Abstract
Physicians are often approached by young women with a BRCA mutation and a recent history of breast cancer who wish to have a baby. They wish to know if pregnancy impacts upon their future risks of cancer recurrence and survival. To date, there is little information on the survival experience of women who carry a mutation in one of the BRCA genes and who become pregnant. From an international multi-center cohort study of 12,084 women with a BRCA1 or BRCA2 mutation, we identified 128 case subjects who were diagnosed with breast cancer while pregnant or who became pregnant after a diagnosis of breast cancer. These women were age-matched to 269 mutation carriers with breast cancer who did not become pregnant (controls). Subjects were followed from the date of breast cancer diagnosis until the date of last follow-up or death from breast cancer. The Kaplan–Meier method was used to estimate 15-year survival rates. The hazard ratio for survival associated with pregnancy was calculated using a left-truncated Cox proportional hazard model, adjusting for other prognostic factors. Among women who were diagnosed with breast cancer when pregnant or who became pregnant thereafter, the 15-year survival rate was 91.5 %, compared to a survival of 88.6 % for women who did not become pregnant (adjusted hazard ratio = 0.76; 95 % CI 0.31–1.91; p = 0.56). Pregnancy concurrent with or after a diagnosis of breast cancer does not appear to adversely affect survival among BRCA1/2 mutation carriers.
Keywords: BRCA1, BRCA2, Breast cancer, Pregnancy, Survival
Introduction
A proportion of young women with a BRCA1 or BRCA2 mutation will be diagnosed with breast cancer during the course of a pregnancy and others may wish to become pregnant after a diagnosis of breast cancer. This is a consequence of the typical early age-of-onset of hereditary breast cancer and the desire of many women to delay childbearing until their late thirties. The majority of women with early-stage breast cancer have their disease cured [1] and many breast cancer survivors will later have children.
Pregnancy-associated breast cancer is defined as a breast cancer diagnosed during pregnancy or within 1 year of delivery [2, 3] and has been reported to have a relatively poor prognosis [4–9]. If the survival of women with pregnancy-associated breast cancer is, in fact, inferior to that of women with breast cancer and no pregnancy, then this might be for several reasons. First, the pathologic and clinical features of a cancer which is diagnosed during pregnancy (such as grade, size, nodal status, and hormone receptors) may be worse than expected; in this case, adjusting for covariates will diminish the difference. In support of this, pregnancy-associated breast cancers are often diagnosed at a relatively advanced stage and are often HER2-positive and hormone receptor-negative [10]. Second, the hormonal surges which occur during pregnancy may impact on the metastatic behavior of the cancer—possibly with different consequences for estrogen receptor (ER)+ and ER− breast cancers. Third, pregnant women may avoid or delay treatment, e.g., chemotherapy or hormonal therapies or ovarian ablation. Last, there may be a delay in the diagnosis of breast cancer in pregnant women, as a result of enlarged and dense breasts [10–13].
A second class of patients are those who have a baby one or more years after a diagnosis of breast cancer. Physicians are often approached by women of childbearing age with recent diagnoses of breast cancer who ask about the advisability of pregnancy and the potential harmful effects of cancer treatments which could impair their fertility (such as ovarian ablation, chemotherapy, and hormone therapies) and the effect of late pregnancy on the recurrence of the earlier breast cancer. It has been proposed that the time elapsed from completion of breast cancer treatment to birth is relevant and that the longer the length between breast cancer diagnosis and pregnancy, the better the long-term prognosis [14, 15].
Pregnancy around the time of breast cancer diagnosis presents challenging clinical issues. To date, there is little information on the survival experience of women who carry a mutation in one of the BRCA genes and who become pregnant. The purpose of this study is to evaluate the impact of pregnancy at the time of breast cancer or following a diagnosis of breast cancer on recurrence and mortality in this high-risk group of women.
Materials and methods
We conducted a multicenter, historical cohort study of women known to carry a BRCA1 or BRCA2 mutation. The study included subjects from 52 centers from Canada, the United States, Asia, and Europe. Subjects were selected from a database of 12,084 BRCA1 and BRCA2 mutation carriers from the ongoing international multicenter cohort study. Since 1995, we have been collecting data on incident cases of breast and ovarian cancer including primary surgery, chemotherapy, cancer recurrences, and survival. Mutation detection was performed using several techniques and all mutations were confirmed by direct sequencing. Every host center obtained approval from their ethics review board.
The data for this study were obtained from three sources: (1) the study questionnaires, (2) the medical chart, and (3) the surgical pathology report. The questionnaire contained information on date of birth, country of residence, type of BRCA mutation, date of disclosure of genetic result, obstetrical and gynecological history (onset of menarche and menopause, parity, pregnancy outcome and length, fertility treatments, contraception methods, and hormonal therapies), breast cancer screening (mammography and MRI), and breast cancer history (date of diagnosis, primary surgery, adjuvant treatment, recurrence, and reconstructions). The women in the cohort study are followed every 2 years with a standard follow-up questionnaire, which details new cancers, second primary cancers and dates of local, regional, and distal recurrences.
The medical chart review was performed for members of the study cohort with the goal of collecting detailed information on obstetric history, including date of delivery, gestational age, pregnancy outcome, duration of lactation, type of breast cancer surgery, salpingo-oophorectomy, chemotherapy (yes/no), radiotherapy (yes/no), tamoxifen (yes/no), date and cause of death, and breast cancer recurrence (distant/local). Pathology reports were requested from the corresponding research center or directly from the hospital where the surgery was performed, and were used to help establish tumor size, grade, lymph node status, and hormone receptor status.
Case subjects and comparison subjects
We defined a case subject as a woman with a pregnancy-associated breast cancer (breast cancer diagnosed during pregnancy or within 1 year of delivery) or pregnancy-following breast cancer (pregnancy occurring a minimum of 1 year after a breast cancer diagnosis). A potential control subject was a woman with breast cancer who was pre-menopausal at the time of breast cancer diagnosis but who did not have a pregnancy 1 year prior to diagnosis or thereafter (controls). Pregnancies resulted in live births (therapeutic and spontaneous abortions excluded).
Inclusion and exclusion criteria
The potential study subjects included women: (1) diagnosed from 1985 to 2010 with premenopausal invasive breast cancer between the ages of 20 and 45 years, (2) enrolled in the parent cohort study and known to carry a BRCA1 or BRCA2 mutation, and (3) completed a baseline questionnaire and at least one follow-up questionnaire. Patients were followed until June 2012. Additional exclusions included women who have been diagnosed with ovarian cancer or another cancer (except skin cancer) in the past or in the follow-up period or have Stage IV breast cancer at diagnosis.
Ascertainment of subjects
Of the 11,874 subjects in the database, we excluded 7,042 subjects who had no breast cancer diagnosis, 590 subjects who were diagnosed with breast cancer prior to 1985, 1,826 subjects who were more than 45 years old at the time of breast cancer diagnosis, 2 subjects with stage IV breast cancer at presentation, 201 subjects who had natural menopause prior to diagnosis, 64 subjects who had an oophorectomy prior to breast cancer, and 131 subjects who had ovarian cancer. For 37 subjects, information on key variables was missing (i.e., date of delivery, date of breast cancer diagnosis, or breast cancer treatment), and these women were also excluded. In total, 1,981 women were eligible for the study; 161 had a pregnancy-associated or pregnancy-following breast cancer and 1,820 women did not. Eleven participants had both a pregnancy-associated breast cancer and a pregnancy-following breast cancer. These cases were assigned to the pregnancy-associated breast cancer group.
Matching strategy
We attempted to identify three controls for each case subject. Case and control subjects were matched by age (±2 years), BRCA mutation type (BRCA1 vs. BRCA2), country of residency, date of breast cancer diagnosis (±2 years), and date of completion of baseline questionnaire (date of study entry ±2 years). Cases could not have had a hysterectomy or oophorectomy or have undergone natural menopause prior to diagnosis. They could not have experienced a distant recurrence prior to the date of pregnancy (if they experienced a local recurrence they remained eligible). To be eligible and matched to a given case, the control had not to have an oophorectomy or experienced a distant recurrence prior to the date of birth for the index pregnancy in the matched case. We identified 128 matched sets (128 cases and 269 controls). Seventy-five case subjects were diagnosed with a pregnancy-associated breast cancer and 53 case subjects were diagnosed with a pregnancy-following breast cancer. We retrieved pathology reports for 55 % of the 128 cases and for 53 % of the 269 matched controls.
Statistical analyses
The patient was followed from the date of diagnosis until death from breast cancer, date of last follow-up or death from another cause. Because (by definition) patients were alive at the time of completion of the baseline questionnaire, we used a left-truncated survival analysis, whereby the time period between diagnosis and completion of the baseline questionnaire was censored. To adjust for possible differences in the baseline characteristics of the cases and controls and treatments received, we performed a multivariate analysis and adjusted for age at diagnosis, tumor size (<2, 2–5, >5 cm), lymph node status (positive, negative, missing), ER status (positive, negative, missing), use of chemotherapy (yes/no), and oophorectomy (yes/no, time-dependent-variable).
We also performed a survival analysis using breast cancer recurrence as the endpoint. We considered only regional and distant recurrences (local recurrences were not considered). We followed the patients from the date of breast cancer diagnosis until the date of the breast cancer recurrence, using a left-truncated analytic approach. The data was analyzed using SAS statistical software for survival analysis (Kaplan–Meier method) and Cox-proportional hazards model. We estimated the adjusted hazard ratio associated with pregnancy as well as with other tumor characteristics and treatments (chemotherapy yes/no) and oophorectomy (yes/no). In all survival analyses, oophorectomy was treated as a time-dependent covariate.
Results
We identified 397 eligible subjects, including 75 women with pregnancy-associated breast cancers (58.6 %), 53 women with pregnancies following breast cancer (41.4 %), and 269 matched controls (no pregnancy). For each case subject, we identified from one to three matched controls (mean 2.1) (Table 1).
Table 1.
Characteristic of cases and controls
| Characteristic | Cases n = 128 | Controls n = 269 | p value |
|---|---|---|---|
| Date of birth (mean) (range) | 1965.2 (1947–82) | 1964.5 (1946–81) | 0.44 |
| Year of diagnosis (range) | 1997.3 (1985–2009) | 1997.8 (1985–2011) | 0.47 |
| Age of breast cancer (range) | 32.5 (25–42) | 33.8 (26–44) | 0.009 |
| Date of baseline questionnaire (range) | 2,003.5 (1996–2011) | 2,003.7 (1996–2012) | 0.84 |
| Mutation n (%) | |||
| BRCA1 | 106 (81.3 %) | 227 (84.4 %) | Matched |
| BRCA2 | 24 (18.8 %) | 42 (15.6 %) | |
| Place of residence | 96 (75.0 %) | 210 (78.1 %) | Matched |
| North America | 20 (15.6 %) | 43 (16.0 %) | |
| Poland | 12 (9.4 %) | 16 (6.0 %) | |
| Others | |||
| Parous | |||
| No | 0 | 78 (29.5 %) | 0.62 |
| Yes | 128 (100 %) | 191 (71.0 %) | |
| Mean Parity | 2.1(1–5) | 2.0 (1–4) | |
| Age of menarche (years) | 12.8 (9–17) | 12.8 (10–21) | 0.73 |
| Age at first birth (years) | 30.7 (18–42) | 25.6 (18–33) | < 0.0001 |
| Age at last birth (years) | 35.0 (27–44) | 28.7 (20–38) | < 0.0001 |
| Time from diagnosis to last childbirth (years) | 2.4 (0–13) | NA | < 0.0001 |
| Salpingo-oophorectomy after breast cancer diagnosis | |||
| No | 55 (43.0 %) | 127 (47.2 %) | |
| Yes | 73 (57.0 %) | 142 (52.8 %) | 0.43 |
| Vital status | |||
| Alive | 120 (92.8 %) | 246 (91.5 %) | |
| Dead | 8 (6.3 %) | 23 (8.6 %) | 0.42 |
| Cause of death | |||
| Breast cancer | 7 | 19 | |
| Unknown | 1 | 4 | |
The mean time elapsed from breast cancer diagnosis to pregnancy in the case group was 2.4 years (range 0–13 years). The mean time from last pregnancy to breast cancer in the comparison group was 5.8 years (range from 1 to 21). No control had an oophorectomy prior to the date of the delivery of the index case. Approximately one-half of the cases and matched controls underwent a prophylactic salpingo-oophorectomy at some time after childbirth in the index case.
We obtained a pathology report for 54 % of the subjects (Tables 2, 3). Based on these reports, the mean tumor size among case subjects was 24.6 mm and among controls was 27.7 mm. Forty-three percent of control subjects and 40 % of case subjects had positive lymph nodes. In both groups (pregnant and non-pregnant), the majority of tumors were estrogen receptor negative (control subjects 68.6 %; case subjects 78.0 %). None of these differences were statistically significant. The various breast cancer treatments are presented in Table 2. We found no significant differences in the surgical treatments for the non-pregnant and the pregnant patient sub-groups. We also evaluated breast cancer characteristics between breast cancer controls, pregnancy-associated breast cancers, and pregnancies following breast cancer. We found no significant differences (Table 3, supplemental).
Table 2.
Breast cancer characteristics and treatments used among study subjects
| Characteristic | Cases n = 128 | Controls n = 269 | p-value |
|---|---|---|---|
| Breast cancer | |||
| Unilateral | 97 (75.8 %) | 214 (79.6 %) | 0.39 |
| Bilateral | 31 (24.2 %) | 55 (20.1 %) | |
| Primary surgery | |||
| Lumpectomy | 57 (44.9 %) | 136 (51.3 %) | |
| Unilateral mastectomy | 69 (53.9 %) | 125 (47.2 %) | |
| Bilateral mastectomy | 1 (0.8 %) | 4 (1.5 %) | 0.37 |
| Chemotherapy | |||
| No | 21 (16.5 %) | 47 (17.6 %) | |
| Yes | 106 (83.5 %) | 220 (82.4 %) | 0.79 |
| Radiotherapy | |||
| No | 60 (47.2 %) | 120 (45.1 %) | |
| Yes | 67 (52.8 %) | 146 (54.9 %) | 0.69 |
| Tamoxifen | |||
| No | 105 (83.3 %) | 201 (75.3 %) | |
| Yes | 21 (16.7 %) | 66 (24.7 %) | 0.07 |
Table 3.
Hazard ratios at 15 years of breast cancer-specific mortality, all subjects
| Variables | Univariate
|
Multivariateb
|
||
|---|---|---|---|---|
| HR (95 % CI) | p-value | HR (95 % CI) | p-value | |
| Birth after diagnosis | ||||
| No | 1 | 0.83 | 1 | |
| Yes | 0.91 (0.38–2.18) | 0.76 (0.31–1.91) | 0.56 | |
| Birth after diagnosis | ||||
| No pregnancy | 1 | 1 | ||
| Pregnancy associated | 0.89 (0.30–2.65) | 0.84 | 0.79 (0.25–2.44) | 0.68 |
| Pregnancy following | 0.93 (0.27–3.17) | 0.91 | 0.73 (0.21–2.68) | 0.64 |
| Age at diagnosis (trend per year) | 0.91 (0.82–1.01) | 0.08 | 0.92 (0.82–1.04) | 0.18 |
| Chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 0.53 (0.22–1.29) | 0.16 | 0.39 (0.14–0.09) | 0.07 |
| Bilateral salpingo-oophorectomya | ||||
| No | 1 | 1 | ||
| Yes | 0.19 (0.06–0.57) | 0.003 | 0.20 (0.06–0.62) | 0.006 |
| Tumor size | ||||
| < 2 cm | 1 | 1 | ||
| 2–5 cm | 1.78 (0.51–6.33) | 0.37 | 2.08 (0.55–7.96) | 0.28 |
| > 5 cm | 5.70 (1.03–31.4) | 0.05 | 8.980 (1.32–61.6) | 0.02 |
| Lymph nodes | ||||
| Negative | 1 | 1 | ||
| Positive | 1.67 (0.51–5.48) | 0.40 | 1.79 (0.50–6.44) | 0.37 |
| Receptor status | ||||
| ER− | 1 | 1 | ||
| ER+ | 0.42 (0.05–3.39) | 0.41 | 0.39 (0.04–3.63) | 0.41 |
HR hazard ratio, CI confidence interval
Oophorectomy is time dependent
All variables used in the regression
Breast cancer survival analysis
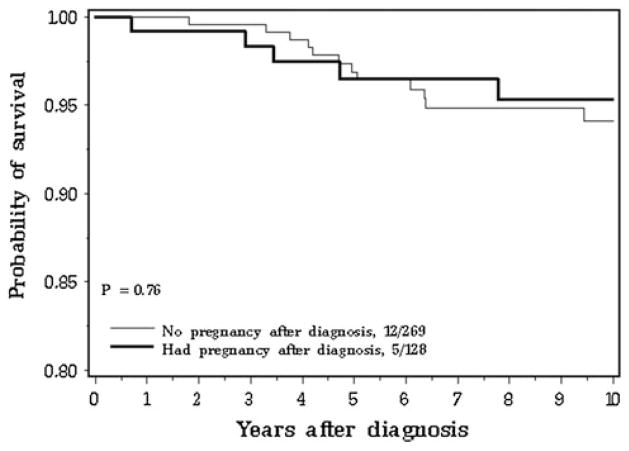
After a mean of 10.2 years of follow-up from diagnosis (range 0.2–26 years), 7 of the 128 case subjects (5.5 %), and 19 of the 269 matched controls (7.1 %) had died of breast cancer (Fig. 1). The actuarial 15-year survival rates were 89.1 % for the pregnancy-associated breast cancer subjects, were 93.6 % for the pregnancy-following breast cancer subjects, and were 88.6 % for the non-pregnant controls. Eleven of the 128 case subjects (8.6 %) and 20 of the 269 matched controls (7.4 %) experienced a distant breast cancer recurrence.
Fig. 1.
Breast cancer-specific survival for subjects with and without a pregnancy: from date of breast cancer
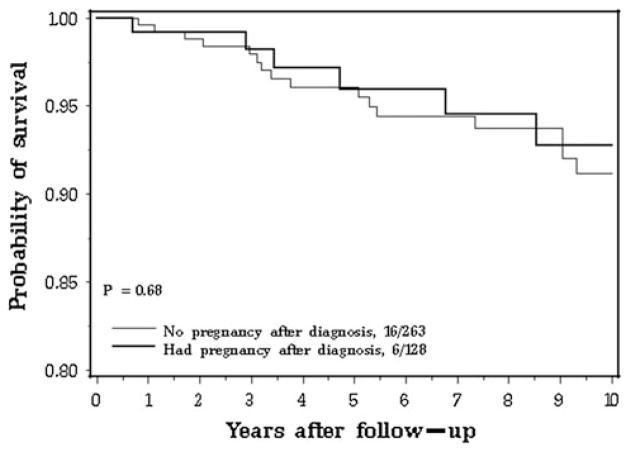
The unadjusted hazard ratio for breast cancer-specific mortality at 15 years from breast cancer diagnosis was 0.91 (95 % CI 0.38–2.18) for all pregnant versus non-pregnant subjects. The unadjusted hazard ratio was 0.89 (95 % CI 0.30–2.65) for pregnancy-associated breast cancer (p = 0.83) and was 0.93 (95 % CI 0.27–3.17) for pregnancy-following breast cancer (p = 0.91), compared to matched controls. The unadjusted hazard ratio was 0.81 (95 % CI 0.32–2.05) for BRCA1 carriers (all pregnant vs. non-pregnant). There were no deaths among the BRCA2 carriers. The mortality curves for women with a pregnancy-associated breast cancer, a pregnancy-following breast cancer and no pregnancy are compared in Fig. 2.
Fig. 2.
Breast cancer-specific survival for subjects with and without a pregnancy: from date of diagnosis
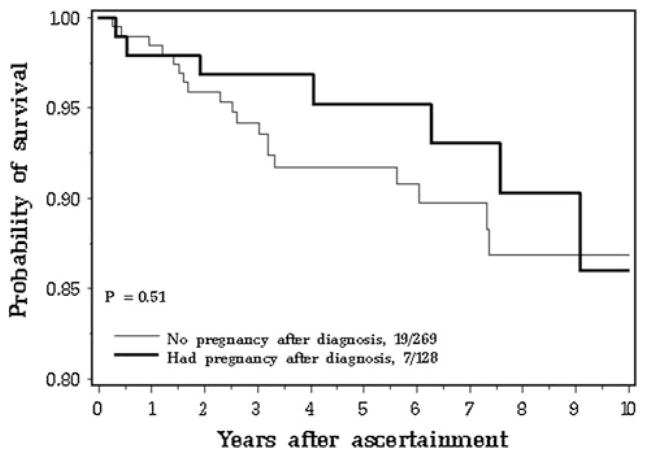
The Kaplan–Meier survival curve from breast cancer diagnosis to death is presented in Fig. 1; from the date of childbirth until death is presented in Fig. 3 and from the date of ascertainment until death is presented in Fig. 4 of electronic supplementary material. There were no signifi-cant differences between groups for any of the analyses. Likewise, the Kaplan–Meier survival curves from the date of breast cancer diagnosis and the date breast cancer recurrence did not show a difference for pregnant and non-pregnant women (Fig. 5 electronic supplementary material). At 15 years after the birth of the index child, the survival rate was 90.0 % for the pregnancy-associated case subjects and was 87.1 % for the matched controls. At 15 years after the date of ascertainment, the survival rate was 86.0 % for the pregnancy-associated case subjects and was 86.9 % for the matched controls. At 15 years from the date of breast cancer, 84.6 % of the pregnant cases and 91.9 % of the controls were free of regional or distant recurrence (p = 0.64).
Fig. 3.
Breast cancer-specific survival in subjects with and without a pregnancy after breast cancer: follow-up from date of last birth
After adjustment for age at breast cancer diagnosis, chemotherapy use (yes/no), tumor size (<2 cm, 2–5 cm, >5 cm), lymph node status (Ŧ), estrogen receptor status, and oophorectomy (Ŧ), the hazard ratio for mortality from the time of breast cancer diagnosis was 0.76 (95 % CI 0.31–1.91) for pregnant cases versus non-pregnant controls. The adjusted hazard ratio was 0.72 for BRCA1 carriers (95 % CI 0.27–1.90) (there were no deaths among the BRCA2 carrier case group). The adjusted hazard ratio was 0.79 (95 % CI 0.25–2.44) for the pregnancy-associated breast cancer sub-group and was 0.73 (95 % CI 0.21–2.68) for the pregnancy-following breast cancer sub-group (Table 4). In this multivariate analysis, tumor size and bilateral salpingo-oophorectomy were both significant prognostic factors (Table 4). Women who underwent bilateral oophorectomy had a much lower risk of death than women who had two ovaries intact (adjusted HR = 0.20; 95 % CI 0.06–0.62; p = 0.006). Women who received chemotherapy were less likely to die of their disease than women who did not receive chemotherapy (adjusted HR = 0.39; 95 % CI 0.14–1.09), but this did not achieve statistical significance (p = 0.07).
Table 4.
Hazard ratios at 15 years of breast cancer-specific mortality, all subjects
| Variables | Univariate
|
Multivariateb
|
||
|---|---|---|---|---|
| HR (95 % CI) | p value | HR (95 % CI) | p value | |
| Birth after diagnosis | ||||
| No | 1 | 1 | ||
| Yes | 0.91 (0.38–2.18) | 0.83 | 0.76 (0.31–1.91) | 0.56 |
| Birth after diagnosis | ||||
| No pregnancy | 1 | 1 | ||
| Pregnancy associated | 0.89 (0.30–2.65) | 0.84 | 0.79 (0.25–2.44) | 0.68 |
| Pregnancy following | 0.93 (0.27–3.17) | 0.91 | 0.73 (0.21–2.68) | 0.64 |
| Age at diagnosis (trend per year) | 0.91 (0.82–1.01) | 0.08 | 0.92 (0.82–1.04) | 0.18 |
| Chemotherapy | ||||
| No | 1 | 1 | ||
| Yes | 0.53 (0.22–1.29) | 0.16 | 0.39 (0.14–1.09) | 0.07 |
| Bilateral salpingo-oophorectomya | ||||
| No | 1 | 1 | ||
| Yes | 0.19 (0.06–0.57) | 0.003 | 0.20 (0.06–0.62) | 0.006 |
| Tumor size | ||||
| < 2 cm | 1 | 1 | ||
| 2–5 cm | 1.78 (0.51–6.33) | 0.37 | 2.08 (0.55–7.96) | 0.28 |
| > 5 cm | 5.70 (1.03–31.4) | 0.05 | 8.980 (1.32–61.6) | 0.02 |
| Lymph nodes | ||||
| Negative | 1 | 1 | ||
| Positive | 1.67 (0.51–5.48) | 0.40 | 1.79 (0.50–6.44) | 0.37 |
| Receptor status | ||||
| ER− | 1 | 1 | ||
| ER+ | 0.42 (0.05–3.39) | 0.41 | 0.39 (0.04–3.63) | 0.41 |
HR hazard ratio, CI confidence interval
Oophorectomy is time dependent
All variables used in the regression
Discussion
These data show that the survival of women who carry a BRCA1 or BRCA2 mutation and who become pregnant at the time of, or after a breast cancer diagnosis does not appear to be unduly affected by the pregnancy. After adjustment for other prognostic factors, the survival experiences of the pregnant cases in each of the two subgroups and non-pregnant controls were similar.
Among the patients in this study who became pregnant, the mean tumor size was 2.5 cm; 40 % were lymph node-positive and 79 % were ER-negative. Despite the high prevalence of these three adverse prognostic features, the 15-year survival rate for these patients was 93 %. Women with low-risk breast cancers may choose to become pregnant more often than women with more aggressive cancers (“healthy mother effect”) [16] but, in our study, the distribution of stage and other prognostic features of the cancers in the women who did and who did not become pregnant after breast cancer was similar.
The breast cancer patients in the study are a highly selected group. By definition, the women had to be alive and without distant recurrence at the date of parturition to be eligible and this will select for survivors, which may explain in part our excellent 15-year survival rate. On average, 2.4 years had passed from diagnosis to birth. However, we also selected our controls be alive and recurrence-free at the time of delivery of the baby in the matched case, so the survivorship bias should apply equally to cases and controls. We adjusted for potential survivor-ship bias by using a left-truncated survival analysis, wherein we only considered person-years in the follow-up period after the date of ascertainment [17]. Finally, we compared survival three ways: from the date of breast cancer, from the date of parturition, and from the date of ascertainment and for all three analyses the mortality experiences of the cases and comparison groups were similar.
There are several limitations to our study. The sample size of exposed women was small (n = 128 women with breast cancer and a pregnancy) and the subgroups of women with pregnancy-associated breast cancers and pregnancy-following breast cancers were small. However, considering that very few breast cancer patients carry a BRCA mutation and even fewer are associated with pregnancy, this combination is a very rare event and this is the only study to date to address the association. We were able to retrieve pathology reports for only one-half of the index cases and this limited our ability to adjust for cofactors, but an analysis of the samples for which pathology data were available indicated that the cancer features were similar for cases and controls. Subgroups were small and for the main analyses, it was necessary to combine women with BRCA1 mutations and BRCA2 mutations and women with pregnancy-associated breast cancers and pregnancy-following breast cancers. Subjects were enrolled from 11 countries, and treatment was not standardized.
Previous research on pregnancy and breast cancer mortality in the general population is inconsistent [18–36]. Breast cancer diagnosed shortly after delivery has been associated with a worse prognosis [4], but if a pregnancy occurs long after diagnosis it is associated with a neutral or a protective effect [14, 15, 36]. The Society of Obstetricians and Gynaecologist of Canada (SOGC) recommendation is to postpone pregnancy for 3 years after diagnosis but if lymph nodes are involved, they extend this period to 5 years [37].
In this study, we found that carriers in the pregnancy group who underwent oophorectomy had a significant reduction in the risk of dying of their breast cancer compared to women with intact ovaries (HR = 0.20; 95 % CI 0.06–0.62). We recently reported that BRCA1 mutation carriers with breast cancer in Poland who had an oophorectomy experienced a relative mortality reduction of 70 % [38]. We conclude that pregnancy after breast cancer does not adversely affect survival per se, but because oophorectomy results in infertility, it is important to discuss the potential risk of delaying oophorectomy for a breast cancer patient who wishes to have a baby. It is important that more studies be conducted on the impact of oophorectomy on the survival from BRCA1 and BRCA2-associated breast cancer in pre-menopausal women.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the study coordinators, Roxana Bucur Marcia Llacuachaqui, Alejandra Ragone, Jennifer Ng, Sara Elmi and Linda Steele. Supported by the Canadian Breast Cancer Foundation and the Women’s College Hospital. Also supported by NIH RO1CA74415 (S.L.N.) and P30 CA033752. S.L.N. is the Morris and Horowitz Families Endowed Professor. Recruitment of cases from the City of Hope Clinical Cancer Genetics Community Research Network is supported by Award Number RC4A153828 (PI: J.W.) from the National Cancer Institute and the Office of the Director, National Institutes of Health. The study conducted complies with the current laws of the country in which they were performed.
Appendix: Other members of the Hereditary Breast Cancer Clinical Study Group
Jacek Gronwald, Cezary Cybulski, Tomasz Huzarski, Andre Robidoux, Kenneth Offit, Ruth Gershoni-Baruch, Claudine Isaacs, Nadine Tung, Barry Rosen, Rochelle Demsky, Jeanna McCuaig, Andrea Eisen, Louise Bordeleau, Beth Karlan, Judy Garber, Dawna Gilchrist, Charis Eng, Fergus Couch, Gareth Evans, Ava Kwong, Lovise Maehle, Eitan Friedman, Wendy McKinnon, Marie Wood, Mary Daly, Joanne L. Blum, Mark Robson, Albert Chud-ley, Seema Panchal, Jane McLennan, Barabara Pasini, Gad Rennert, John Lunn, Taya Fallen, Daniel Rayson, Marissa Smith, Ophira Ginsburg, Edmond Lemire, Wendy Me-schino, Tuya Pal, Susan Vadaparampil, David Euhus, Josephine Wagner Costalas, Talia Donenberg, Raluca N. Kurz, Susan Friedman (on behalf of FORCE), Kevin Sweet, Carey A. Cullinane, Robert E. Reilly, Joanne Kotsopoulos, Sonia Nanda, Kelly Metcalfe.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (doi:10.1007/s10549-013-2729-1) contains supplementary material, which is available to authorized users.
The other members of the Hereditary Breast Cancer Clinical Study Group are given in Appendix.
Contributor Information
Adriana Valentini, Women’s College Research Institute, Toronto, ON, Canada.
Jan Lubinski, Hereditary Cancer Center, Pomeranian Medical University, Szczecin, Poland.
Tomasz Byrski, Hereditary Cancer Center, Pomeranian Medical University, Szczecin, Poland.
Parviz Ghadirian, Epidemiology Research Unit, Research Center of the University of Montreal Hospital Centre (CRCHUM), Montreal, QC, Canada.
Pal Moller, Research Group on Inherited Cancer, Department of Medical Genetics, Oslo University Hospital, Radiumhosptialet, Norway.
Henry T. Lynch, Department of Preventive Medicine and Public Health, Creighton University School of Medicine, Omaha, NE, USA
Peter Ainsworth, London Regional Cancer Program, London, ON, Canada.
Susan L. Neuhausen, Department of Population Sciences, Beckman Research Institute of City of Hope, Duarte, CA, USA
Jeffrey Weitzel, City of Hope National Medical Center, Duarte, CA, USA.
Christian F. Singer, Department of Obstetrics and Gynaecology and Comprehensive Cancer Center, Medical University of Vienna, Vienna, Austria
Olufunmilayo I. Olopade, Center for Clinical Cancer Genetics, University of Chicago, Chicago, IL, USA
Howard Saal, Hereditary Cancer Program, Division of Human Genetics, Children’s Hospital Medical Center, Cincinnati, OH, USA.
Dominique Stoppa Lyonnet, Service de Génétique Oncologique, Institut Curie Hôpital, Institut Curie Centre de Recherche, Paris, France.
William D. Foulkes, Department of Medical Genetics, McGill University, Montreal, QC, Canada
Charmaine Kim-Sing, BC Cancer Agency, Vancouver, BC, Canada.
Siranoush Manoukian, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Dana Zakalik, Cancer Genetics Program, Beaumont Hospital, Royal Oak, MI, USA.
Susan Armel, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Princess Margaret Hospital, University of Toronto, Toronto, ON, Canada.
Leigha Senter, Division of Human Genetics, The Ohio State University Medical Center, Columbus, OH, USA.
Charis Eng, Genomic Medicine Institute and Center for Personalized Genetic Healthcare, Cleveland Clinic, Cleveland, OH, USA. Department of Genetics and Genome Sciences and Comprehensive Cancer Center, Case Western Reserve University, Cleveland, OH, USA.
Eva Grunfeld, Department of Family and Community Medicine, University of Toronto, Toronto, ON, USA.
Anna M. Chiarelli, Dalla Lana School of Public Health, University of Toronto, Toronto, ON, USA. Cancer Care Ontario, Toronto, ON, USA
Aletta Poll, Women’s College Research Institute, Toronto, ON, Canada.
Ping Sun, Women’s College Research Institute, Toronto, ON, Canada.
Steven A. Narod, Email: steven.narod@wchospital.ca, Women’s College Research Institute, Toronto, ON, Canada. Dalla Lana School of Public Health, University of Toronto, Toronto, ON, USA. Women’s College Research Institute, 790 Bay Street, Room 750, Toronto, ON M5G 1N8, Canada
References
- 1.Hayat MJ, Howlader N, Reichman ME, Edwards BK. Cancer statistics, trends, and multiple primary cancer analyses from the surveillance, epidemiology, and end results (SEER) program. Oncologist. 2007;12:20–37. doi: 10.1634/theoncologist.12-1-20. [DOI] [PubMed] [Google Scholar]
- 2.Petrek JA. Breast cancer and pregnancy. J Natl Cancer Inst Monogr. 1994;16:113–121. [PubMed] [Google Scholar]
- 3.Nugent P, O’Connell TX. Breast cancer and pregnancy. Arch Surg. 1985;120:1221–1224. doi: 10.1001/archsurg.1985.01390350007001. [DOI] [PubMed] [Google Scholar]
- 4.Bonnier P, Romain S, Dilhuydy JM, Bonichon F, Julien JP, Charpin C, Lejeune C, Martin PM, Piana L. Influence of pregnancy on the outcome of breast cancer: a case–control study. Societe Francaise de Senologie et de Pathologie Mammaire Study Group. Int J Cancer. 1997;72:720–727. doi: 10.1002/(sici)1097-0215(19970904)72:5<720::aid-ijc3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Rodriguez AO, Chew H, Cress R, Xing G, McElvy S, Danielsen B, Smith L. Evidence of poorer survival in pregnancy-associated breast cancer. Obstet Gynecol. 2008;112:71–78. doi: 10.1097/AOG.0b013e31817c4ebc. [DOI] [PubMed] [Google Scholar]
- 6.Ali SA, Gupta S, Sehgal R, Vogel V. Survival outcomes in pregnancy associated breast cancer: a retrospective case control study. Breast J. 2012;18:139–144. doi: 10.1111/j.1524-4741.2011.01201.x. [DOI] [PubMed] [Google Scholar]
- 7.Bladström A, Anderson H, Olsson H. Worse survival in breast cancer among women with recent childbirth: results from a Swedish population-based register study. Clin Breast Cancer. 2003;4:280–285. doi: 10.3816/cbc.2003.n.033. [DOI] [PubMed] [Google Scholar]
- 8.Guinee VF, Olsson H, Möller T, Hess KR, Taylor SH, Fahey T, Gladikov JV, van den Blink JW, Bonichon F, Dische S, et al. Effect of pregnancy on prognosis for young women with breast cancer. Lancet. 1994;343:1587–1589. doi: 10.1016/s0140-6736(94)93054-6. [DOI] [PubMed] [Google Scholar]
- 9.Azim HA, Jr, Botteri E, Renne G, Dell’orto P, Rotmensz N, Gentilini O, Sangalli C, Pruneri G, Di Nubila B, Locatelli M, Sotiriou C, Piccart M, Goldhirsch A, Viale G, Peccatori FA. The biological features and prognosis of breast cancer diagnosed during pregnancy: a case–control study. Acta Oncol. 2012;51:653–661. doi: 10.3109/0284186X.2011.636069. [DOI] [PubMed] [Google Scholar]
- 10.Genin AS, Lesieur B, Gligorov J, Antoine M, Selleret L, Rouzier R. Pregnancy-associated breast cancers: do they differ from other breast cancers in young women? Breast. 2012;4:550–555. doi: 10.1016/j.breast.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Ishida T, Yokoe T, Kasumi F, Sakamoto G, Makita M, Tominaga T, Simozuma K, Enomoto K, Fujiwara K, Nanasawa T, et al. Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case–control study in Japan. Jpn J Cancer Res. 1992;83:1143–1149. doi: 10.1111/j.1349-7006.1992.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardonick E, Dougherty R, Grana G, Gilmandyar D, Ghaffar S, Usmani A. Breast cancer during pregnancy: maternal and fetal outcomes. Cancer J. 2010;16:76–82. doi: 10.1097/PPO.0b013e3181ce46f9. [DOI] [PubMed] [Google Scholar]
- 13.Zemlickis D, Lishner M, Degendorfer P, Panzarella T, Burke B, Sutcliffe SB, Koren G. Maternal and fetal outcome after breast cancer in pregnancy. Am J Obstet Gynecol. 1992;166:781–787. doi: 10.1016/0002-9378(92)91334-7. [DOI] [PubMed] [Google Scholar]
- 14.Dodds L, Fell DB, Joseph KS, Dewar R, Scott H, Platt R, Aronson KJ. Relationship of time since childbirth and other pregnancy factors to premenopausal breast cancer prognosis. Obstet Gynecol. 2008;111:1167–1173. doi: 10.1097/AOG.0b013e31816fd778. [DOI] [PubMed] [Google Scholar]
- 15.Albrektsen G, Heuch I, Hansen S, Kvale G. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br J Cancer. 2005;17:167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sankila R, Heinavaara S, Hakulinen T. Survival of breast cancer patients after subsequent term pregnancy: “healthy mother effect”. Am J Obstet Gynecol. 1994;170:818–823. doi: 10.1016/s0002-9378(94)70290-x. [DOI] [PubMed] [Google Scholar]
- 17.Keilding N. Delayed entry. In: Armitage P, Colton T, editors. Encyclopedia of biostatistics. Wiley; Hoboken, NJ: 2005. [Google Scholar]
- 18.Beadle BM, Woodward WA, Middleton LP, Tereffe W, Strom EA, Litton JK, Meric-Bernstam F, Theriault RL, Buchholz TA, Perkins GH. The impact of pregnancy on breast cancer outcomes in women <or = 35 years. Cancer. 2009;115:1174–1184. doi: 10.1002/cncr.24165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blakely LJ, Buzdarm AU, Lozada JA, Shullaih SA, Hoy E, Smith TL, Hortobagyi GN. Effects of pregnancy after treatment for breast carcinoma on survival and risk of recurrence. Cancer. 2004;100:465–469. doi: 10.1002/cncr.11929. [DOI] [PubMed] [Google Scholar]
- 20.Von Schoultz E, Johansson H, Wilking N, Rutgvist LE. Influence of prior and subsequent pregnancy on breast cancer prognosis. J Clin Oncol. 1995;13:430–434. doi: 10.1200/JCO.1995.13.2.430. [DOI] [PubMed] [Google Scholar]
- 21.Mueller BA, Simon MS, Deapen D, Kamineni A, Malone KE, Daling JR. Childbearing and survival after breast carcinoma in young women. Cancer. 2003;98:1131–1140. doi: 10.1002/cncr.11634. [DOI] [PubMed] [Google Scholar]
- 22.Mignot L. Pregnancy after treated cancer of the breast. Results of a case–control. Pathol Biol (Paris) 1989;37:1002. [PubMed] [Google Scholar]
- 23.Hemminki K, Försti A, Sundquist J, Ji J. Risk of familial breast cancer is not increased after pregnancy. Breast Cancer Res Treat. 2008;108:417–420. doi: 10.1007/s10549-007-9611-y. [DOI] [PubMed] [Google Scholar]
- 24.Dalberg K, Eriksson J, Holmberg L. Birth outcome in women with previously treated breast cancer—a population-based cohort study from Sweden. PLoS Med. 2006;3:e336. doi: 10.1371/journal.pmed.0030336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kroman N, Jensen MB, Melbye M, Wohlfahrt J, Mouridsen HT. Should women be advised against pregnancy after breast-cancer treatment? Lancet. 1997;350:319–322. doi: 10.1016/S0140-6736(97)03052-3. [DOI] [PubMed] [Google Scholar]
- 26.Kroman N, Mouridsen HT. Prognostic influence of pregnancy before, around, and after diagnosis of breast cancer. Breast. 2003;12:516–521. doi: 10.1016/s0960-9776(03)00159-0. [DOI] [PubMed] [Google Scholar]
- 27.Velentgas P, Daling JR, Malone KE, Weiss NS, Williams MA, Self SG, Mueller BA. Pregnancy after breast carcinoma: outcomes and influence on mortality. Cancer. 1999;85:2424–2432. doi: 10.1002/(sici)1097-0142(19990601)85:11<2424::aid-cncr17>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 28.Ibrahim EM, Ezzat AA, Baloush A, Hussain ZH, Mohammed GH. Pregnancy-associated breast cancer: a case–control study in a young population with a high-fertility rate. Med Oncol. 2000;17:293–300. doi: 10.1007/BF02782194. [DOI] [PubMed] [Google Scholar]
- 29.Keleher A, Wendt R, III, Delpassand E, Stachowiak AM, Kuerer HM. The safety of lymphatic mapping in pregnant breast cancer patients using Tc-99m sulfur colloid. Breast J. 2004;10:492–495. doi: 10.1111/j.1075-122X.2004.21503.x. [DOI] [PubMed] [Google Scholar]
- 30.Murphy CG, Mallam D, Stein S, Patil S, Howard J, Sklarin N, Hudis CA, Gemignani ML, Seidman AD. Current or recent pregnancy is associated with adverse pathologic features but not impaired survival in early breast cancer. Cancer. 2012;118:3254–3259. doi: 10.1002/cncr.26654. [DOI] [PubMed] [Google Scholar]
- 31.Gelber S, Coates AS, Goldhirsch A, castiglione-Gertsch M, Marini G, Lindtner J, Edelmann DZ, Gudgeon A, Harvey V, Gelber RD International Breast Cancer Study Group. Effect of pregnancy on overall survival after the diagnosis of early-stage breast cancer. J Clin Oncol. 2001;19:1671–1675. doi: 10.1200/JCO.2001.19.6.1671. [DOI] [PubMed] [Google Scholar]
- 32.Cooper DR, Butterfield J. Pregnancy subsequent to mastectomy for cancer of the breast. Ann Surg. 1970;171:429–433. doi: 10.1097/00000658-197003000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ives A, Saunders C, Bulsara M, Semmens J. Pregnancy after breast cancer: population based study. BMJ. 2007;334:194. doi: 10.1136/bmj.39035.667176.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Largillier R, Savignoni A, Gligorov J, Chollet P, Guilhaume MN, Spielmann M, Luporsi E, Asselain B, coudert B, Namer M GET(N)A Group. Prognostic role of pregnancy occurring before or after treatment of early breast cancer patients aged <35 years: a GET(N)A working group analysis. Cancer. 2009;115:5155–5165. doi: 10.1002/cncr.24608. [DOI] [PubMed] [Google Scholar]
- 35.Valachis A, Tsali L, Pesce LL, Polyzos NP, Dimitriadis C, Tsalis K, Mauri D. Safety of pregnancy after primary breast carcinoma in young women: a meta-analysis to overcome bias of healthy mother effect studies. Obstet Gynecol Surv. 2010;65:786–793. doi: 10.1097/OGX.0b013e31821285bf. [DOI] [PubMed] [Google Scholar]
- 36.Azim HA, Jr, Santoro L, Russell-Edu W, Pentheroudakis G, Pavlidis N, Peccatori FA. Prognosis of pregnancy-associated breast cancer: a meta-analysis of 30 studies. Cancer Treat Rev. 2012;38:834–842. doi: 10.1016/j.ctrv.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Davies GA, Wolfe LA, Mottola MF, MacKinnon C Society of Obstetricians and gynecologists of Canada, SOGC Clinical Practice Obstetrics Committee . Joint SOGC/CSEP clinical practice guideline: exercise in pregnancy and the postpartum period. Can J Appl Physiol. 2003;28:330–341. [PubMed] [Google Scholar]
- 38.Huzarski T, Byrski T, Gronwald J, Gorski B, Domagala P, Cybulski C, Oszurek O, Szwiec M, Gugala K, Stawicka M, Morawiec Z, Mierzwa T, Janiszewska H, Kilar E, Marczyk E, Kozak-Klonowska B, Siolek M, Surdyka D, Wisniowski R, Posmyk M, Sun P, Lubinski J, Narod SA Polish Breast Cancer Consortium. Ten year survival in BRCA1-negative and BRCA1-positive breast cancer patients. J Clin Oncol. 2013;31:3191–3196. doi: 10.1200/JCO.2012.45.3571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.