Abstract
This protocol describes a high through put colorimetric method that relies on the formation of a complex between iodine and chains of glucose molecules in starch. Iodine forms complexes with both amylose and long chains within amylopectin. After the addition of iodine to a starch sample, the maximum absorption of amylose and amylopectin occurs at 620 and 550 nm, respectively. The amylose/amylopectin ratio can be estimated from the ratio of the 620 and 550 nm absorbance values and comparing them to a standard curve in which specific known concentrations are plotted against absorption values. This high throughput, inexpensive method is reliable and reproducible, allowing the evaluation of large populations of potato clones.
Keywords: Chemistry, Issue 80, Technology, Industry, and Agriculture, Life Sciences (General), Potato, amylose, amylopectin, colorimetric assay, iodine
Introduction
Approximately 80% of the fresh weight of a potato tuber is water; nearly all of the remaining dry matter is starch1. Most of the starch (70%) is composed of amylopectin, while the remainder is amylose. The ratio between amylose and amylopectin is the most important property influencing the physical properties of starch. Amylose is a linear alpha 1-4 glucose chain, while amylopectin is a linear alpha 1-4 chain with alpha 1-6 branches2. Methods such as iodine binding, differential scanning calorimetry (DSC), high performance size exclusion chromatography (HPSEC) and concanavalin A interactions have been developed for starch determination in different crop species3,4. Each protocol requires special skills and equipment, making it difficult to scale up to quantify numerous samples at a time. The method we present here is a modified protocol from Hovenkamp-Hermelink et al.5 which is a dual wavelength iodine binding method based on stained starch granules. The advantages of this method versus others include the amylose determination from raw starch without purifying it and the use of the dual wavelength system to increase the precision of the method4,6.
Protocol
1. Amylose Determination from Potato Tuber Starch
Peel and cut fresh tubers into small cubes.
Place potato cubes in small brown bags and store overnight at -80 °C.
Transfer potato cubes to nylon bags and for freeze drying.
After freeze dried, grind the potato sample into powder using mortar and pestle or a Wiley Mill.
In a 50 ml tube, add 20-30 mg of the freeze-dried, ground tuber sample.
Prepare a solution of 45% (w/v) perchloric acid (mix 24.4 ml of 60% perchloric acid and 25.6 ml of ultra-pure water.
Add 500 μl of 45% (w/v) perchloric acid and shake or swirl it to disperse starch granules.
After a four-minute incubation period at room temperature, add 16 ml of ultra-pure water to the solution and mix by vortexing.
After non-soluble material has settled to the bottom of the tube (7-10 min later), transfer 40 μl of the solution to a microtiter plate (avoiding the pipetting of any particles).
Add 50 μl of iodine solution (2 g KI + 1 g I2 in 900 ml of ultra-pure water) and mix the sample by pipetting.
Read the absorbance at 550 nm and 620 nm immediately.
Determine the amylose percentage after comparing the amylose/amylopectin ratio of each sample (620 nm/550 nm absorbance) with a standard curve generated from amylose and amylopectin solutions at a range of concentrations. This procedure will be described next. Read the blank (iodine solution and perchloric acid) along with the test samples. Use blanked data for the analyses.
2. Amylose/amylopectin Curve
In separate tubes weigh 12.5 mg of amylose and 12.5 mg of amylopectin.
Add to each tube 5 ml of 45% (w/v) perchloric acid and dissolve completely.
For each of the solutions (amylose and amylopectin), bring to a final volume of 50 ml with ultra-pure water.
Mix 6.25 ml of amylose stock from the previous step with 18.75 ml ultra-pure water. This will make a 6.25 mg/ml amylose solution.
Repeat the procedure for the amylopectin solution.
Using the amylose and amylopectin standard solutions from steps 2.4 and 2.5, prepare the percent amylose standards which are 0-100% amylose (100-0% amylopectin) to a final volume of 5 ml to create the standard curve in 10% intervals. For example, to prepare: The 0% amylose / 100% amylopectin standard, pipette 5 ml amylopectin solution. The 10% amylose / 90% amylopectin standard, combine 0.5 ml amylose and 4.5 ml amylopectin solutions. The 20% amylose / 80% amylopectin standard, combine 1.0 ml amylose and 4.0 ml amylopectin solutions. The 30% amylose / 70% amylopectin standard, combine 1.5 ml amylose and 3.5 ml amylopectin solutions. The 40% amylose / 60% amylopectin standard, combine 2.0 ml amylose and 2.0 ml amylopectin solutions. The 50% amylose / 50% amylopectin standard, combine 2.5 ml amylose and 2.5 ml amylopectin solutions. The 60% amylose / 40% amylopectin standard, combine 3.0 ml amylose and 2.0 ml amylopectin, and so on).
Transfer 40 μl of each standard mixture to a microtiter plate. Include a well with 40 μl 45% (w/v) perchloric acid as the blank.
Add 50 μl of iodine solution (2 g KI + 1 g I2 in 900 ml of ultra-pure water) and mix each sample (including the blank) by pipetting.
Read absorbances at 550 nm and 620 nm immediately and calculate the amylose/ amylopectin ratio for each concentration.
Representative Results
The standard curve constructed from blanked data of different amylose/amylopectin concentration solutions shows approximate absorbance ratios ranging from 0.7 to 1.6 (Table 1). A linear regression trend line from these data is later used for inferring amylose content in freeze dried potato samples (Figure 1).
The plant production environment has some effect on the amylose content in the potato tuber7. Replicated amylose content determinations in two separate field locations showed low variation among potato tubers (Table 2). Pigments in colored flesh potatoes do not seem to affect the amylose determination assay.
| % Amylosesolution1 | 550 nm absorbance | 620 nm absorbance | Ratio2 620 nm/550 nm |
| 0 | 0.223 | 0.156 | 0.700 |
| 10 | 0.225 | 0.181 | 0.804 |
| 20 | 0.221 | 0.203 | 0.919 |
| 30 | 0.220 | 0.219 | 0.995 |
| 40 | 0.223 | 0.246 | 1.103 |
| 60 | 0.216 | 0.280 | 1.296 |
| 70 | 0.231 | 0.319 | 1.381 |
| 90 | 0.225 | 0.347 | 1.542 |
| 100 | 0.219 | 0.357 | 1.630 |
Table 1.Relationship between amylose concentration and absorbance ratio (620 nm/550 nm). Absorbance ratio vs. percent amylose is plotted in Figure 1 for amylose determination1. Percentage amylose solution was made from amylose and amylopectin stock solutions at different concentrations2. Calculated ratio from blanked data absorbances at 620 nm and 550 nm.
| Cultivar | Loc | 550 nm | 620 nm | Ratio | Amylose % |
| Adirondack Blue | 1 | 1.86 | 1.78 | 0.955 | 25.5 |
| 2 | 2.28 | 2.15 | 0.946 | 24.4 | |
| Early Rose | 1 | 2.17 | 2.12 | 0.977 | 27.8 |
| 2 | 2.22 | 2.14 | 0.967 | 26.7 | |
| Freedom Russet | 1 | 1.99 | 1.92 | 0.965 | 26.5 |
| 2 | 1.98 | 1.93 | 0.973 | 27.4 | |
| Inca Gold | 1 | 1.95 | 2.00 | 1.021 | 32.6 |
| 2 | 2.27 | 2.28 | 1.004 | 30.7 | |
| Ranger Russet | 1 | 1.88 | 1.84 | 0.975 | 27.6 |
| 2 | 2.15 | 2.08 | 0.967 | 26.7 | |
| Russet Norkotah | 1 | 1.84 | 1.79 | 0.972 | 27.3 |
| 2 | 1.90 | 1.86 | 0.975 | 27.6 | |
| Snowden | 1 | 2.23 | 2.13 | 0.956 | 25.5 |
| 2 | 1.64 | 1.61 | 0.979 | 28.0 | |
| White Pearl | 1 | 2.32 | 2.28 | 0.986 | 28.7 |
| 2 | 2.22 | 2.18 | 0.983 | 28.5 |
Table 2. Replicated evaluation of amylose content in potato cultivars from two different field production locations (Loc).
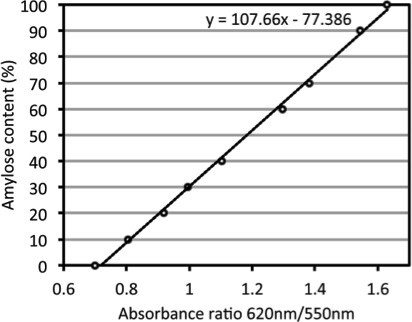 Figure 1.
Amylose concentration standard curve used to infer amylose percent content in potato tubers.
Figure 1.
Amylose concentration standard curve used to infer amylose percent content in potato tubers.
Discussion
Amylose content in potato tubers typically ranges from 20-35%7,8. If most values are outside this range, consider these possible errors: 1) raw data were used instead of blanked data for the construction of the amylose/amylopectin standard curve; 2) raw data were used instead of blanked data for the determination of the amylose/amylopectin ratios from the potato samples; or 3) the water used for the analysis was not pure enough. We noticed that by using distilled water and not ultra-pure water in the analysis, the values obtained were not reproducible. An easy way to verify that the iodine solution is prepared correctly or if it is still too old to be used is to check whether the raw absorbance value of the blank sample at 550 nm is around 0.1.
Precision in the preparation of the iodine and the amylopectin/amylose solutions is crucial to getting reliable amylose determinations. Since we are determining the proportion of amylose content rather than the exact amount of amylose in the potato starch, there is no purification step for the isolation of pure starch from the potato powder as presented by other determination methods.
For the determination of the amylose content in potato cultivars, two independent freeze dried tubers from each cultivar were processed at each locality. We found that the variation for amylose content between the potato tubers was small (less than one percentage point) and not significant.
There was no difference in the amylose content determination due to the initial amount of potato starch, between 20-30 mg. Outside this range, the results became unreliable or undetectable by our plate reader. Even though Hovenkamp-Hermelink et al.5 suggest adding a volume of water if the sample is too concentrated, we recommend reweighing the sample and repeating the procedure.
This study presents data based on white fleshed potatoes. In addition, we have evaluated this method with colored flesh potatoes (yellow, red and purple). No interference with the iodine/amylose complex was detected. However, as with any iodine binding assay, the proportion of amylose is likely to be overestimated somewhat due to the binding of iodine to straight chains in the amylopectin molecules. This assay is intended for screening large numbers of samples. Once high amylose individuals are identified, we recommend re-testing with an amylose-specific assay, such as concanavalin A9,10. This assay is more expensive and labor-intensive than the iodine binding assay, but it more precisely determines amylose levels.
Disclosures
We have nothing to disclose.
Acknowledgments
Funding for this research was provided in part by the USDA-ARS Research Associate Program and the USDA Crop Germplasm Committee.
References
- Composition Hoover R. molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr. 2001;45(3):253–267. [Google Scholar]
- Bertoft E, Blennow A. Advances in Potato Chemistry and Technology. 1st ed. Elsevier Ltd; 2009. Structure of Potato Starch; pp. 83–98. [Google Scholar]
- Campbell MR, Yeager H, Abdubek N, Pollak L, Glover D. Comparison of methods for amylose screening in maize starches from exotic backgrounds. Cereal Chem. 2002;79:317–321. [Google Scholar]
- Zhu T, Jackson DS, Wehling RL, Geera B. Comparison of amylose determination methods and the development of a dual wavelength iodine binding technique. Cereal Chem. 2008;85:51–58. [Google Scholar]
- Hovenkamp-Hermelink J, Devries J, Adamse P, Jacobsen E, Witholt B, Feenstra W. Rapid estimation of the amylose amylopectin ratio in small amounts of tuber and leaf tissue of the potato. Potato Res. 1988;31:241–246. [Google Scholar]
- Shannon JC, Garwood DL, Boyer CD. Starch. 3rd ed. Elsevier Inc; 2009. Genetics and Physiology of Starch Development; pp. 23–82. [Google Scholar]
- Haase NU, Plate J. Properties of potato starch in relation to varieties and environmental factors. Stärke. 1996;48(5):167–171. [Google Scholar]
- Johnston FB, Urbas B, Khanzada G. Effect of storage on the size distribution and amylose/amylopectin ratio in potato starch granules. Am. Pot. J. 1968;45:315–321. [Google Scholar]
- Gibson TS, Solah VA, McCleary BV. A procedure to measure amylose in cereal starches and flours with concanavalin A. J. Cereal Sci. 1997;25(2):111–119. [Google Scholar]
- Sun S, Matheson N, Yun S-H. Estimation of amylose content of starches after precipitation of amylopectin by concanavalin A. Starch. 1990;42:302–305. [Google Scholar]


