Abstract
Glycan microarrays have become indispensable tools for studying protein-glycan interactions. Along with chemo-enzymatic synthesis, glycans isolated from natural sources have played important roles in array development and will continue to be a major source of glycans. N- and O-glycans from glycoproteins, and glycans from glycosphingolipids can be released from corresponding glycoconjugates with relatively mature methods, although isolation of large numbers and quantities of glycans are still very challenging. Glycosylphosphatidylinositol (GPI)-anchors and glycosaminoglycans (GAGs) are less represented on current glycan microarrays. Glycan microarray development has been greatly facilitated by bifunctional fluorescent linkers, which can be applied in a “Shotgun Glycomics” approach to incorporate isolated natural glycans. Glycan presentation on microarrays may affect glycan binding by GBPs, often through multivalent recognition by the GBP.
Introduction
Glycoproteins, proteoglycans, and glycolipids within the glycocalyx, defined as the assortment of complex glycoconjugates on the plasma membrane and associated with the surface of an animal cell, are involved in myriad molecular interactions. Functional Glycomics is the systematic study of the structurally and functionally important glycans, which often involve glycan-binding proteins (GBPs) that recognize specific glycan sequences and thereby “decode” the complex structural information in glycans. A major technical breakthrough in glycosciences that facilitated decoding glycan functions was the development of printed glycan microarrays, in which many defined glycan structures are simultaneously presented to GBPs or microorganisms including viruses and bacteria. While the applications of glycan microarrays have been extensively reviewed in the last decade [1–5], this article provides some highlights and perspectives of the chemistry, current challenges and promises of natural glycan microarray technology.
Glycan libraries for glycan microarrays
Glycan microarrays are essentially a simultaneous presentation of a library of defined glycans in a resolvable pattern for the purpose of defining binding specificities of GBPs. GBP specificities are determined by comparing binding to all glycans presented on the microarray, including bound and unbound glycans and deciphering the key glycan determinants associated with the highest degree of binding [4]. Therefore, the number and diversity of defined glycans on a microarray is paramount for this application. It has been estimated that the human glycome has >7,000 glycan recognition determinants comprised of penta- and hexasaccharide sequences and their modifications, e.g. phosphorylation, sulfation, etc. [6]. The full potential of defined glycan microarrays will only be realized when the libraries of glycans used for their production are comprised of sufficient glycans to represent the complete glycomes of an organism, tissue, or cell. The chemistries related to glycan synthesis, the harvesting of natural glycans, and their subsequent immobilization to generate arrays are important for technology development.
Chemo-enzymatic synthesis versus isolation and separation from natural sources
A major key to the future of functional glycomics is the expansion of the glycan libraries for glycan microarray production. Extensive progress has been made in the synthesis of glycans by development of protection/deprotection reactions of saccharide building blocks, anomeric activation and glycosidic bond formation with stereochemistry control. Glycan synthesis has been significantly improved in terms of pace and throughput using glycosyltransferases from a variety of sources, and many structurally challenging glycans have been synthesized [7]. In fact, the majority of the glycans incorporated into the CFG glycan microarray of the Consortium for Functional Glycomics (CFG) (www.functionalglycomics.org/), the largest publicly-available glycan microarray and associated database, arose from chemo-enzymatic synthesis. As additional chemical reactions and enzymes become available, this approach holds great promise in the future development of glycan microarrays.
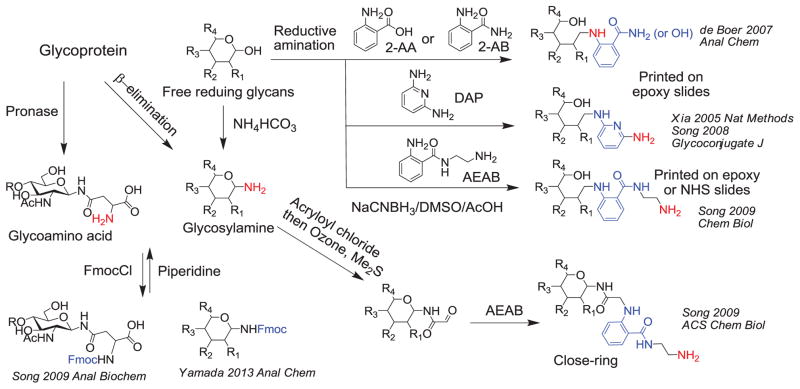
Despite these advances [8], the glycan synthetic approaches lag far behind the explosive need for more complex and biologically relevant glycans for biomedical research; the synthesis of any individual complex glycan is still challenging. More importantly, synthesis is target-driven, but often the actual biological target is unknown and can only be defined after significant structural and functional studies on the natural products. Therefore, natural glycans represent the most imminent and significant resource for Functional Glycomics, and it is logical to isolate natural glycans and immobilize them on glycan microarrays (Fig. 1). Thus, it is now possible to utilize a wide variety of natural glycans for microarray development [9–13] (Fig. 2). Fluorescent tags such as 2-aminobenzamide (2-AB), 2-aminobenzoic acid (2-AA) [9] and 2,6-diaminopyridine [14,15] are especially useful in this regard, since they allow sensitive detection of glycans; however, less-reactive aromatic amines restricts immobilization of these conjugates to only epoxy-derivatized slides. The 2-amino-N-(2-aminoethyl)benzamide (AEAB) label is a more versatile, bifunctional fluorescent tag for labeling free reducing glycans, and the reactive primary amine is suitable for most coupling reactions through a wide variety of chemistries [13]. The sensitivity of fluorescent tags for detection and the efficient coupling of the primary amine allow access to even extremely complex glycans for functional studies. Thus, in spite of their low quantities and expense, hundreds of commercially available natural glycans can now be easily incorporated into glycan microarrays. Furthermore, mixtures of natural glycans that have been chemically or enzymatically released from natural sources can be labeled with AEAB and directly separated and purified by multi-dimensional HPLC [12]. Cleavable, fluorescent tags such as fluorenylmethyloxycarbonyl (Fmoc) have also been used to tag amino-functionalized glycans, either glycoamino acids [10] or glycosylamines [16]. This cleavable linkage strategy has recently been developed to simply regenerate free reducing glycans from reductively aminated conjugates of 2-AA, 2AB, and AEAB [17]. These chemistries provide the versatility required for generating glycan microarrays from synthetic and natural glycans and for removing the tags from natural conjugates to simplify their structural analysis in a Shotgun Glycomics approach [11,18], where detailed structural analysis is reserved for functionally relevant glycans identified by glycan microarray analysis [19].
Fig. 1.

The generation of a natural glycan microarray. Glycans from various natural glycoconjugates on cells/tissues/organs can be extract, tagged and separated to individual glycans. These natural glycans can be immobilized onto solid surfaces to prepare natural glycan microarrays. These arrays are invaluable tools for the elucidation of GBP binding specificities.
Fig. 2.
The functional fluorescent tagging of natural glycans for glycan microarray preparation. In the reductive amination route, several fluorescent tags, 2-AA, 2-AB, DAP and AEAB have been used. The aromatic amines of 2-AA, 2-AB and DAP can only be printed on epoxy slides to obtain acceptable immobilization efficiency while the alkylamine of AEAB can react with both epoxy and NHS slides efficiently. A route to prepare closering glycan-AEAB conjugates was also provided. Fmoc can serve as a cleavable fluorescent tag for amino groups in both glycosylamines (generated from free reducing sugar or β-elimination of O-glycans from glycoproteins) and glycoamino acids (generated by exhaustive pronase digestion) to facilitate isolation, characterization and quantification. The Fmoc tag can be removed and the exposed amino groups can be used for solid phase immobilization.
De novo synthesis and isolation of glycans from natural sources are very different approaches to obtain glycans but can be complementary. In the near future natural glycans may be a more convenient source for the development of extensive glycan microarrays. In certain cases, natural glycans can be obtained in sufficient amounts to serve as substrates for enzymatic modification. This strategy was applied to the synthesis of mannose-6-phosphate N-glycans [20] and modified sialic acids [21] on complex glycans to produce specialized glycan microarrays that were very useful for analyzing M6P receptors [22,23], lectins, and viruses. For biologically active glycans with known structures, synthesis is likely to be the favored approach to provide enough material for detailed functional studies; however, it is also important to generate structural analogs of glycans with directed modifications that may lead to further insight into biological recognition and provide translational opportunities as inhibitors or activators of GBPs and enzymes that modify glycans.
N- and O-glycans from glycoproteins
Glycoprotein-derived glycans have received much attention in biomedical studies, in terms of both developmental changes in structure and expression, as well as alterations in disease processes largely due to the availability of enzymes such as PNGase F (N-glycanase) [24–26] and PNGase A [27] that effect their release. Hydrazinolysis [28,29], the classic chemical method to release N-glycans, is used primarily for analytical purposes. Both of these processes generate free reducing glycans for further derivatizations and subsequent modification using the recently described asymmetric chemo-enzymatic synthesis to produce novel complex N-glycans [8]. Another simple, inexpensive approach utilizes Pronase to digest glycoproteins, generating single Asn-linked glycans [10] that can be temporarily protected with Fmoc to facilitate detection for HPLC purification and MS analysis. The purified Fmoc labeled glycans can then be regenerated to glycoamino acids or glycopeptides for printing microarrays.
While O-glycans are generally released using β-elimination under basic conditions, and often with reducing reagents, e.g. sodium borohydride, to limit glycan degradation, recent methods have been developed for milder and more efficient O-glycan release [30–33]. Currently, O-glycans from natural sources are less studied on microarrays, presumably due to the lack of efficient and specific releasing methods. No general O-glycanases are available, and O-glycans released using chemical methods are often highly contaminated with N-glycans. A specific, efficient method to release reducing O-glycans would greatly enrich current glycan libraries for producing microarrays.
Glycosphingolipid-related glycans
Glycosphingolipids (GSLs) can be isolated from cells and tissues, and purified GSLs can be printed onto nitrocellulose-coated glass slides, similar to neoglycolipids [34]. Recently GSLs have been fluorescently labeled by insertion of a small group into the sphingosine moiety followed by addition of an amino function to effect covalent attachment to a microarray [11], where the anomeric configuration of the reducing monosaccharide and polar head group of the ceramide are retained. The release of free glycans from GSLs for fluorescent labeling, separation and microarray preparation can be accomplished using ceramidases [35]; however, these enzymes are expensive and their specificities are not sufficiently broad for use in general procedures. Ozonolysis to oxidize the double bond present in the sphingosine moiety of most ceramides, followed by base-catalyzed β-elimination was traditionally used to release glycans from GSL, but this method is best used in the presence of NaBH4 to reduce the free glycan and prevent alkaline degradation. It was recently shown that glycans can be released from ozonized GSL without strong alkali [36] and this may be the method of choice for preparing libraries of free glycans from GLS in the future.
Glycans derived from glycosaminoglycans
Glycosaminoglycans (GAGs) represent the most challenging class of glycans for study due to their complexity. Although GAG chains are linear, they are heterogeneous with respect to sulfation and highly negatively charged, making the structural characterization and chemical/enzymatic synthesis challenging. A theoretical calculation estimated thousands of possible structural determinants for pentasaccharides and this number grows exponentially with chain length [6]. There has been limited work on glycan microarrays incorporating GAGs and GAG fragments, which contained only limited numbers of synthetic structures, small oligomers such as di- and tetra-saccharide or larger, but less defined fractions isolated from natural sources [37–39]. Although these glycan microarrays have shown great success, there has been no large-scale GAG microarray for general screening of GAG-binding proteins. Based on new developments in chemical synthesis of GAGs and many new chromatographic developments on the separation of GAG oligosaccharides, including ion-exchange HPLC, reverse phase ion-paring HPLC and continuous elution polyacrylamide gel electrophoresis [40–42], it is likely that more comprehensive GAG microarrays will become available in the near future.
Other special classes of glycans, such as GPI-anchors [43] and polysaccharides from bacteria and plants [44,45], have been less studied. Again the paucity of information is directly related to the lack of appropriate methods to obtain and characterize these glycans. As the field of glycosciences expands to support the rapidly growing interest in functional glycomics, glycan microarrays will continue to play an important role in the definition of GBP specificities, and immobilized glycans coupled to a variety of solid surfaces will be used to discover novel GBPs that have not yet been identified. While generating significant quantities of the diverse sets of glycans that comprise the glycomes of cells, tissues, and organisms remains a challenge, efficient derivatizations and coupling chemistries must be areas of continued investigation.
Presentation of glycans on glycan microarrays
The analyses of hundreds of GBPs on the CFG defined glycan microarray over the past decade have generated interesting and paradigm-changing data (www.functionalglycomics.org under ‘CFG paradigm pages’ and ‘CFG Library’). For example, previously influenza viruses were thought to simply distinguish α2–6 from α2–3 sialylated glycans, but glycan microarray studies indicated that each virus strain has specific and unique glycan specificity, and that sialic acid and its linkages are necessary, but not sufficient, for high affinity and specific binding [46–49]. Similarly, earlier studies had suggested that rotaviruses generally recognized sialic acid-containing residues, but studies sparked by glycan microarray analyses led to the identification of non-sialylated ligands that bind specific types of rotaviruses, and crystal structures confirmed the specificity [50]. Interestingly and perhaps not surprisingly, not all GBPs applied to the CFG microarray are bound. With over 600 glycans being interrogated on the array, it is tempting to presume that most of the important glycans are present. However, considering the size of the glycome, the most probable reason for GBPs not binding to an array is the absence of appropriate ligands. An alternative explanation is that lack of GBP binding is due to improper presentation of the glycans. The issue of glycan presentation may actually raise more questions than it answers, which is not surprising since we know so little about how glycans are presented in nature. Nevertheless, it is clear that presentation of glycans on a microarray is an important factor in the binding of a GBP.
Glycan presentation on microarrays involves two major parameters, the solid surface immobilization chemistry and the linker/spacer/carrier of glycans, which are often confused with each other. Currently there are several platforms being used for printing glycan microarrays, described below. Since most glycan arrays are printed on commercial proprietary slides, we know very little about their surface coating, manufacturing processes, or the quality control processes that ensure reproducible products. Various mechanisms for immobilizing glycans on activated solid surfaces are shown in Fig. 3. While many of them are based on covalent attachment, others rely on hydrophobic or other interaction for non-covalent attachment. Theoretically any reactions that generate covalent linkages and non-covalent interactions that are strong enough to hold biomolecules together under normal experimental conditions can be used to prepare glycan microarrays. Each immobilizing method provides unique advantages over others, but there is little systematic experimental data showing the effects of these surfaces on glycan array performance.
Fig. 3.

The various chemistry/physical immobilization strategies for glycan microarray, including covalent and non-covalent attachment. Covalent immobilization requires efficient conjugation between amino groups and NHS ester or epoxy; alkyne and azide; sulfhydryl and maleimide; ene and diene. Non-covalent attachment includes fluorous-fluorous interaction, lipid-assisted hydrophobic interaction, and the annealing power of oligonucleotides.
The microarray from the CFG is based on the printing of amino functionalized glycans onto NHS-activated surfaces that are commercially available and has been shown to be relatively consistent and reliable. Other types of surfaces or platforms used for printing of glycan microarrays include epoxy-activated slides [9,14], fluorous surface-derivatized slides [51,52], and nitrocellulose-coated slides [53–55], and it should not be surprising that certain platforms are more suitable than others under certain circumstances. Preliminary studies showed that platforms differ in a number of properties including printing efficiency, spot morphology, sensitivity and signal/noise ratio. Such differences are likely due to the nature of the reactions; for example, an amine reacting with an NHS-ester generates a natural and inert amide linkage, while its reaction with an epoxide is likely faster, generating a less inert secondary imine. Although careful examination of glycan microarrays printed on either platform generate valid results in terms of GBP specificities [56], the direct comparison of signals from two different platforms is often misleading. In addition to epoxy and NHS, there are other covalent attachments applied to the preparation of glycan microarrays [1] as shown in Fig. 3, including sulfhydryl-maleimide and alkyne-azide or “click” reactions. It is likely that all of these products show slight variations in the actual microarray experimental results. Printing platforms such as nitrocellulose [34,57,58] and fluorous slides [59,60] present more complex chemistry/physics in glycan immobilization, generally thought to be through hydrophobic interactions requiring addition of a lipid to the glycans that normally lack them. A potential merit of nitrocellulose membranes is the existence of three-dimensional structure, compared to the two-dimensional surface of other glass surfaces. However, since little is known about most of the glass surfaces and good cross-platform comparisons are not available, this advantage has not been conclusively confirmed. DNA-directed immobilization (DDI) of glycans developed recently shows great potential [61–64]. This approach takes advantage of the annealing specificity of DNA oligomers to immobilize glycans attached with a DNA oligomer. The current DDI array incorporates a very limited number of glycans, presumably due to the technical challenge in preparation, purification and characterization of glycan-oligonucleotide conjugates.
The other parameters affecting GBP binding to glycans on a microarray surface is the nature of the linker or coupling structure between the reducing end of the glycan and the coated surface. Minor changes in linkers have resulted in significant differences in GBP binding on the CFG glycan array [65] as well as certain natural glycan arrays. In many cases these differences are observed for relatively small glycans, where the determinant required for binding is close to the linker [12]. While these observations raise concerns about array fabrication, they are for the most part only observed empirically and insufficient data are available to predict linker behavior.
Glycan-BSA conjugates have been used to print glycan microarrays, and such conjugates probably present glycans in a more dense arrangement than direct covalent coupling, thus increasing the potential avidity toward GBPs and enhancing detection of lower affinity binding. Such properties may be advantageous in some situations [66,67], but for general screening, this approach might lead to confusing data when attempting to decipher GBP specificities of individual glycans; i.e., it might promote detection of low affinity cross reactions that might not be biologically relevant. In addition, glycan-BSA conjugates are structurally less defined, varied in density, and difficult to quality control. Incorporating a polymer/dendrimer between glycan and solid surfaces has been applied in the preparation of glycan microarrays [68–71], and these arrays significantly increased glycan density and avidity of GBP binding in a well-defined construct. Other approaches to increasing glycan density and avidity of GBP binding include self-assembled monolayers [72–74] and fluidic glycan microarrays with glycans embedded in a supported lipid bilayer [75]. Clearly, multiple types of defined presentations of glycans are needed in the future and should help to generate hypotheses about the physiological recognition determinants required for GBP interactions.
Conclusions
Defined glycan microarrays are generally used to determine the glycan-binding specificity of GBPs, and the effectiveness of any array is more dependent on the number and diversity of the glycans on the array than other parameters such as density or presentation. Since little is known about the presentation of glycans in nature, it is not possible to define a single best presentation or density of glycans on an array. Nevertheless, it will certainly be important to carry out well-designed cross-platform comparisons, but such comparisons must be performed using identical libraries of glycan species and large assortments of GBPs. Expanding the number and diversity of glycans on microarrays to represent animal glycomes is clearly an important objective and may best be accomplished in the near future by using natural glycan sources. Development of simple, reliable chemical methods to harvest natural glycans for printing arrays, expanding the application of Shotgun Glycomics, and defining selected glycomes, along with chemo-enzymatic synthesis to help refine the molecular nature of these protein-glycan interactions, should be major priorities in the glycosciences.
Highlights.
Natural glycan microarray is a crucial technology for protein/glycan interactions
Expanding natural glycan libraries is the most important task for the field
Chemo-enzymatic synthesis applied to natural glycans will expand glycan array diversity
Presentation of glycans could affect sensitivity of detection of GBP binding
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- *1.Rillahan CD, Paulson JC. Glycan microarrays for decoding the glycome. Annu Rev Biochem. 2011;80:797–823. doi: 10.1146/annurev-biochem-061809-152236. A recent general review for the field of glycan arrays. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin I, Tae J, Park S. Carbohydrate microarray technology for functional glycomics. Curr Chem Biol. 2007;1:187–199. [Google Scholar]
- 3.Oyelaran O, Gildersleeve JC. Glycan arrays: recent advances and future challenges. Curr Opin Chem Biol. 2009;13:406–413. doi: 10.1016/j.cbpa.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith DF, Song X, Cummings RD. Use of glycan microarrays to explore specificity of glycan-binding proteins. Methods Enzymol. 2010;480:417–444. doi: 10.1016/S0076-6879(10)80033-3. [DOI] [PubMed] [Google Scholar]
- 5.de PJL, Seeberger PH. Recent advances and future challenges in glycan microarray technology. Methods Mol Biol (N Y, NY, U S) 2012;808:1–12. doi: 10.1007/978-1-61779-373-8_1. [DOI] [PubMed] [Google Scholar]
- *6.Cummings RD. The repertoire of glycan determinants in the human glycome. Mol Biosyst. 2009;5:1087–1104. doi: 10.1039/b907931a. A review with many glycan determinant structures and perspective analysis on glycomics. [DOI] [PubMed] [Google Scholar]
- 7.Hsu C-H, Hung S-C, Wu C-Y, Wong C-H. Toward Automated Oligosaccharide Synthesis. Angew Chem, Int Ed. 2011;50:11872–11923. doi: 10.1002/anie.201100125. [DOI] [PubMed] [Google Scholar]
- *8.Wang Z, Chinoy ZS, Ambre SG, Peng W, McBride R, de VRP, Glushka J, Paulson JC, Boons G-J. A General Strategy for the Chemoenzymatic Synthesis of Asymmetrically Branched N-Glycans. Science (Washington, DC, U S) 2013;341:379–383. doi: 10.1126/science.1236231. A most recent achievement by chemists on chemoenzymatic synthesis of glycans. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *9.de Boer AR, Hokke CH, Deelder AM, Wuhrer M. General microarray technique for immobilization and screening of natural glycans. Anal Chem. 2007;79:8107–8113. doi: 10.1021/ac071187g. A description of the application of 2-AA and 2-AB tagged glycans for glycan microarray preparation on epoxy slides. [DOI] [PubMed] [Google Scholar]
- *10.Song X, Lasanajak Y, Rivera-Marrero C, Luyai A, Willard M, Smith DF, Cummings RD. Generation of a natural glycan microarray using 9-fluorenylmethyl chloroformate (FmocCl) as a cleavable fluorescent tag. Anal Biochem. 2009;395:151–160. doi: 10.1016/j.ab.2009.08.024. A description of the combination of pronase digestion and Fmoc-tagging for the isolation of natural N-glycans for microarray printing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **11.Song X, Lasanajak Y, Xia B, Heimburg-Molinaro J, Rhea JM, Ju H, Zhao C, Molinaro RJ, Cummings RD, Smith DF. Shotgun glycomics: a microarray strategy for functional glycomics. Nat Methods. 2011;8:85–90. doi: 10.1038/nmeth.1540. The introduction of the concept of “Shotgun Glycomics” which demonstrates its utility using a new fluorescent derivatization of glycosphingolipids. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song X, Lasanajak Y, Xia B, Smith DF, Cummings RD. Fluorescent Glycosylamides Produced by Microscale Derivatization of Free Glycans for Natural Glycan Microarrays. ACS Chem Biol. 2009;4:741–750. doi: 10.1021/cb900067h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **13.Song X, Xia B, Stowell SR, Lasanajak Y, Smith DF, Cummings RD. Novel Fluorescent Glycan Microarray Strategy Reveals Ligands for Galectins. Chem Biol (Cambridge, MA, U S) 2009;16:36–47. doi: 10.1016/j.chembiol.2008.11.004. The introduction of a novel bifunctional linker that is ideal for natural glycan microarray preparation due to its selective chemical conjugation and efficient solid phase immobilization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song X, Xia B, Lasanajak Y, Smith DF, Cummings RD. Quantifiable fluorescent glycan microarrays. Glycoconjugate J. 2008;25:15–25. doi: 10.1007/s10719-007-9066-8. [DOI] [PubMed] [Google Scholar]
- *15.Xia B, Kawar ZS, Ju T, Alvarez RA, Sachdev GP, Cummings RD. Versatile fluorescent derivatization of glycans for glycomic analysis. Nat Methods. 2005;2:845–850. doi: 10.1038/nmeth808. The introduction of bifunctional fluorescent tagging for potential usages in glycomics. [DOI] [PubMed] [Google Scholar]
- 16.Yamada K, Hirabayashi J, Kakehi K. Analysis of O-glycans as 9-fluorenylmethyl derivatives and its application to the studies on glycan array. Anal Chem. 2013;85:3325–3333. doi: 10.1021/ac303771q. [DOI] [PubMed] [Google Scholar]
- *17.Song X, Johns BA, Ju H, Lasanajak Y, Zhao C, Smith DF, Cummings RD. Novel Cleavage of Reductively Aminated Glycan-Tags by N-Bromosuccinimide to Regenerate Free, Reducing Glycans. ACS Chem Biol. 2013 doi: 10.1021/cb400513k. The introduction of a new and simple chemical method to cleave all reductively aminated glycan tag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Diepen A, Smit CH, van Egmond L, Kabatereine NB, Pinot de Moira A, Dunne DW, Hokke CH. Differential anti-glycan antibody responses in Schistosoma mansoni-infected children and adults studied by shotgun glycan microarray. PLoS Negl Trop Dis. 2012;6:e1922. doi: 10.1371/journal.pntd.0001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *19.Yu Y, Mishra S, Song X, Lasanajak Y, Bradley KC, Tappert MM, Air GM, Steinhauer DA, Halder S, Cotmore S, et al. Functional Glycomic Analysis of Human Milk Glycans Reveals the Presence of Virus Receptors and Embryonic Stem Cell Biomarkers. J Biol Chem. 2012;287:44784–44799. doi: 10.1074/jbc.M112.425819. An application of the “Shotgun Glycomics” concept to the human milk glycome and demonstration of the utility of this strategy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *20.Song X-Z, Lasanajak Y, Olson LJ, Boonen M, Dahms NM, Kornfeld S, Cummings RD, Smith DF. Glycan Microarray Analysis of P-type Lectins Reveals Distinct Phosphomannose Glycan Recognition. J Biol Chem. 2009;284:35201–35214. doi: 10.1074/jbc.M109.056119. Natural glycans and enzymatic modifications were combined to synthesize natural Mannose-6-Phosphate glycans that are difficult to access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Song X, Yu H, Chen X, Lasanajak Y, Tappert MM, Air GM, Tiwari VK, Cao H, Chokhawala HA, Zheng H, et al. A sialylated glycan microarray reveals novel interactions of modified sialic acids with proteins and viruses. J Biol Chem. 2011;286:31610–31622. doi: 10.1074/jbc.M111.274217. Natural glycans and enzymatic modifications were combined to synthesize glycans modified with various sialic acids that are difficult to access. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Castonguay AC, Lasanajak Y, Song X, Olson LJ, Cummings RD, Smith DF, Dahms NM. The glycan-binding properties of the cation-independent mannose 6-phosphate receptor are evolutionary conserved in vertebrates. Glycobiology. 2012;22:983–996. doi: 10.1093/glycob/cws058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohnsack RN, Song X, Olson LJ, Kudo M, Gotschall RR, Canfield WM, Cummings RD, Smith DF, Dahms NM. Cation-independent mannose 6-phosphate receptor: a composite of distinct phosphomannosyl binding sites. J Biol Chem. 2009;284:35215–35226. doi: 10.1074/jbc.M109.056184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plummer TH, Jr, Elder JH, Alexander S, Phelan AW, Tarentino AL. Demonstration of peptide:N-glycosidase F activity in endo-beta-N-acetylglucosaminidase F preparations. J Biol Chem. 1984;259:10700–10704. [PubMed] [Google Scholar]
- 25.Plummer TH, Jr, Tarentino AL. Purification of the oligosaccharide-cleaving enzymes of Flavobacterium meningosepticum. Glycobiology. 1991;1:257–263. doi: 10.1093/glycob/1.3.257. [DOI] [PubMed] [Google Scholar]
- 26.Tarentino AL, Gomez CM, Plummer TH., Jr Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985;24:4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- 27.Plummer TH, Jr, Tarentino AL. Facile cleavage of complex oligosaccharides from glycopeptides by almond emulsin peptide: N-glycosidase. J Biol Chem. 1981;256:10243–10246. [PubMed] [Google Scholar]
- 28.Takasaki S, Mizuochi T, Kobata A. Hydrazinolysis of asparagine-linked sugar chains to produce free oligosaccharides. Methods Enzymol. 1982;83:263–268. doi: 10.1016/0076-6879(82)83019-x. [DOI] [PubMed] [Google Scholar]
- 29.Patel TP, Parekh RB. Release of oligosaccharides from glycoproteins by hydrazinolysis. Methods Enzymol. 1994;230:57–66. doi: 10.1016/0076-6879(94)30007-0. [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Mechref Y, Novotny MV. Microscale nonreductive release of O-linked glycans for subsequent analysis through MALDI mass spectrometry and capillary electrophoresis. Anal Chem. 2001;73:6063–6069. doi: 10.1021/ac015534c. [DOI] [PubMed] [Google Scholar]
- *31.Miura Y, Kato K, Takegawa Y, Kurogochi M, Furukawa J, Shinohara Y, Nagahori N, Amano M, Hinou H, Nishimura S. Glycoblotting-assisted O-glycomics: ammonium carbamate allows for highly efficient o-glycan release from glycoproteins. Anal Chem. 2010;82:10021–10029. doi: 10.1021/ac101599p. The most recent β-elimination method for free reducing O-glycan release. [DOI] [PubMed] [Google Scholar]
- *32.Wang C, Fan W, Zhang P, Wang Z, Huang L. One-pot nonreductive O-glycan release and labeling with 1-phenyl-3-methyl-5-pyrazolone followed by ESI-MS analysis. Proteomics. 2011;11:4229–4242. doi: 10.1002/pmic.201000677. A one-pot combination of β-elimination and PMP-conjugation for efficient release and tagging for O-glycan analysis. [DOI] [PubMed] [Google Scholar]
- *33.Zauner G, Koeleman CA, Deelder AM, Wuhrer M. Mass spectrometric O-glycan analysis after combined O-glycan release by beta-elimination and 1-phenyl-3-methyl-5-pyrazolone labeling. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbagen.2011.07.004. A combination of β-elimination and PMP-conjugation for O-glycan analysis. [DOI] [PubMed] [Google Scholar]
- 34.Liu Y, Childs RA, Palma AS, Campanero-Rhodes MA, Stoll MS, Chai W, Feizi T. Neoglycolipid-based oligosaccharide microarray system: preparation of NGLs and their noncovalent immobilization on nitrocellulose-coated glass slides for microarray analyses. Methods Mol Biol (N Y, NY, U S) 2012;808:117–136. doi: 10.1007/978-1-61779-373-8_8. [DOI] [PubMed] [Google Scholar]
- 35.Manzi AE. Enzymatic release of oligosaccharides from glycolipids. Curr Protoc Mol Biol. 2001;Chapter 17(Unit17):17A. doi: 10.1002/0471142727.mb1717as32. [DOI] [PubMed] [Google Scholar]
- *36.Song X, Smith DF, Cummings RD. Nonenzymatic release of free reducing glycans from glycosphingolipids. Anal Biochem. 2012;429:82–87. doi: 10.1016/j.ab.2012.06.029. An efficient method to release free reducing glycans without enzyme and base treatment. [DOI] [PubMed] [Google Scholar]
- 37.de PJL, Spillmann D, Seeberger PH. Microarrays of heparin oligosaccharides obtained by nitrous acid depolymerization of isolated heparin. Chem Commun (Cambridge, U K) 2006:3116–3118. doi: 10.1039/b605318a. [DOI] [PubMed] [Google Scholar]
- 38.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA, III, Nishi A, Hsieh-Wilson LC. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- *39.Rogers CJ, Clark PM, Tully SE, Abrol R, Garcia KC, Goddard WA, 3rd, Hsieh-Wilson LC. Elucidating glycosaminoglycan-protein-protein interactions using carbohydrate microarray and computational approaches. Proc Natl Acad Sci U S A. 2011;108:9747–9752. doi: 10.1073/pnas.1102962108. One of the limited GAG microarray studies demonstrating the utility of this method. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo YC, Conrad HE. Analysis of oligosaccharides from heparin by reversed-phase ion-pairing high-performance liquid chromatography. Anal Biochem. 1988;168:54–62. doi: 10.1016/0003-2697(88)90009-7. [DOI] [PubMed] [Google Scholar]
- 41.Laremore TN, Ly M, Solakyildirim K, Zagorevski DV, Linhardt RJ. High-resolution preparative separation of glycosaminoglycan oligosaccharides by polyacrylamide gel electrophoresis. Anal Biochem. 2010;401:236–241. doi: 10.1016/j.ab.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rice KG, Kim YS, Grant AC, Merchant ZM, Linhardt RJ. High-performance liquid chromatographic separation of heparin-derived oligosaccharides. Anal Biochem. 1985;150:325–331. doi: 10.1016/0003-2697(85)90518-4. [DOI] [PubMed] [Google Scholar]
- *43.Kamena F, Tamborrini M, Liu X, Kwon YU, Thompson F, Pluschke G, Seeberger PH. Synthetic GPI array to study antitoxic malaria response. Nat Chem Biol. 2008;4:238–240. doi: 10.1038/nchembio.75. Although only a handful of synthetic structures, this demonstrates the utility of a GPI glycan microarray. [DOI] [PubMed] [Google Scholar]
- 44.Wang D, Liu S, Trummer BJ, Deng C, Wang A. Carbohydrate microarrays for the recognition of cross-reactive molecular markers of microbes and host cells. Nat Biotechnol. 2002;20:275–281. doi: 10.1038/nbt0302-275. [DOI] [PubMed] [Google Scholar]
- 45.Pedersen HL, Fangel JU, McCleary B, Ruzanski C, Rydahl MG, Ralet M-C, Farkas V, Schantz Lv, Marcus SE, FAMC, et al. Versatile High Resolution Oligosaccharide Microarrays for Plant Glycobiology and Cell Wall Research. J Biol Chem. 2012;287:39429–39438. doi: 10.1074/jbc.M112.396598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci U S A. 2004;101:17033–17038. doi: 10.1073/pnas.0407902101. The fundamental work for the glycan microarray of the Consortium of Functional Glycomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulati S, Smith DF, Cummings RD, Couch RB, Griesemer SB, St George K, Webster RG, Air GM. Human H3N2 Influenza Viruses Isolated from 1968 To 2012 Show Varying Preference for Receptor Substructures with No Apparent Consequences for Disease or Spread. PLoS One. 2013;8:e66325. doi: 10.1371/journal.pone.0066325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stevens J, Blixt O, Paulson JC, Wilson IA. Glycan microarray technologies: tools to survey host specificity of influenza viruses. Nat Rev Microbiol. 2006;4:857–864. doi: 10.1038/nrmicro1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walther T, Karamanska R, Chan RW, Chan MC, Jia N, Air G, Hopton C, Wong MP, Dell A, Malik Peiris JS, et al. Glycomic analysis of human respiratory tract tissues and correlation with influenza virus infection. PLoS Pathog. 2013;9:e1003223. doi: 10.1371/journal.ppat.1003223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Hu L, Crawford SE, Czako R, Cortes-Penfield NW, Smith DF, Le Pendu J, Estes MK, Prasad BV. Cell attachment protein VP8* of a human rotavirus specifically interacts with A-type histo-blood group antigen. Nature. 2012;485:256–259. doi: 10.1038/nature10996. A paradigm changing example of glycan microarray analysese leading to the identification of new ligands for rotaviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *51.Pohl NL. Fluorous tags catching on microarrays. Angew Chem Int Ed Engl. 2008;47:3868–3870. doi: 10.1002/anie.200704801. The DNA-directed immobilzation provides a unique attachment that may greatly increase the capacity of glycan microarrays. [DOI] [PubMed] [Google Scholar]
- 52.Ko KS, Jaipuri FA, Pohl NL. Fluorous-based carbohydrate microarrays. J Am Chem Soc. 2005;127:13162–13163. doi: 10.1021/ja054811k. [DOI] [PubMed] [Google Scholar]
- 53.Feizi T, Chai W. Oligosaccharide microarrays to decipher the glyco code. Nat Rev Mol Cell Biol. 2004;5:582–588. doi: 10.1038/nrm1428. [DOI] [PubMed] [Google Scholar]
- 54.Feizi T, Fazio F, Chai W, Wong CH. Carbohydrate microarrays - a new set of technologies at the frontiers of glycomics. Curr Opin Struct Biol. 2003;13:637–645. doi: 10.1016/j.sbi.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 55.Fukui S, Feizi T, Galustian C, Lawson AM, Chai W. Oligosaccharide microarrays for high-throughput detection and specificity assignments of carbohydrate-protein interactions. Nat Biotechnol. 2002;20:1011–1017. doi: 10.1038/nbt735. [DOI] [PubMed] [Google Scholar]
- 56.Padler-Karavani V, Song X, Yu H, Hurtado-Ziola N, Huang S, Muthana S, Chokhawala HA, Cheng J, Verhagen A, Langereis MA, et al. Cross-comparison of Protein Recognition of Sialic Acid Diversity on Two Novel Sialoglycan Microarrays. J Biol Chem. 2012;287:22593–22608. doi: 10.1074/jbc.M112.359323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang D. Carbohydrate antigen microarrays. Methods Mol Biol (N Y, NY, U S) 2012;808:241–249. doi: 10.1007/978-1-61779-373-8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feizi T, Chai W. Innovation: Oligosaccharide microarrays to decipher the glyco code. Nat Rev Mol Cell Biol. 2004;5:582–588. doi: 10.1038/nrm1428. [DOI] [PubMed] [Google Scholar]
- 59.Edwards HD, Nagappayya SK, Pohl NLB. Probing the limitations of the fluorous content for tag-mediated microarray formation. Chem Commun (Cambridge, U K) 2012;48:510–512. doi: 10.1039/c1cc16022b. [DOI] [PubMed] [Google Scholar]
- 60.Nagappayya SK, Pohl NLB. Production of fluorous-based microarrays with uncharged carbohydrates. Methods Mol Biol (N Y, NY, U S) 2012;808:149–153. doi: 10.1007/978-1-61779-373-8_10. [DOI] [PubMed] [Google Scholar]
- *61.Chevolot Y, Bouillon C, Vidal S, Morvan F, Meyer A, Cloarec J-P, Jochum A, Praly J-P, Vasseur J-J, Souteyrand E. DNA-based carbohydrate biochips: a platform for surface glyco-engineering. Angew Chem, Int Ed. 2007;46:2398–2402. doi: 10.1002/anie.200604955. The DNA-directed immobilzation provides a unique attachment that may greatly increase the capacity of glycan microarrays. [DOI] [PubMed] [Google Scholar]
- 62.Gerland B, Goudot A, Pourceau G, Meyer A, Dugas V, Cecioni S, Vidal S, Souteyrand E, Vasseur J-J, Chevolot Y, et al. Synthesis of a library of fucosylated glycoclusters and determination of their binding toward Pseudomonas aeruginosa lectin B (PA-IIL) using a DNA-based carbohydrate microarray. Bioconjugate Chem. 2012;23:1534–1547. doi: 10.1021/bc2006434. [DOI] [PubMed] [Google Scholar]
- 63.Goudot A, Pourceau G, Meyer A, Gehin T, Vidal S, Vasseur J-J, Morvan F, Souteyrand E, Chevolot Y. Quantitative analysis (Kd and IC50) of glycoconjugates interactions with a bacterial lectin on a carbohydrate microarray with DNA Direct Immobilization (DDI) Biosens Bioelectron. 2013;40:153–160. doi: 10.1016/j.bios.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 64.Morvan F, Chevolot Y, Zhang J, Meyer A, Vidal S, Praly J-P, Vasseur J-J, Souteyrand E. Glycoarray by DNA-directed immobilization. Methods Mol Biol (N Y, NY, U S) 2012;808:195–219. doi: 10.1007/978-1-61779-373-8_14. [DOI] [PubMed] [Google Scholar]
- 65.Grant OC, Smith HM, Firsova D, Fadda E, Woods RJ. Presentation, presentation, presentation! Molecular-level insight into linker effects on glycan array screening data. Glycobiology. 2014;24:17–25. doi: 10.1093/glycob/cwt083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Li Q, Rodriguez LG, Gildersleeve JC. An Array-Based Method To Identify Multivalent Inhibitors. J Am Chem Soc. 2010;132:9653–9662. doi: 10.1021/ja100608w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Oyelaran O, Li Q, Farnsworth D, Gildersleeve JC. Microarrays with Varying Carbohydrate Density Reveal Distinct Subpopulations of Serum Antibodies. J Proteome Res. 2009;8:3529–3538. doi: 10.1021/pr9002245. This paper demonstrates that mutivalent presentation of glycans increase the detection sensitivity of GBPs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Branderhorst HM, Ruijtenbeek R, Liskamp RMJ, Pieters RJ. Multivalent carbohydrate recognition on a glycodendrimer-functionalized flow-through chip. ChemBioChem. 2008;9:1836–1844. doi: 10.1002/cbic.200800195. [DOI] [PubMed] [Google Scholar]
- 69.Parera PN, Branderhorst HM, Kooij R, Maierhofer C, van dKM, Liskamp RMJ, Wittmann V, Ruijtenbeek R, Pieters RJ. Rapid Screening of Lectins for Multivalency Effects with a Glycodendrimer Microarray. ChemBioChem. 2010;11:1896–1904. doi: 10.1002/cbic.201000340. [DOI] [PubMed] [Google Scholar]
- 70.Godula K, Rabuka D, Nam KT, Bertozzi CR. Synthesis and microcontact printing of dual end-functionalized mucin-like glycopolymers for microarray applications. Angew Chem Int Ed Engl. 2009;48:4973–4976. doi: 10.1002/anie.200805756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Godula K, Bertozzi CR. Synthesis of Glycopolymers for Microarray Applications via Ligation of Reducing Sugars to a Poly(acryloyl hydrazide) Scaffold. J Am Chem Soc. 2010;132:9963–9965. doi: 10.1021/ja103009d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tantakitti F, Burk-Rafel J, Cheng F, Egnatchik R, Owen T, Hoffman M, Weiss DN, Ratner DM. Nanoscale Clustering of Carbohydrate Thiols in Mixed Self-Assembled Monolayers on Gold. Langmuir. 2012;28:6950–6959. doi: 10.1021/la300444h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Huang K-T, Gorska K, Alvarez S, Barluenga S, Winssinger N. Combinatorial Self-Assembly of Glycan Fragments into Microarrays. ChemBioChem. 2011;12:56–60. doi: 10.1002/cbic.201000567. [DOI] [PubMed] [Google Scholar]
- 74.Zhi Z-L, Powell AK, Turnbull JE. Fabrication of Carbohydrate Microarrays on Gold Surfaces: Direct Attachment of Nonderivatized Oligosaccharides to Hydrazide-Modified Self-Assembled Monolayers. Anal Chem. 2006;78:4786–4793. doi: 10.1021/ac060084f. [DOI] [PubMed] [Google Scholar]
- 75.Zhu XY, Holtz B, Wang Y, Wang L-X, Orndorff PE, Guo A. Quantitative Glycomics from Fluidic Glycan Microarrays. J Am Chem Soc. 2009;131:13646–13650. doi: 10.1021/ja902783n. [DOI] [PMC free article] [PubMed] [Google Scholar]