals from a mixed model are displayed. For visits V1-V12, serum was collected after morning study drug intake, except for the ‘trough’ sample at week 12 (V05). The shaded range of serum urate concentrations represents exclusionary values at the screening visits (SC1 and SC2). C, CSF urate concentrations and ranges (bars; with boxes and dots representing the interquartile and median values, respectively) after 12 weeks on study drug. P < 0.001 for Mild and Moderate inosine groups compared to placebo. D, Correlation between CSF and serum urate at the 12-week visit for individuals identified by their treatment groups and gender.
URATE ELEVATION
Participants randomized to mild or moderate elevation treatment arms were titrated to an average inosine dose of 1.18 or 1.51 gm/day,19 and achieved average increases in serum urate of 2.3 and 3.0 mg/dL, respectively (Figure 2B and eTables 10 and 11; p<0.001). Serum urate levels were significantly elevated above placebo as soon as the 2-week visit (V01, Figure 2B). They were relatively constant starting at the 2-week visit among those in the mild elevation group and continued to rise during titration until the 4-week visit (V02) among those in the moderate elevation group. The 12-week visit (V05) was the only one for which participants were asked not to take their study drug beforehand, accounting for the apparent dip in serum urate at the time of this trough measurement. Serum urate had fully reverted to baseline levels by the time of the safety visit, one month after discontinuation of study drug. Increases in serum urate were observed in both women and men, although the increase was slightly greater in women (eFigure 2) consistent with their lower mean baseline values, as expected.
CSF urate levels were measured once (at the 12-week visit) in 44 (59%) of the participants. The others did not consent to lumbar puncture (29%) or lumbar punctures were contraindicated (e.g., participants on warfarin; 4%) or were attempted but failed (7%). Among those measured, levels were 40% and 50% higher in mild and moderate elevation treatment groups, respectively, relative to placebo participants (p=0.006 and p<0.001, respectively; Figure 2C and eTables 12 and 13). There was evidence of a difference by gender. CSF urate levels were lower among female than male placebo participants, and were significantly elevated in the active arms relative to placebo only among female participants. Twelve-week serum and CSF urate levels in women and men were modestly correlated (r = 0.43, Figure 2D).
SECONDARY ANALYSES
Although not powered to determine the effects of inosine on long-term changes in clinical measures, preliminary data were collected. Time to need for dopaminergic therapy, which was the primary endpoint in DATATOP10 and PRECEPT9, was reached in 47 (63%) of the randomized participants during the study and did not differ significantly among the treatment groups (Figure 3A; eTable 14).
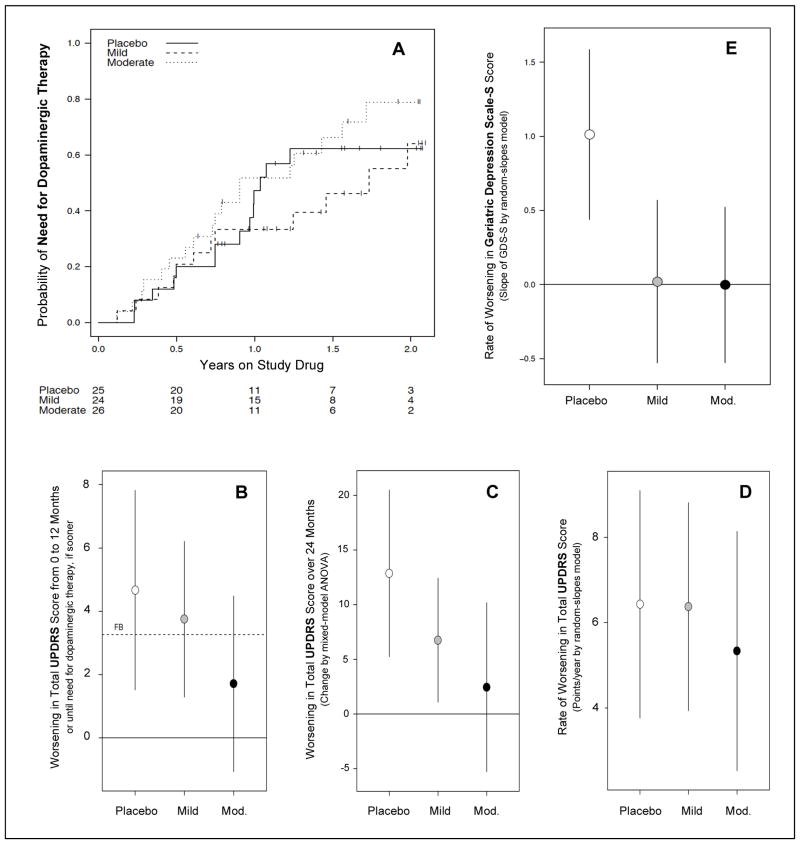
Figure 3. Secondary analyses of clinical outcomes in SURE-PD.
A, Kaplan-Meier curves showing time to disability warranting dopaminergic therapy for up to two years of follow-up for each of the three treatment groups. B, Futility analysis of the change in total UPDRS scores over 12 months or until need for dopaminergic therapy, based on NET-PD methodology.25,26 Much or most of the 95% confidence interval for the Mild or Moderate inosine treatment groups, respectively, falls below the futility boundary (FB) defined as 70% of the placebo group’s mean rate of change. C, 24-month change of total UDPRS score estimated from a mixed model ANOVA allowing unstructured profiles over time suggests a trend of decreasing rate with increasing inosine dose. D, A weaker trend is observed when employing a complementary random-slopes model incorporating gender-specific effects and assuming linearity in change over time. E, Rates of mood change during the study as assessed by differences in GDS-S scores over an average of 18 months follow-up. On either dose of inosine the rate appears slower (comparison-wise p < 0.001) compared to placebo.
Plausible efficacy of serum urate elevation to delay symptomatic progression was preliminarily assessed using a futility analysis approach equivalent to that employed for the primary analysis in the National Institutes of Health Exploratory Trials in PD (NET-PD) program25,26 except that the active groups were compared to our own placebo group rather than to historical controls as in NET-PD26. The two active groups were compared to a futility boundary (FB) calculated as 70% of the estimated progression among our placebo participants over 12 months. Both mild and moderate elevation treatments were non-futile based on this comparison for six parkinsonism (sub)scales (eTable 15) including total UPDRS scores, which worsened at an average rate of 1.7 points/year for participants in the moderate elevation treatment group compared to 4.7 points/year for those on placebo (Figure 3B; eTable 15).
To reduce the bias introduced by carrying forward the last UPDRS score for participants who developed need for dopaminergic treatment before the end of the observation period, we also employed two random-slopes models with follow-up truncated at the time of dopaminergic therapy initiation: one with no treatment x gender interaction but allowing unstructured profiles over time (i.e., separate treatment x visit estimates; Figure 3C; eTable 16) and one including gender-specific effects of treatment but assuming linear trends in symptom scores over time (Figure 3D; eTable 17). Like the futility analysis, these complementary approaches suggested attenuated clinical progression with increasing inosine doses, although the treatment differences were not significant.
As demonstration of disease modification by putative neuroprotectants in PD is simpler when not confounded by symptomatic effects, we estimated the effects of inosine on parkinsonian features and disability during gradual wash-in of study drug (from baseline to week 12) and abrupt wash-out (from study drug discontinuation to the safety visit one month later). Neither active treatment demonstrated an acute symptomatic change during either wash-in or wash-out based on UPDRS (parts I-III), Schwab & England, or modified Hoehn & Yahr scores (eTable 18).
There was no evidence of an effect of active treatment on cognitive function as assessed by MoCA Rasch scores27 (eTable 19), although only non-demented individuals were enrolled and the placebo group showed no cognitive decline during the study. Mood as assessed on the GDS-S worsened slightly on average during the trial only among placebo participants, suggesting a possible preventative effect on depression of urate-elevating inosine (Figure 3E and eTable 19; comparison-wise p <0.001 for each inosine group versus placebo), although only three participants had scores outside the normal range by the end of follow-up (2 placebo participants and 1 moderate elevation participant).
DISCUSSION
The results of the SURE-PD trial demonstrate that oral inosine treatment in early PD is clinically safe, tolerable, and produces an increase in serum and CSF urate. Participants comprised recently diagnosed PD patients at greater risk of clinical and radiographic progression of PD based on having a serum urate below the population median of 6 mg/dL.11,12 In this population we found that treatment with inosine for up to 24 months was clinically safe and well tolerated at doses that elevated serum urate concentrations from a mean of 4.5 mg/dL to 6–7 and 7–8 mg/dL in the two dosing regimens. In observational studies,11,12 these higher but still relatively normal urate levels were predictive of favorable outcomes in PD. The present findings support the development of a more definitive trial to investigate the ability of inosine treatment to slow clinical progression among persons with early PD who have lower urate.
We did not observe any increase in risk of SAEs associated with urate elevation in this population, the oldest to date to be chronically exposed to urate-elevating treatment. Our data strengthen the evidence against a hypertensive effect of urate elevation by inosine28 and do not support the contention that chronically elevated urate contributes to the hypertensive, hyperglycemic, dyslipidemic and obesity components of metabolic syndrome,18 or to other cardiovascular disease29 associated with higher urate. Although overall safety of urate-elevating inosine treatment of 50 participants for an average of 1.5 years appeared at least as good as that of control participants, a small or delayed increase in risk of SAEs related to the cardiovascular system remains a possibility.
By contrast, the risk of urate-related crystallopathies increases with increasing urate concentration in blood or urine. Our findings suggest that these risks can be adequately managed for inosine treatment. Although no participant developed gout during the study, symptomatic urolithiasis did occur in three inosine-treated participants, one of whom had a documented uric acid stone. Exploratory data suggest that monitoring for both uric acid crystal formation and urine acidity in addition to close monitoring of serum urate may further reduce the risk of urolithiasis related to inosine treatment.
The results provide proof-of-principle of the ability of oral inosine to raise urate to concentrations in CSF (>0.50 mg/dL) and serum (>6.0 mg/dL) predictive of slower disease progression in prior studies.11,12 This chronic ‘target engagement’ in relevant peripheral and central nervous system compartments at safe and tolerable doses of inosine greatly strengthens the rationale for conducting disease modification studies using the higher dosing regimen for inosine. Whereas our findings support the safety of raising serum urate elevation to either 6.1–7.0 or 7.1–8.0 mg/dL ranges, the latter was associated with a slower rate of clinical11,12 and particularly radiographic11 decline in prior PD studies.
Refinements to the dose titration regimen employed here should take into account our findings that the extent of the actual urate elevation is influenced by gender and the timing of serum sampling relative to dosing. The capacity to increase urate may be related to gender, with women in our trial having achieved greater increases in both serum and CSF because they had lower values than men at baseline (i.e., with women enrolling with mean serum urate levels 0.5 mg/dL lower than those in men, whereas all participants were titrated to the same target ranges). Dosing was tied to urate levels in serum collected at random times after the morning dose. Based on pharmacokinetic data from the trial,19 these sample urate values likely were close to peak levels, explaining why they exceeded trough values by ~1.0 mg/dL (Figure 2B). These data, suggesting trough sampling may provide an improved approach to approximating the intended target ranges for serum urate, illustrate the broader value19 of incorporating the experience gained in the SURE-PD trial into the design of future clinical trials of inosine.
Preliminary assessments of the effects of inosine on clinical outcomes over 8 to 24 months further support clinical development of inosine in PD. A UPDRS-based futility analysis has been employed by the NET-PD program in the past[?] to decide on the value of advancing leading candidate neuroprotectants to full efficacy trials.25,26 For example, a long-term phase 3 PD trial of creatine30 was developed based on its demonstration of non-futility compared to historical UPDRS progression data.26 In the present study, inosine dosed to mildy or moderately elevate urate also suggested non-futility by equivalent NET-PD methodology except for our use of matched contemporary rather than historical controls, albeit with a small sample size. Similarly, efficacy analyses that incorporated UPDRS data over two years in SURE-PD corroborate the suggestion of a dose-dependent attenuation of clinical decline by inosine. Data on time-to-disability did not indicate delayed disability among participants on inosine although power for this secondary analysis was minimal. Interestingly, treatment with inosine appeared to prevent slight worsening of depressive symptoms during the trial, a finding that if substantiated could strengthen the long-standing theory31 and early evidence32 of enhanced motivation as the basis for urate elevation during human evolution.
The SURE-PD trial provides strong evidence that long-term administration of oral inosine can be generally safe and well tolerated by early PD patients, and increases both serum and CSF urate in a dose-dependent fashion. Secondary analyses suggest that a disease-modifying benefit of inosine is plausible. Together with previous findings, these of the present study support a more definitive trial of inosine as a potential treatment to slow the clinical progression of PD.
Acknowledgments
We are grateful for the invaluable contributions of the study participants and their families, as well as for the key contributions and dedication of the
Data and Safety Monitoring Committee Members: Caroline M. Tanner (chair), Bruce Levin, Grace S. Liang and Nina E. Tolkoff-Rubin;
Michael J. Fox Foundation for Parkinson’s Research (MJFF) staff and leadership serving as grantor/non-voting members of the Steering Committee: Brian K. Fiske, Alison Urkowitz and Todd B. Sherer;
Project Advisors to the MJFF: Jeffrey M. Bronstein and David M. Weiner;
Clinical Materials Services Unit (www.clinicalmaterial.com) of the Center of Human Experimental Therapeutics at the University of Rochester Medical Center: Patrick Bolger, Tim Hackett, Cornelia Kamp, Ellen Weinberger and Joan Woodcook; and
Other contributing clinical site staff including Linda Baldwin (deceased).
Funding/Support: This project was funded by a grant from the Michael J. Fox Foundation for Parkinson’s Research. The views, findings and opinions expressed in this publication are those of the authors and do not necessarily represent those of the MJFF. Additional support was provided by the National Institutes of Health (K24NS060991), Harvard NeuroDiscovery Center, the RJG Foundation and the Parkinson’s Disease Foundation’s Advancing Parkinson’s Therapies initiative. No funder had a role in the collection, management, analysis or interpretation of the data; or in the preparation, review or approval of the manuscript. No funder was involved in the design and conduct of the study, other than through the provisions of funds for the study.
Appendix. Authored by The Parkinson Study Group SURE-PD Investigators
Steering committee members
Alberto Ascherio, MD, DrPH (Harvard School of Public Health, Boston, MA; co-chair)
M. Flint Beal, MD (Cornell University / New York, NY)
Merit E. Cudkowicz, MD (Massachusetts General Hospital, Boston, MA)
Gary C. Curhan, MD (Brigham and Women’s Hospital, Boston, MA)
Joshua M. Hare, MD (University of Miami, Miami, FL)
D. Craig Hooper, PhD (Thomas Jefferson University, Philadelphia, PA)
Karl Kieburtz, MD (University of Rochester, Rochester, NY)
Eric A. Macklin, PhD (Massachusetts General Hospital, Boston] (lead statistician)
David Oakes, PhD (University of Rochester, Rochester, NY)
Alice Rudolph, PhD (University of Rochester, Rochester, NY)
Michael A. Schwarzschild, MD, PhD (Massachusetts General Hospital, Boston, MA; chair)
Ira Shoulson, MD (Georgetown University, Washington, DC)
Marsha K. Tennis, RN (Peterborough, NH)
Participating Site Investigators and Coordinators
University of Cincinnati, Cincinnati, OH: Alberto Espay, MD, MSc, Maureen Gartner, RN, M.Ed;
Massachusetts General Hospital, Boston, MA: Albert Hung, MD, PhD, Grace Bwala, MBBS;
Scott & White Hospital/Texas A&M University, Temple, TX: Richard Lenehan, MD, Elmyra Encarnacion, MD, Melissa Ainslie, Richard Castillo;
University of Southern California, Los Angeles, CA: Daniel Togasaki, MD, PhD, Gina Barles;
Butler Hospital, Providence, RI: Joseph Friedman, MD, Lisa Niles, MS;
Oregon Health & Science University, Portland, OR: Julie Carter, RN, MN, ANP, Megan Murray, MA;
Rush University Medical Center, Chicago, IL: Christopher Goetz, MD, Jeana Jaglin, RN, CCRC;
Cleveland Clinic, Cleveland, OH: Anwar Ahmed, MD;
Institute of Neurodegenerative Disorders, New Haven, CT: David Russell, MD, PhD, Candace Cotto, RN;
Michigan State University, East Lansing, MI: John Goudreau, DO, PhD, Doozie Russell;
Struthers Parkinson’s Center, Golden Valley, MN: Sotirios Parashos, MD, PhD, Patricia Ede, RN;
Boston University, Boston, MA: Marie Saint-Hilaire, MD, Cathi-Ann Thomas, RN, MS, Raymond James;
Duke University, Durham, NC: Mark Stacy, MD, Julia Johnson, MD, Lisa Gauger, BA;
Eastern Connecticut Neurology Specialists, Manchester, CT: Antonelle Demarcaida, MD, Sheila Thurlow, MSN, BSN;
Parkinson’s Disease & Movement Disorder Center of Boca Raton, Boca Raton, FL: Stuart Isaacson, MD, Lisbeth Carvajal;
Ochsner Clinic Foundation, New Orleans, LA: Jayaraman Rao, MD, Maureen Cook, RN, BSN, Charlise Hope-Porche, RN.
Other participating Associate Members of the PSG
Lauren McClurg, Daniela Grasso and Robert Logan, MS (Administrative Coordination Center; Massachusetts General Hospital, Boston, MA)
Constance Orme, BA, Tori Ross, Alicia Brocht, Radu Constantinescu, MD, Saloni Sharma, MBBS, Charles Venuto, PharmD, Joe Weber and Ken Eaton (Clinical Coordination Center; University of Rochester, Rochester, NY).
Footnotes
Online-only Material: The eFigures and eTables are available at http://www.jamaneuro.com.
The full text as published in JAMA Neurology can be found at http://archneur.jamanetwork.com/article.aspx?articleid=1790169
Author Contributions:
Study concept and design: Ascherio, Kieburtz, Macklin, Schwarzschild.
Acquisition of data: All participating clinical site coordinators and investigators, and all participating Associate Members of the PSG.
Analysis and interpretation of data: All Steering Committee members.
Drafting of the manuscript: Ascherio, Macklin, Schwarzschild.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Macklin.
Obtaining funding: Ascherio, Kieburtz, Macklin, Schwarzschild.
Administrative, technical, and material support: All participating Associate Members of the PSG, and all participating clinical site coordinators and investigators.
Study supervision: All Steering Committee members.
Macklin and Schwarzschild had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Authors’ potential conflicts of interest: None of direct relevance to the drug development of inosine, the potential therapy under study. Note that in accordance with conflict of interest policy of Parkinson Study Group (http://www.parkinson-study-group.org/parkinson-research/constitution-and-bylaws) all SURE-PD steering committee members, site investigators and site coordinators could have no financial relationship with any involved company during the study, and provided signed attestation annually to this effect. Although the study received no commercial support, Kyowa Hakko U.S.A., Inc., its affiliate Kyowa Pharmaceutical, Inc. and their parent company Kyowa Hakko Kirin Co., Ltd. were designated as the only ‘involved companies’. The designations were based on the use of Kyowa Hakko U.S.A. as the vendor from which inosine was obtained (as the active pharmaceutical ingredient for study drug manufacture) through an unsubsidized retail purchase.
References
- 1.Ames BN, Cathcart R, Schwiers E, et al. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci U S A. 1981;78(11):6858–62. doi: 10.1073/pnas.78.11.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies KJ, Sevanian A, Muakkassah-Kelly SF, et al. Uric acid-iron ion complexes. A new aspect of the antioxidant functions of uric acid. Biochem J. 1986;235(3):747–54. doi: 10.1042/bj2350747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cipriani S, Desjardins CA, Burdett TC, et al. Urate and its transgenic depletion modulate neuronal vulnerability in a cellular model of Parkinson’s disease. PLoS One. 2012;7(5):e37331. doi: 10.1371/journal.pone.0037331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gong L, Zhang QL, Zhang N, et al. Neuroprotection by urate on 6-OHDA-lesioned rat model of Parkinson’s disease: linking to Akt/GSK3β signaling pathway. J Neurochem. 2012 Dec;123(5):876–85. doi: 10.1111/jnc.12038. [DOI] [PubMed] [Google Scholar]
- 5.Chen X, Burdett TC, Desjardins CA, et al. Disrupted and transgenic urate oxidase alter urate and dopaminergic neurodegeneration. Proc Natl Acad Sci U S A. 2013 Jan 2;110(1):300–5. doi: 10.1073/pnas.1217296110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis JW, Grandinetti A, Waslien CI, et al. Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol. 1996;144(5):480–4. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- 7.de Lau LM, Koudstaal PJ, Hofman A, et al. Serum uric acid levels and the risk of Parkinson disease. Ann Neurol. 2005;58(5):797–800. doi: 10.1002/ana.20663. [DOI] [PubMed] [Google Scholar]
- 8.Weisskopf MG, O’Reilly E, Chen H, et al. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166(5):561–7. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parkinson Study Group PRECEPT Investigators. Mixed lineage kinase inhibitor CEP-1347 fails to delay disability in early Parkinson disease. Neurology. 2007 Oct 9;69(15):1480–90. doi: 10.1212/01.wnl.0000277648.63931.c0. [DOI] [PubMed] [Google Scholar]
- 10.The Parkinson Study Group. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med. 1993 Jan 21;328(3):176–83. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- 11.Schwarzschild MA, Schwid SR, Marek K, et al. Serum urate as a predictor of clinical and radiographic progression in Parkinson disease. Arch Neurol. 2008;65(6):716–23. doi: 10.1001/archneur.2008.65.6.nct70003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ascherio A, LeWitt PA, Xu K, et al. Urate predicts rate of clinical decline in Parkinson disease. Arch Neurol. 2009;66(12):1460–8. doi: 10.1001/archneurol.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto T, Moriwaki Y, Cheng J, et al. Effect of inosine on the plasma concentration of uridine and purine bases. Metabolism. 2002 Apr;51(4):438–42. doi: 10.1053/meta.2002.31322. [DOI] [PubMed] [Google Scholar]
- 14.Spitsin S, Hooper DC, Leist T, et al. Inactivation of peroxynitrite in multiple sclerosis patients after oral administration of inosine may suggest possible approaches to therapy of the disease. Mult Scler. 2001 Oct;7(5):313–9. doi: 10.1177/135245850100700507. [DOI] [PubMed] [Google Scholar]
- 15.Toncev G. Therapeutic value of serum uric acid levels increasing in the treatment of multiple sclerosis. Vojnosanit Pregl. 2006 Oct;63(10):879–82. doi: 10.2298/vsp0610879t. [DOI] [PubMed] [Google Scholar]
- 16.Markowitz CE, Spitsin S, Zimmerman V, et al. The treatment of multiple sclerosis with inosine. J Altern Complement Med. 2009 Jun;15(6):619–25. doi: 10.1089/acm.2008.0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonsette RE, Sindic C, D’hooghe MB, et al. ASIIMS study group. Boosting endogenous neuroprotection in multiple sclerosis: the ASsociation of Inosine and Interferon beta in relapsing- remitting Multiple Sclerosis (ASIIMS) trial. Mult Scler. 2010 Apr;16(4):455–62. doi: 10.1177/1352458509360547. [DOI] [PubMed] [Google Scholar]
- 18.Soltani Z, Rasheed K, Kapusta DR, Reisin E. Potential role of uric acid in metabolic syndrome, hypertension, kidney injury, and cardiovascular diseases: is it time for reappraisal? Curr Hypertens Rep. 2013 Jun;15(3):175–81. doi: 10.1007/s11906-013-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macklin EA, et al. Safety of urate elevation in Parkinson’s disease (SURE-PD): clinical trial design and implementation. [in preparation] [Google Scholar]
- 20. [accessed 9/25/2013]; http://clinicaltrials.gov/show/NCT00833690.
- 21.Cameron MA, Sakhaee K. Uric acid nephrolithiasis. Urol Clin North Am. 2007 Aug;34(3):335–46. doi: 10.1016/j.ucl.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 22.Siderowf A, McDermott M, et al. Test-retest reliability of the Unified Parkinson Disease Rating Scale in patients with early Parkinson disease: results from a multicenter clinical trial. Mov Disord. 2002;17:758–763. doi: 10.1002/mds.10011. [DOI] [PubMed] [Google Scholar]
- 23.Zadikoff C, Fox SH, Tang-Wai DF, Thomsen T, de Bie RM, Wadia P, Miyasaki J, Duff-Canning S, Lang AE, Marras C. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23:297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 24.Schrag A, Barone P, Brown RG, Leentjens AF, McDonald WM, Starkstein S, Weintraub D, Poewe W, Rascol O, Sampaio C, Stebbins GT, Goetz CG. Depression rating scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2007;22:1077–1092. doi: 10.1002/mds.21333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tilley BC, Palesch YY, Kieburtz K, et al. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66:628–633. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- 26.NINDS NET-PD Investigators. A randomized, double-blind, futility clinical trial of creatine and minocycline in early Parkinson disease. Neurology. 2006 Mar 14;66(5):664–71. doi: 10.1212/01.wnl.0000201252.57661.e1. [DOI] [PubMed] [Google Scholar]
- 27.Koski L, Xie H, Finch L. Measuring cognition in a geriatric outpatient clinic: Rasch analysis of the Montreal Cognitive Assessment. J Geriatr Psychiatry Neurol. 2009 Sep;22(3):151–60. doi: 10.1177/0891988709332944. [DOI] [PubMed] [Google Scholar]
- 28.Spitsin S, Markowitz CE, Zimmerman V, Koprowski H, Hooper DC. Modulation of serum uric acid levels by inosine in patients with multiple sclerosis does not affect blood pressure. J Hum Hypertens. 2010 May;24(5):359–62. doi: 10.1038/jhh.2009.83. [DOI] [PubMed] [Google Scholar]
- 29.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med. 2008 Oct 23;359(17):1811–21. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elm JJ NINDS NET-PD Investigators. Design innovations and baseline findings in a long-term Parkinson’s trial: the National Institute of Neurological Disorders and Stroke Exploratory Trials in Parkinson’s Disease Long-Term Study-1. Mov Disord. 2012 Oct;27(12):1513–21. doi: 10.1002/mds.25175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orowan E. Origin of Man. Nature. 1955 Apr 16;175(4459):683–4. doi: 10.1038/175683a0. [DOI] [PubMed] [Google Scholar]
- 32.Kasl SV, Brooks GW, Rodgers WL. Serum uric acid and cholesterol in achievement behavior and motivation. JAMA. 1970 Aug 17;213(7):1158–64. Aug 24 213(8):1291–9. [PubMed] [Google Scholar]