Abstract
Wnt signaling pathways play important roles in tumorigenesis and are initiated by binding of Wnt to various receptors including frizzleds (FZDs). FZDs are one of several families of receptors comprised of FZD/LRP/ROR2/RYK in the Wnt signaling pathway. Expression of some FZD receptors are up-regulated, thereby activating the Wnt signaling pathway and is correlated with cancer malignancy and patient outcomes (recurrence and survival) in many cancers. The FZD family contains ten genes in humans and their function has not been completely examined including the regulatory mechanisms of FZD genes in cancer. Knockdown of FZDs may suppress the Wnt signaling pathway resulting in decreased cell growth, invasion, motility and metastasis of cancer cells.
Recently a number of microRNAs (miRNAs) have been identified and reported to be important in several cancers. MiRNAs regulate target gene expression at both the transcription and translation levels. The study of miRNA is a newly emerging field and promises to be helpful in understanding the pathogenesis of FZDs in cancer. Also miRNAs may be useful in regulating FZDs in cancer cells.
Therefore the aim of this review is to discuss current knowledge of the functional mechanisms of FZDs in cancer, including regulation by miRNAs and the potential for possible use of miRNAs and FZDs in future clinical applications.
Keywords: FZD, cancer, microRNA, Wnt signaling
Introduction
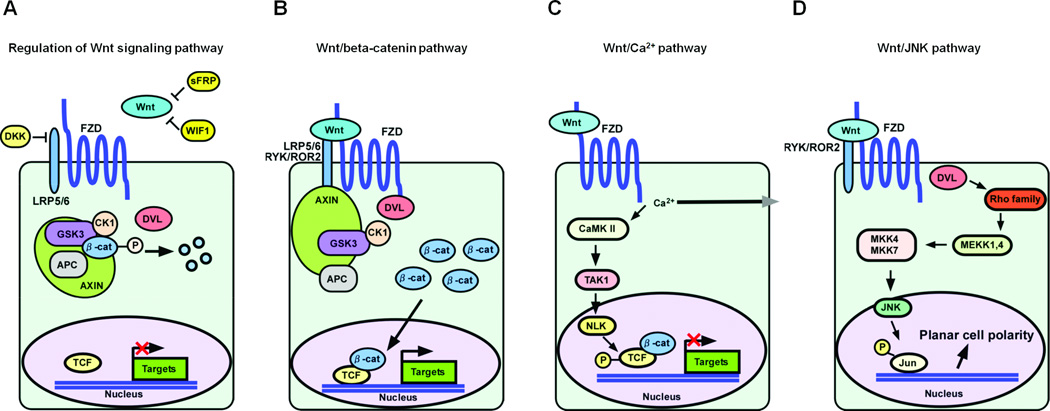
Frizzled homolog protein (FZD) is a seven-pass transmembrane type receptor and 10 members have been identified (FZD1–FZD10) in humans (1) (Figure 1). Wnt is a ligand of FZDs and consists of nineteen family genes in humans. The Wnt signaling pathway is initiated by the binding of Wnt ligands to the complex compromised of FZD and low-density lipoprotein receptor–related proteins 5/6 (LRP5/6)/ROR2/RYK, resulting in regulation of diverse cellular functions (2–5). The Wnt signaling pathway is comprised of canonical and noncanonical signals. In the canonical Wnt signaling pathway, Wnts bind to a complex of FZD and LRP5/6. The resultant signals prevent β-catenin phosphorylation by a multiprotein complex composed of adenomatous polyposis coli (APC), glycogen synthase kinase 3β, casin kinase 1, and axins, causing its proteosomal degradation. The beta-catenin associates with T cell factor (TCF)/lymphocyte enhancer transcription factors to activate target genes involved in cell survival, proliferation, or invasion (Figure 1B). The non-canonical Wnt signaling pathway consists of the Wnt/Ca2+ pathway and Wnt/c-Jun N-terminal kinase (JNK) (planar cell polarity) pathway (6). In the non-canonical Wnt signaling pathway, Wnts bind to a complex comprised of FZD and ROR2/RYK while the Wnt/Ca2+ pathway, Wnt activates intracellular Ca2+ signaling, as well as Ca2+-dependent protein kinases, such as protein kinase C (PKC) and calmodulin dependent protein kinase II (7) (Figure 1C). In the Wnt/JNK pathway, receptor stimulation activates Dishevelled (Dvl), which in turn activates the Rho family of GTPases such as RhoA and Rac. RhoA stimulates c-Jun expression through phosphorylation of c-Jun by Rho associated kinase (ROCK) (8–10) (Figure 1D). Therefore FZD plays a crucial role in both canonical and non-canonical pathways and the expression of FZD has been reported to be up-regulated in some cancer tissues.
Figure 1. Schematics of Wnt signaling pathway in cancer cells.
A. Regulation of Wnt signaling pathway in normal cells. Beta-catenin is usually phosphorylated and degradated by a multiprotein complex composed of APC, GSK3, CK1 and AXIN.
B. Wnt/beta-catenin pathway. Wnt ligands bind to the complex compromised of FZD/LRP/ROR/RYK, resulting in the constitutive stabilization of beta-catenin. The beta-catenin associates with TCF to activate target genes.
C. Wnt/Ca2+ pathway (non-canonical pathway). Wnt activates intracellular Ca2+ signaling as well as Ca2+-dependent protein kinases such as PKC and CaMK II. TAK1 and NLK can interfere with TCF/beta-catenin signaling.
D. Wnt/JNK pathway (non-canonical pathway). Receptor stimulation activates DVL, which in turn activates the Rho family of GTPases. Rho stimulates c-Jun expression through phosphorylation of c-Jun by ROCK.
Non-coding RNAs are very numerous and potentially important in gene and protein regulation. Currently microRNAs are well known as examples of non-coding RNAs (11, 12) and over 1500 human miRNAs have been identified based on miRBase [http://www.mirbase.org/]. miRNAs bind to the 3’UTR of target gene mRNA and repress translation or induce mRNA cleavage, thereby inhibiting translation from mRNA to protein (13). Thus miRNAs regulate target genes including FZDs, resulting in inhibition of diverse Wnt signaling pathways. Aberrant expression of miRNAs has been reported in many types of cancers and can function as tumor suppressor genes or oncogenes (14–16). Decreased expression of tumor suppressor miRNAs result in increased expression of target oncogenes. In contrast, increased expression of oncogenic microRNAs leads to loss or decreased expression of target tumor suppressor genes. So far, there have been few reports regarding the mechanism of FZD gene expression in cancer. Therefore identifying miRNAs regulating FZD expression in cancer tissues will be helpful to understand the mechanisms of FZD gene expression in cancer. Based on previous literature, five miRNAs (miR-204, miR-31, miR-493, miR-194, miR-23b) have been reported to regulate five FZD genes.
In this review, we focus on the function of FZDs (FZD1–FZD10) in cancer and discuss current regulatory mechanisms including miRNAs in the context of understanding their potential roles in tumorigenesis (Table 1).
Table 1.
FZDs and Wnt signaling pathway in Cancer
| FZD | chromosome | Wnt signaling pathway | Over-expressed in Cancer/reference | microRNA/function in cancer/reference | |||
|---|---|---|---|---|---|---|---|
| FZD1 | 7q21 | Canonical | colon, ovarian, breast, neuroblastoma | 19, 20, 21, 22 | miR-204/suppressor | 29, 30, 31 | |
| FZD2 | 17q21.1 | Canonical | Non-canonical (Ca2+) | Wilms’ tumour, melanoma, lung | 33, 34, 35, 36 | ||
| FZD3 | 8p21 | Canonical | Non-canonical (PKA) | lung, leukemia, myeloma, lymphoma, sarcoma | 36, 41, 42 | miR-31/suppressor | 48 |
| FZD4 | 11q14-q21 | Canonical | cervical uterus, leukemia colon, melanoma, pancreatic | 49 | miR-493/suppressor | 55 | |
| FZD5 | 2q33.3-q34 | Canonical | kidney, prostate | 57, 58 | |||
| FZD6 | 8q22.3-q23.1 | Non-canonical (Ca2+) | squamous cell carcinomas | 64 | miR-194/unknown | 70 | |
| FZD7 | 2q.33 | Canonical | Non-canonical (JNK) | esophageal, gastric, nasopharyngeal, adenoid cystic, hepatocellular, colon, Wilms’ tumour | 71, 72, 73, 74, 75, 76, 77, 78 | miR-23b/suppressor | 88, 89, 90 |
| FZD8 | 10p11.2 | Canonical | cervical uterus, kidney, leukaemia, lung | 41, 57, 91, 92 | |||
| FZD9 | 7q11.23 | Non-canonical (ERK) | glioblastoma, astrocytoma | 96 | |||
| FZD10 | 12q24.33 | Canonical | Non-canonical (JNK) | colon, lung, sarcoma | 104, 105, 106, 107 | ||
FZD1 (Frizzled-1) and miRNA
Human FZD1 was first cloned and mapped to chromosome 7q21 by Sagara et al (17). Human FZD1 interacts with Wnts1-3 and Wnt3a increasing Wnt/beta-catenin signaling and resulting in stimulation of diverse tumorigenic processes. (18). FZD1 expression has been reported to be up-regulated in several cancers including colon, ovarian, breast neuroblastoma (19–22). In one study, Wang et al used thiazolidinediones, a novel cancer drug for breast cancer, and found that the drug decreased mRNA expression of both FZD1 and LRP6, inhibited beta-catenin mediated transactivation, and resulted in the inhibition of cell growth in breast cancer cell lines (23).
Two doxorubicin resistant neuroblastoma cancer cell lines had amplification of the chromosome 7q21 region (24) and overexpression of FZD1 and MDR1 (multidrug resistant gene) was found (22) in these doxorubicin resistant neuroblastoma cell lines. In this study, FZD1 silencing dramatically reduced MDR1 expression (22). Since this report showed the association of FZD1 and MDR1, these results may be helpful in understanding the function of FZD1 in chemo-resistant cancer cells.
In contrast, one report showed LOH in the same region (chromosome 7q21) in follicular thyroid carcinoma and FZD1 expression was downregulated in these tumors. Cell growth and invasion ability were also decreased in follicular thyroid carcinoma cell lines (25). Therefore depending on FZD1 expression levels in cancer tissues or condition, the function of FZD1 may vary.
To date, one paper has documented the association of FZD1 with miRNA-204. MiR-204 is one of several down-regulated miRNAs in senescent human trabecular meshwork cells (26, 27). Over-expression of miR-204 decreased FZD1 mRNA expression in two primary human trabecular meshwork cell lines and there was lower luciferase activity using plasmid containg the FZD1 3’-UTR sequence in HEK 293 cells (28), suggesting that miR-204 regulates FZD1 expression directly.
Regarding miR-204 function, several reports have shown it to be a tumor suppressor gene in head and neck, endometrial and renal cancers (29–31). Additional research will be required to elucidate the regulation of FZD1 by miR-204.
FZD2
Human FZD2 has been mapped to chromosome 17q21.1 (32). Since FZD2 mRNA is expressed in most human adult and fetal tissues, FZD2 expression is up-regulated in several cancers including primary Wilms’ tumour, melanoma and lung squamous cell carcinoma. (33–36). Wnt5a binds to FZD2 and activates the WNT/Ca2+ signaling pathway in melanoma cell lines (35). In the presence of Wnt3a, FZD2 also activates Wnt/beta-catenin signaling in pulmonary carcinoma (37). These reports suggest that FZD2 in the presence of Wnts may activate both canonical and non-canonical Wnt signaling pathways in cancer. So far no studies have reported the association of miRNAs with FZD2 regulation.
FZD3 and miRNA
Human FZD3 was mapped to chromosome 8p21 by Kirikoshi et al (38) and Sala et al (39). Kirikoshi et al reported that FZD3 mRNA is expressed in normal tissues (skeletal muscle, kidney, pancreas, cerebellum and cerebral cortex) and cancer cell lines and Sala et al also reported that FZD3 mRNA was expressed in most normal tissues. Although FZD3 mRNA was down-regulated during progression of ovarian carcinoma (40), FZD3 expression was up-regulated in several cancers (lung squamous cell carcinoma tissues, primary acute and lymphoblastic leukemia, myeloma, lymphoma, Ewing sarcoma) (36, 41, 42).
In one report, expression of sFRP1 (secreted Frizzled-Related Protein 1), a Wnt antagonist, was significantly decreased by DNA methylation in acute leukemia and interestingly over-expression of FZD3 mRNA correlated with hypermethylation of the sFRP1 (secreted Frizzled-Related Protein 1) (43). This result suggested that activation of aberrant Wnt signalling may be caused by the cooperation of SFRP1 down-regulation and FZD3 over-expression (43).
High FZD3 expression levels correlated with Wnt target gene, c-Myc and Cyclin D1 in sporadic adenoma, familial adenomatous polyposis and chronic lymphocytic leukemia (44, 45). FZD3 activated several Wnt/beta-catenin signaling pathways in the presence of Wnt3 and LRP6 compared to Wnt3 only in chronic lymphocytic leukemia (45). FZD3 regulated Wnt-3a-dependent neurite outgrowth in Ewing sarcoma (46), activated Galpha(s)/cAMP/PKA signaling pathway in the presence of Wnt5a and inhibited cell migration in breast cancer cell lines (47). As these reports show that FZD3 activates or inhibits cancer cells in the presence Wnt ligands and LRP, if FZD3 were to be used in cancer as a biomarker, both FZD3 and Wnt ligand expression should be analyzed. Thus FZD3 function as oncogene and has potential as a therapeutic target gene. With regards to miRNA-FZD3, one report has been published about breast cancer. In this study, Valastyan et al focused on miR-31 in breast cancers since it was expressed in primary normal human mammary epithelial cells and non-metastatic breast tumor cells, but not expressed in metastatic breast cancer cell lines. Cell invasion/migration and lung metastasis were inhibited in miR-31 transfected MDA-MB-231 cells compared to controls. FZD3 protein level and luciferase activity using a plasmid vector with the FZD3 3’-UTR sequence in miR-31 transfected MDA-MB-231 cells was decreased compared to control transfectants. Knockdown of FZD3 decreased MDA-MB-231 cell invasion (48). So far this study is the only one showing miR-31 as a novel microRNA targeting FZD3.
FZD4 and miRNA
Human FZD4 was mapped to chromosome 11q14-q21 (49) and was reported to be expressed in most normal human tissues. Sagara et al also reported that FZD4S, a splicing variant of the FZD4 gene (50), was expressed in adult heart, lung, fetal kidney and lung using RNA dot blot analysis. FZD4S inhibited and activated Wnt/beta-catenin signaling in the presence of Wnts in Xenopus (51). FZD4 activated the Wnt/beta-catenin signaling pathway and is related to epithelial to mesenchymal transition marker, E-cadherin and Snail1 expression in VaP cells (prostate cancer cell line) with TMPRSS2-ERG gene fusion and U87R4 cell lines being highly invasive (52, 53). Primers and antibodies able to differentiate between FZD4 and FZD4S should be used in the analysis of FZD4 expression because FZD4 and FZD4S may function differently in cancer. High methylation at the FZD4 loci was associated with progression-free survival in epithelial ovarian cancer (54). Though no studies have reported about FZD4 expression in cancer and normal tissues, methylation at the FZD4 locus may be a good cancer marker. One report has shown possible regulation of FZD4 by miR-493. MiR-493 was down regulated in bladder cancer tissues and bladder cancer cell lines (J82, T24, TCC-SUP) compared to normal tissues and cell lines (SV-HUC-1). Over-expression of miR-493 decreased T24 and J82 cell migration and motility and FZD4 protein levels and luciferase activity in miR-493 transfected T24 cells was decreased compared to controls, indicating that FZD4 is a target gene of miR-493. Also knockdown of FZD4 decreased cell migration and motility in T24 and J82 cells (55). As it is possible that FZD4S shares the same 3’UTR sequence with FZD4, both may be targets of miR-493, but there have been no reports regarding FZD4S in mammalian cells including cancer (51).
FZD5
Human FZD5 was mapped to chromosome 2q33.3-q34 (56) and its expression was reported to be up-regulated in renal cell carcinoma and advanced prostate cancer tissues compared to normal kidney and benign prostatic hyperplasia (BPH) tissues, respectively (57, 58). Additionally FZD5 protein levels correlated with the Wnt target gene, cyclin D1 protein expression levels in renal cell carcinoma (57). In another report, Wnt7a, a ligand of FZD5, activated the Wnt/beta-catenin signaling pathway and cell motility/invasion in metastatic melanoma, endometrial and ovarian cancer cell lines (59, 60, 61, 62). These reports suggest that FZD5 may be a biomarker and potential therapeutic target gene in cancer.
FZD6
Human FZD6 is on chromosome 8q22.3-q23.1 (63) and expressed in most adult normal tissues, fetal brain, liver, lung and kidney and cancer cell lines. FZD6 expression has been reported to be higher in cancers such as squamous cell sarcoma and some adenomas (64). According to array comparative genomic hybridization (aCGH) data, chromosome 8q22.3 in 61% of prostate cancer cases was amplified and FZD6 expression correlated with amplification of 8q22.3 in prostate cancer (65). Also in another report, high FZD6 expression was significantly correlated with poor survival in human neuroblastoma (66).
In contrast, FZD6 is a negative regulator of the Wnt/beta-catenin signaling pathway through the CaMKII-mediated TAK1-NLK pathway (Wnt/Ca2+ signaling pathway) (7). However mouse FZD6 interacted with Wnt4 through mouse FZD6 CRD (conserved cysteine-rich domain) (67) and activated the Wnt/JNK signaling pathway (68). In another report, FZD6 and Wnt4 protein were widely expressed in several adenoma tissues however localization of beta-catenin in the nuclei was not observed. ERK1/2, which is a non-canonical related gene, was highly activated in GHomas and TSHomas (69). Considering these reports, FZD6 may be involved in the non-canonical Wnt pathway in cancer but not in the Wnt/beta-catenin pathway.
Transcription factor 1 [Tcf1; hepatocyte nuclear factor 1a (HNF1a)] plays an important role in human hepatocytes. Down-regulation of the miR-192/-194 cluster was found in the livers of Tcf1−/− mice. Over-expression of miR-194 decreased FZD6 mRNA and luciferase activity with plasmids containing FZD6 3’-UTR sequence in miR-194 transfected HEK 293 cells compared to controls (70).
FZD7 and miRNA
Human FZD7 resides on chromosome 2q.33 (17) and is expressed in adult normal skeletal muscle, heart, brain, placenta, kidney, fetal kidney and lung. FZD7 mRNA is up-regulated in several cancers including esophageal, gastric, nasopharyngeal, adenoid cystic, hepatocellular, colon, Wilms’ tumour (71) (FzE3 primers shown in this paper were FZD7 based on BLAST [http://blast.ncbi.nlm.nih.gov/Blast.cgi].) (72–78). Additionally high FZD7 expression correlated with a significantly shorter survival time in colon and gastric cancer (10, 33, 79). FZD7 activates Wnt/beta-catenin signaling in several cancers including hepatocellular carcinoma, colon cancer and TNBC (triple negative breast cancer) (75–78, 80). It was reported that Wnt3 is a ligand of FZD7 (81). FZD7 also regulates Wnt/JNK signaling in colon cancer (10) and therefore may be involved in both canonical and non-canonical signaling pathways in cancer. Knockdown of FZD7 decreased cell growth in colon cancer cell lines with APC or CTNNB1 gene mutations (75), caused an increase in SNAI2 mRNA, a decrease in E-cadherin mRNA and inhibited MET (mesenchymal–epithelial transition) in non-adherent colorectal cancer cell carcinoid cell lines LIM1863-Mph. (82, 83). Expression levels of FZD7 mRNA were higher compared to other FZD family genes in colon cancer cell lines. It was report that the activity of the FZD7 gene promoter was regulated by beta-catenin in colorectal cancers (84). FZD7 mRNA levels were significantly higher in stage II, III or IV colon cancer tissues (10). Expression levels of Wnt11 mRNA were significantly higher in stage I-IV tumor tissues than in non-tumor tissues and correlated with those of FZD7 mRNA. Patient groups with high FZD7 and high Wnt11 were significantly associated with shorter disease free survival compared to low FZD7 and low Wnt11 groups (85).
Thus FZD7 may be a novel oncogene and several therapeutic options have been described. For example, compounds such as FJ9 and small interfering peptides (RHPDs) suppressed cell growth and activity of the Wnt/beta-catenin signaling pathway by inhibiting interaction between FZD7 and Dishevelled (DVL) in cancer cell lines (86, 87).
Currently miRNA has emerged as another FZD7 regulator. A previous study indicates that miR-23b targets FZD7 in colon cancer (88). MiR-23b down-regulated cell migration, invasion and induced apoptosis based on genome-wide functional screening (88). Primary tumor growth and lung metastasis were inhibited in miR-23b transfected colon cancer cells (HCT–116) compared to controls. FZD7 protein levels and luciferase activity with the FZD7 3’-UTR in miR-23b transfected HCT-116 cells were decreased compared to mock transfectants, indicating that miR-23 directly targets FZD7. Also knockdown of FZD7 decreased cell invasion in HCT-116 cells (88). The expression of miR-23b was significantly downregulated in several cancer tissues (89) and studies indicate a miR-23b tumor suppressor function targeting cMET in hepatocellular carcinoma (90).
FZD8
Human FZD8 is located on chromosome 10p11.2 (91) and expressed in fetal kidney and brain and in adult kidney, heart, pancreas, and skeletal muscle. In some cancers (renal cell carcinoma, acute lymphoblastic leukemia and lung cancer), FZD8 expression was found to be up-regulated (41, 57, 92) and related to lung cancer cell growth though activity of the Wnt/beta-catenin signaling pathway (92). FZD8 activate Wnt/beta-catenin signaling in the presence of LRP6 and Wnt-3a in cancer (93), though there are few reports about its role and mechanism.
FZD9
Deletion of a part of chromosome band 7q11.23 was reported in Williams syndrome and human FZD9, previously reported as FZD3, in the deletion region was cloned by Wang et al. (94, 95). FZD9 was reported to be expressed in normal brain, testis, eye, skeletal muscle and kidney and FZD9 expression was up-regulated in glioblastoma and astrocytoma (96). Intensity of FZD9 immunostaining was strongly associated with tumor grade in glioblastoma and astrocytoma (96). The FZD9 promoter is methylated in glioblastoma multiforme, myelodysplastic syndromes and acute myeloid leukemia (97, 98) and hypermethylation was associated with poor survival in these diseases (98).
The degree of promoter methylation and expression of FZD9 may be a tumor marker in cancer. Although FZD9 or Wnt7a individually did not inhibit growth of non-small cell lung cancer, co-expression of FZD9 and Wnt7a decreased cell growth and promoted cell differentiation through ERK-5-dependent activation of PPARγ and Sprouty-4 (99, 100, 101). Knockdown of FZD9 decreased cyclin D1 protein expression and suppressed cell growth/motility in hepatoma cell lines (102). Thus FZD9 may be a tumor suppressor or oncogene in the presence of Wnt ligands in different kinds of cancer.
FZD10
Human FZD10 has been mapped to chromosome 12q24.33 (103) and found to be expressed in adult normal placenta, brain, heart, lung, skeletal muscle, pancreas, spleen, and prostate and fetal kidney, lung and brain. Similar to others in the FZD family, FZD10 expression was reported to be higher in some cancer tissues (colon, lung squamous cell carcinoma, synovial sarcoma) (104–107). Concerning the regulatory mechanism of FZD10, HIG2 (hypoxia-inducible protein-2) activated Wnt/beta-catenin signaling by binding to FZD10 in renal cell carcinoma (108). FZD10 increased the phosphorylation level of c-jun in endometrial cancer in the presence of Wnt7A (61) and activated the Wnt/Dvl-Rac-JNK signaling pathway. These reports show that FZD10 regulates both the canonical and non-canonical signaling pathways in the presence of different ligands in cancer. Based on the recent literature, some groups have documented the effectiveness of anti-FZD10 antibody therapy to synovial sarcomas (SS) since FZD10 increases cell growth in synovial sarcoma (107, 109).
Although there are no reports about miRNAs targeting FZD10, the success of antibody based therapeutics will be helpful for miRNA replacement therapy in cancer treatment.
Relationship of FZD with co-receptors and other Wnt related genes in cancer
LRP5/6, RYK and ROR2 have been known as co-receptors of FZDs (110, 111). When exposed to Wnts, LRP5/6 forms a complex with Wnt and Fzs (Wnt/FZD/LRP complex), resulting in activation of the Wnt/beta-catenin pathway (112–118) (Figure 1B). The Wnt/FZD/LRP complex recruits axin to the plasma membrane and inhibiting destruction of the complex. Based on previous literature, LRP5 or LRP6 have been regarded as biomarkers in several cancers such as osteosarcoma (119) and breast cancer (120–122) and anti-LRP6 antibody blocks the Wnt/beta-catenin pathway inhibiting cell proliferation in cancer cells (123, 124). Two additional receptors such as receptor-like tyrosine kinase (RYK) and receptor tyrosine kinase-like orphan receptor 2 (ROR2) can bind to Wnts (125). RYK is a PTKs (protein tyrosine kinase) family protein (126–128) and is required to activate the Wnt/beta-catenin and Wnt/JNK signaling pathway (147–149) (Figure1B and 1D). It has been reported that RYK is a tumor marker in ovarian cancer (129, 130) and the RYK gene is truncated in leukemia (131). ROR2 has a tyrosine kinase-like domain (132) and activates the Wnt/beta-catenin pathway in the presence of FZD2/Wnt3a in lung cancer cells (133). ROR2 knockdown blocks the Wnt/JNK pathway (134) and inhibits cell metastasis in several cancer cells (osteosarcoma, melanoma, kidney and prostate cancer) (134–137) (Figure1B and 1D). ROR2 is a prognostic tumor marker and therapeutic target in leiomyosarcoma and gastrointestinal stromal cancer (138). Although it is highly possible that these co-receptors play an important role in Wnt signaling in cooperation with FZDs, there have been few reports regarding the relationship and interaction between FZDs and LRP/RYK/ROR2 in cancer.
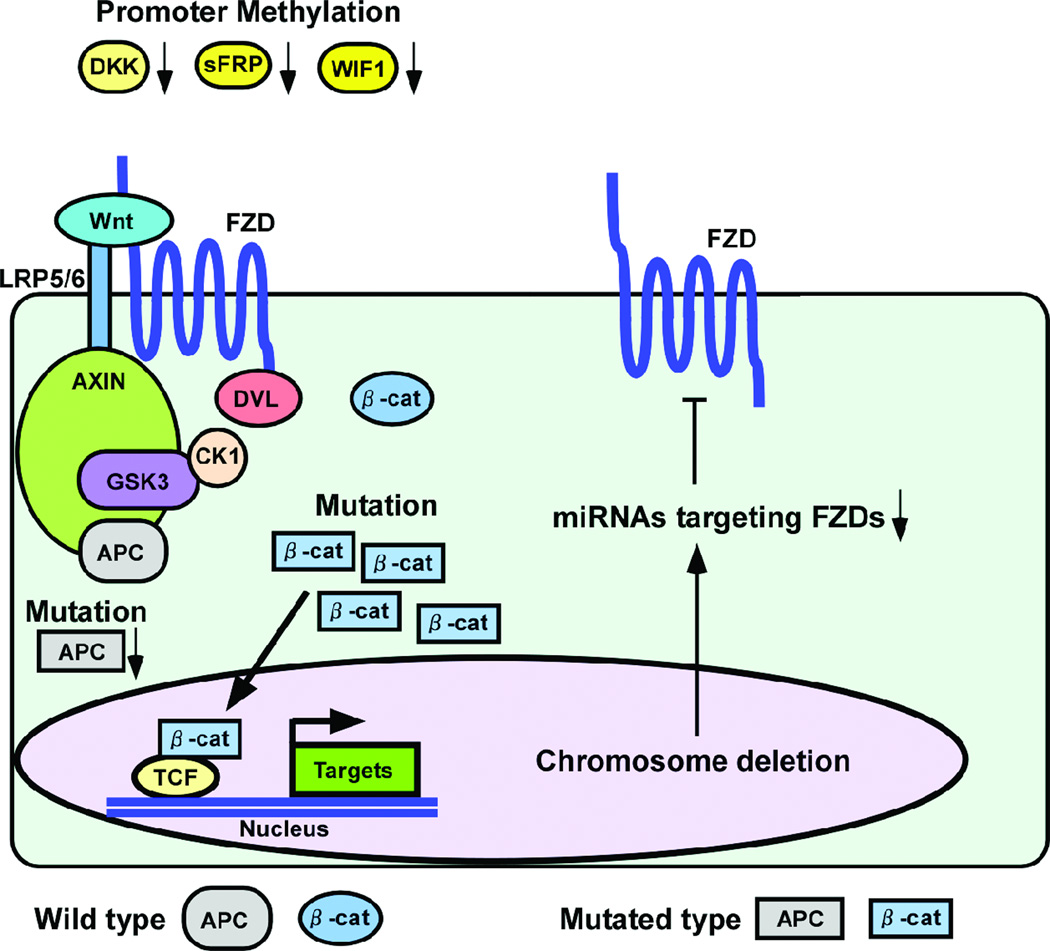
Apart from co-receptors, several Wnt antagonists have been identified and reported as inhibitors of Wnt signaling pathways. Conventional Wnt antagonists such as sFRP (secreted Frizzled-Related Protein), Dkk (Dickkopf) and Wif1 (Wnt inhibitory factor 1) bind to Wnt and LRP and inhibit the Wnt signaling pathway (Figure 1A) (139). Based on previous reports, expression of sFRP, Dkk and Wif1 is down-regulated in many cancers because of promoter hypermethylation (140–145). In the Wnt/beta-catenin signaling pathways in cancer cells, the function of APC gene, a crucial tumor suppressor, is decreased or lost because of gene mutation. In contrast, the beta-catenin gene is mutated at phosphorylation sites, resulting in inhibition of beta-catenin degradation (146). Down regulation of the Wnt antagonists and mutation of APC/beta-catenin genes is often paralleled with up-regulation of FZDs (Table 1), but their direct relationships are not currently understood.
As mentioned above, miRNAs may be involved in the regulation of FZD protein expression. Interestingly some of hose miRNAs are mapped to deleted chromosomal regions based on previous reports as follows: 1. chromosome 9p21 (miR-31 targeting FZD3), 2. 9q21 (miR-204 targeting FZD1), 3. 9q22 (miR-23b targeting FZD7) and 4. 14q32 (miR-493 targeting FZD4) (147–153). These specific chromosome deletions may cause over-expression of these FZDs thorough loss of a particular miRNA (Figure 2).
Figure 2.
Association of FZD with other Wnt related genes and proteins. Chromosome deletions may cause over-expression of some FZDs thorough loss of a particular miRNA. Expression of sFRP, Dkk and Wif1 is down-regulated because of promoter hypermethylation. Mutation of APC/beta-catenin genes is detected in many cancers.
As shown in Figure 2, FZD plays an important role with other co-receptors such as LRP5/LRP6, RYK and ROR2 in Wnt signaling in cancer cells. Of course the expression pattern and function of FZD and other Wnt related genes are different depending on the cancer types. However many diverse factors are involved in Wnt signaling, thus it is important to focus on the various genes and proteins to identify the regulatory mechanisms of Wnt signaling in cancer cells.
Conclusions and future perspectives
As described above, currently ten FZD receptors have been identified. Based on previous literature, FZDs expression is higher in cancer tissues suggesting that FZDs will be potentially valuable therapeutic targets. As a therapeutic approach, some groups have used anti-human FZD10 antibody for cancer treatment. For instance, mouse anti-human FZD10 monoclonal antibody labeled with Yttrium-90 (90Y) decreased in vivo tumor growth in mice with FZD10-positive synovial sarcoma cell lines SYO-1 and FZD10-transfected colon cancer cell line DLD-1 (109, 154).
Additionally new compounds such as FJ9 and small interfering peptides (RHPDs) have emerged as potential therapeutic tools by inhibiting the interaction between FZD7 and Dishevelled (DVL) in cancer cell lines (86, 87). Another study showed that rat anti-human FZD7 monoclonal antibody decreased the number of spheres, colonies and in vivo proliferation of the chick chorio-allantoic-membrane in primary FZD7-positive Wilms’ tumor (155). Antibody treatment against other FZDs may also affect cancer cell growth and metastasis. In a basic research setting, targeting therapy with small RNAs has been shown to be effective. For example, siRNA (small interfering RNA) and shRNA (short hairpin RNA) are loaded onto RISC (RNA inducible silencing complex) to induce mRNA cleavage of target genes (156) and clinical trials of siRNA and double-stranded RNA treatments have been performed in cancer therapy (156).
Recently microRNA has emerged as a new treatment option. MicroRNAs trigger mRNA cleavage or inhibition of target gene translation after being loaded onto RISC (156). So far, numerous labs have focused on the study of miRNAs and found that they dose-dependently inhibit cell proliferation and metastasis in an in vivo models (157–159). Thus miRNAs have been used as therapeutic agents in cancer therapy. In spite of much progress in miRNAs replacement therapy or siRNA therapy both in vitro and in vivo, there are still several problems to be addressed for clinical application. The most important issue is the safety of miRNA or siRNA delivery systems when these replacement therapies are performed in a clinical setting. Whether miRNA replacement therapy or siRNA therapy will be effective as novel anti-cancer therapy will to depend on the success of the delivery system.
Over-expression of tumor suppression miRNAs targeting FZDs, results in suppression of cell growth and metastasis through the Wnt signaling pathway. Since there are few reports describing miRNAs targeting FZDs for use in therapeutics, the antibody therapeutic successes will provide helpful information in this regard. Additional FZDs research will also contribute to uncovering and utilizing microRNAs as therapeutic options for cancer treatment.
Acknowledgements
We thank Dr. Roger Erickson for his support and assistance with the preparation of the manuscript. This study was supported by National Center for Research Resources of the National Institutes of Health through Grant Number R01CA138642, R01CA130860, R01CA160079, VA Merit Review grants and VA Program Project.
Footnotes
Conflict of Interest: The authors disclose no potential conflicts of interest.
References
- 1.Huang HC, Klein PS. The Frizzled family: receptors for multiple signal transduction pathways. Genome Biol. 2004;5:234. doi: 10.1186/gb-2004-5-7-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signaling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]
- 3.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127:469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 4.Katoh M, Katoh M. WNT signaling pathway and stem cell signaling network. Clin Cancer Res. 2007;13:4042–4045. doi: 10.1158/1078-0432.CCR-06-2316. [DOI] [PubMed] [Google Scholar]
- 5.Lai SL, Chien AJ, Moon RT. Wnt/Fz signaling and the cytoskeleton: potential roles in tumorigenesis. Cell Res. 2009;19:532–545. doi: 10.1038/cr.2009.41. [DOI] [PubMed] [Google Scholar]
- 6.Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–798. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- 7.Golan T, Yaniv A, Bafico A, Liu G, Gazit A. The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt. beta-catenin signaling cascade. J Biol Chem. 2004;279:14879–14888. doi: 10.1074/jbc.M306421200. [DOI] [PubMed] [Google Scholar]
- 8.Marinissen MJ, Chiariello M, Tanos T, Bernard O, Narumiya S, Gutkind JS. The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol Cell. 2004;14:29–41. doi: 10.1016/s1097-2765(04)00153-4. [DOI] [PubMed] [Google Scholar]
- 9.Fukukawa C, Nagayama S, Tsunoda T, Toguchida J, Nakamura Y, Katagiri T. Activation of the non-canonical Dvl-Rac1-JNK pathway by Frizzled homologue 10 in human synovial sarcoma. Oncogene. 2009;28:1110–1120. doi: 10.1038/onc.2008.467. [DOI] [PubMed] [Google Scholar]
- 10.Ueno K, Hazama S, Mitomori S, Nishioka M, Suehiro Y, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Down-regulation of frizzled-7 expression decreases survival, invasion and metastatic capabilities of colon cancer cells. Br J Cancer. 2009;101:1374–1381. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ying SY, Chang DC, Lin SL. The microRNA (miRNA): overview of the RNA genes that modulate gene function. Mol Biotechnol. 2008;38:257–268. doi: 10.1007/s12033-007-9013-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 13.McManus MT, Sharp PA. Gene silencing in mammals by small interfering RNAs. Nat Rev Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, Downing JR, Jacks T, Horvitz HR, Golub TR. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 15.Esquela-Kerscher A, Slack FJ. Oncomirs - microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 16.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sagara N, Toda G, Hirai M, Terada M, Katoh M. Molecular cloning, differential expression, and chromosomal localization of human frizzled-1, frizzled-2, and frizzled-7. Biochem Biophys Res Commun. 1998;252:117–122. doi: 10.1006/bbrc.1998.9607. [DOI] [PubMed] [Google Scholar]
- 18.Gazit A, Yaniv A, Bafico A, Pramila T, Igarashi M, Kitajewski J, Aaronson SA. Human frizzled 1 interacts with transforming Wnts to transduce a TCF dependent transcriptional response. Oncogene. 1999;18:5959–5966. doi: 10.1038/sj.onc.1202985. [DOI] [PubMed] [Google Scholar]
- 19.Holcombe RF, Marsh JL, Waterman ML, Lin F, Milovanovic T, Truong T. Expression of Wnt ligands and Frizzled receptors in colonic mucosa and in colon carcinoma. Mol Pathol. 2002;55:220–226. doi: 10.1136/mp.55.4.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badiglian Filho L, Oshima CT, De Oliveira Lima F, De Oliveira Costa H, De Sousa Damião R, Gomes TS, Gonçalves WJ. Canonical and noncanonical Wnt pathway: a comparison among normal ovary, benign ovarian tumor and ovarian cancer. Oncol Rep. 2009;21:313–320. [PubMed] [Google Scholar]
- 21.Milovanovic T, Planutis K, Nguyen A, Marsh JL, Lin F, Hope C, Holcombe RF. Expression of Wnt genes and frizzled 1 and 2 receptors in normal breast epithelium and infiltrating breast carcinoma. Int J Oncol. 2004;25:1337–1342. [PubMed] [Google Scholar]
- 22.Flahaut M, Meier R, Coulon A, Nardou KA, Niggli FK, Martinet D, Beckmann JS, Joseph JM, Mühlethaler-Mottet A, Gross N. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta-catenin pathway. Oncogene. 2009;28:2245–2256. doi: 10.1038/onc.2009.80. [DOI] [PubMed] [Google Scholar]
- 23.Wang PS, Chou FS, Bloomston M, Vonau MS, Saji M, Espinosa A, Pinzone JJ. Thiazolidinediones downregulate Wnt/beta-catenin signaling via multiple mechanisms in breast cancer cells. J Surg Res. 2009;153:210–216. doi: 10.1016/j.jss.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 24.Flahaut M, Mühlethaler-Mottet A, Martinet D, Fattet S, Bourloud KB, Auderset K, Meier R, Schmutz NB, Delattre O, Joseph JM, Gross N. Molecular cytogenetic characterization of doxorubicin-resistant neuroblastoma cell lines: evidence that acquired multidrug resistance results from a unique large amplification of the 7q21 region. Genes Chromosomes Cancer. 2006;45:495–508. doi: 10.1002/gcc.20312. [DOI] [PubMed] [Google Scholar]
- 25.Ulivieri A, Lavra L, Dominici R, Giacomelli L, Brunetti E, Sciacca L, Trovato M, Barresi G, Foukakis T, Jia-Jing L, Larsson C, Bartolazzi A, et al. Frizzled-1 is down-regulated in follicular thyroid tumours and modulates growth and invasiveness. J Pathol. 2008;215:87–96. doi: 10.1002/path.2331. [DOI] [PubMed] [Google Scholar]
- 26.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev. 2009;130:731–741. doi: 10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Modulation of inflammatory markers by miR-146a during replicative senescence in trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2010;51:2976–2985. doi: 10.1167/iovs.09-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Role of miR-204 in the regulation of apoptosis, endoplasmic reticulum stress response, and inflammation in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011;52:2999–3007. doi: 10.1167/iovs.10-6708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung TK, Lau TS, Cheung TH, Yim SF, Lo KW, Siu NS, Chan LK, Yu MY, Kwong J, Doran G, Barroilhet LM, Ng AS, et al. Dysregulation of microRNA-204 mediates migration and invasion of endometrial cancer by regulating FOXC1. Int J Cancer. 2012;130:1036–1045. doi: 10.1002/ijc.26060. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y, Yang X, Huang Y, Fan H, Zhang Q, Wu Y, Li J, Hasina R, Cheng C, Lingen MW, Gerstein MB, Weichselbaum RR, et al. Network modeling identifies molecular functions targeted by miR-204 to suppress head and neck tumor metastasis. PLoS Comput Biol. 2010;6:e1000730. doi: 10.1371/journal.pcbi.1000730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.White NM, Khella HW, Grigull J, Adzovic S, Youssef YM, Honey RJ, Stewart R, Pace KT, Bjarnason GA, Jewett MA, Evans AJ, Gabril M, et al. miRNA profiling in metastatic renal cell carcinoma reveals a tumour-suppressor effect for miR-215. Br J Cancer. 2011;105:1741–1749. doi: 10.1038/bjc.2011.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao Z, Lee CC, Baldini A, Caskey CT. A human homologue of the Drosophila polarity gene frizzled has been identified and mapped to 17q21.1. Genomics. 1995;27:370–373. doi: 10.1006/geno.1995.1060. [DOI] [PubMed] [Google Scholar]
- 33.Pode-Shakked N, Metsuyanim S, Rom-Gross E, Mor Y, Fridman E, Goldstein I, Amariglio N, Rechavi G, Keshet G, Dekel B. Developmental tumourigenesis: NCAM as a putative marker for the malignant renal stem/progenitor cell population. J Cell Mol Med. 2009;13:1792–1808. doi: 10.1111/j.1582-4934.2008.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rhee CS, Sen M, Lu D, Wu C, Leoni L, Rubin J, Corr M, Carson DA. Wnt and frizzled receptors as potential targets for immunotherapy in head and neck squamous cell carcinomas. Oncogene. 2002;21:6598–6605. doi: 10.1038/sj.onc.1205920. [DOI] [PubMed] [Google Scholar]
- 35.Bazhin AV, Tambor V, Dikov B, Philippov PP, Schadendorf D, Eichmüller SB. cGMP-phosphodiesterase 6, transducin and Wnt5a/Frizzled-2-signaling control cGMP and Ca(2+) homeostasis in melanoma cells. Cell Mol Life Sci. 2010;67:817–828. doi: 10.1007/s00018-009-0214-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Lee EH, Chari R, Lam A, Ng RT, Yee J, English J, Evans KG, Macaulay C, Lam S, Lam WL. Disruption of the non-canonical WNT pathway in lung squamous cell carcinoma. Clin Med Oncol. 2008;2008:169–179. [PMC free article] [PubMed] [Google Scholar]
- 37.Li C, Chen H, Hu L, Xing Y, Sasaki T, Villosis MF, Li J, Nishita M, Minami Y, Minoo P. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirikoshi H, Koike J, Sagara N, Saitoh T, Tokuhara M, Tanaka K, Sekihara H, Hirai M, Katoh M. Molecular cloning and genomic structure of human frizzled-3 at chromosome 8p21. Biochem Biophys Res Commun. 2000;271:8–14. doi: 10.1006/bbrc.2000.2578. [DOI] [PubMed] [Google Scholar]
- 39.Sala CF, Formenti E, Terstappen GC, Caricasole A. Identification, gene structure, and expression of human frizzled-3 (FZD3) Biochem Biophys Res Commun. 2000;273:27–34. doi: 10.1006/bbrc.2000.2882. [DOI] [PubMed] [Google Scholar]
- 40.Tapper J, Kettunen E, El-Rifai W, Seppälä M, Andersson LC, Knuutila S. Changes in gene expression during progression of ovarian carcinoma. Cancer Genet Cytogenet. 2001;128:1–6. doi: 10.1016/s0165-4608(01)00386-7. [DOI] [PubMed] [Google Scholar]
- 41.Khan NI, Bradstock KF, Bendall LJ. Activation of Wnt/beta-catenin pathway mediates growth and survival in B-cell progenitor acute lymphoblastic leukaemia. Br J Haematol. 2007;138:338–348. doi: 10.1111/j.1365-2141.2007.06667.x. [DOI] [PubMed] [Google Scholar]
- 42.Qiang YW, Endo Y, Rubin JS, Rudikoff S. Wnt signaling in B-cell neoplasia. Oncogene. 2003;22:1536–1545. doi: 10.1038/sj.onc.1206239. [DOI] [PubMed] [Google Scholar]
- 43.Reins J, Mossner M, Neumann M, Platzbecker U, Schumann C, Thiel E, Hofmann WK. Transcriptional down-regulation of the Wnt antagonist SFRP1 in haematopoietic cells of patients with different risk types of MDS. Leuk Res. 2010;34:1610–1616. doi: 10.1016/j.leukres.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 44.Caldwell GM, Jones CE, Ashley AM, Wei W, Hejmadi RK, Morton DG, Matthews GM. Wnt signaling in adenomas of familial adenomatous polyposis patients. Br J Cancer. 2010;103:910–917. doi: 10.1038/sj.bjc.6605790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu D, Zhao Y, Tawatao R, Cottam HB, Sen M, Leoni LM, Kipps TJ, Corr M, Carson DA. Activation of the Wnt signaling pathway in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 2004;101:3118–3123. doi: 10.1073/pnas.0308648100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Endo Y, Beauchamp E, Woods D, Taylor WG, Toretsky JA, Uren A, Rubin JS. Wnt-3a and Dickkopf-1 stimulate neurite outgrowth in Ewing tumor cells via a Frizzled3- and c-Jun N-terminal kinase-dependent mechanism. Mol Cell Biol. 2008;28:2368–2379. doi: 10.1128/MCB.01780-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen C, Howlin J, Tengholm A, Dyachok O, Vogel WF, Nairn AC, Greengard P, Andersson T. Wnt-5a-induced phosphorylation of DARPP-32 inhibits breast cancer cell migration in a CREB-dependent manner. J Biol Chem. 2009;284:27533–27543. doi: 10.1074/jbc.M109.048884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szász AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Kirikoshi H, Sagara N, Koike J, Tanaka K, Sekihara H, Hirai M, Katoh M. Molecular cloning and characterization of human Frizzled-4 on chromosome 11q14-q21. Biochem Biophys Res Commun. 1999;264:955–961. doi: 10.1006/bbrc.1999.1612. [DOI] [PubMed] [Google Scholar]
- 50.Sagara N, Kirikoshi H, Terasaki H, Yasuhiko Y, Toda G, Shiokawa K, Katoh M. FZD4S, a splicing variant of frizzled-4, encodes a soluble-type positive regulator of the WNT signaling pathway. Biochem Biophys Res Commun. 2001;282:750–756. doi: 10.1006/bbrc.2001.4634. [DOI] [PubMed] [Google Scholar]
- 51.Swain RK, Katoh M, Medina A, Steinbeisser H. Xenopus frizzled-4S, a splicing variant of Xfz4 is a context-dependent activator and inhibitor of Wnt/beta-catenin signaling. Cell Commun Signal. 2005;3:12. doi: 10.1186/1478-811X-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, Rantala J, Alanen K, Nees M, Kallioniemi O. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70:6735–6745. doi: 10.1158/0008-5472.CAN-10-0244. [DOI] [PubMed] [Google Scholar]
- 53.Jin X, Jeon HY, Joo KM, Kim JK, Jin J, Kim SH, Kang BG, Beck S, Lee SJ, Kim JK, Park AK, Park WY, et al. Frizzled 4 regulates stemness and invasiveness of migrating glioma cells established by serial intracranial transplantation. Cancer Res. 2011;71:3066–3075. doi: 10.1158/0008-5472.CAN-10-1495. [DOI] [PubMed] [Google Scholar]
- 54.Dai W, Teodoridis JM, Zeller C, Graham J, Hersey J, Flanagan JM, Stronach E, Millan DW, Siddiqui N, Paul J, Brown R. Systematic CpG islands methylation profiling of genes in the wnt pathway in epithelial ovarian cancer identifies biomarkers of progression-free survival. Clin Cancer Res. 2011;17:4052–4062. doi: 10.1158/1078-0432.CCR-10-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ueno K, Hirata H, Majid S, Yamamura S, Shahryari V, Tabatabai ZL, Hinoda Y, Dahiya R. Tumor suppressor microRNA-493 decreases cell motility and migration ability in human bladder cancer cells by downregulating RhoC and FZD4. Mol Cancer Ther. 2012;11:244–253. doi: 10.1158/1535-7163.MCT-11-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saitoh T, Hirai M, Katoh M. Molecular cloning and characterization of human Frizzled-5 gene on chromosome 2q33.3-q34 region. Int J Oncol. 2001;19:105–110. doi: 10.3892/ijo.19.1.105. [DOI] [PubMed] [Google Scholar]
- 57.Janssens N, Andries L, Janicot M, Perera T, Bakker A. Alteration of frizzled expression in renal cell carcinoma. Tumour Biol. 2004;25:161–171. doi: 10.1159/000081098. [DOI] [PubMed] [Google Scholar]
- 58.Thiele S, Rauner M, Goettsch C, Rachner TD, Benad P, Fuessel S, Erdmann K, Hamann C, Baretton GB, Wirth MP, Jakob F, Hofbauer LC. Expression profile of WNT molecules in prostate cancer and its regulation by aminobisphosphonates. J Cell Biochem. 2011;112:1593–1600. doi: 10.1002/jcb.23070. [DOI] [PubMed] [Google Scholar]
- 59.Weeraratna AT, Jiang Y, Hostetter G, Rosenblatt K, Duray P, Bittner M, Trent JM. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 60.Carmon KS, Loose DS. Wnt7a interaction with Fzd5 and detection of signaling activation using a split eGFP. Biochem Biophys Res Commun. 2008;368:285–291. doi: 10.1016/j.bbrc.2008.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Carmon KS, Loose DS. Secreted frizzled-related protein 4 regulates two Wnt7a signaling pathways and inhibits proliferation in endometrial cancer cells. Mol Cancer Res. 2008;6:1017–1028. doi: 10.1158/1541-7786.MCR-08-0039. [DOI] [PubMed] [Google Scholar]
- 62.Yoshioka S, King ML, Ran S, Okuda H, Maclean JA, 2nd, McAsey ME, Sugino N, Brard L, Watabe K, Hayashi K. WNT7A Regulates Tumor Growth and Progression in Ovarian Cancer through the WNT/β-Catenin Pathway. Mol Cancer Res. 2012;10:469–482. doi: 10.1158/1541-7786.MCR-11-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tokuhara M, Hirai M, Atomi Y, Terada M, Katoh M. Molecular cloning of human Frizzled-6. Biochem Biophys Res Commun. 1998;243:622–627. doi: 10.1006/bbrc.1998.8143. [DOI] [PubMed] [Google Scholar]
- 64.Haider AS, Peters SB, Kaporis H, Cardinale I, Fei J, Ott J, Blumenberg M, Bowcock AM, Krueger JG, Carucci JA. Genomic analysis defines a cancer-specific gene expression signature for human squamous cell carcinoma and distinguishes malignant hyperproliferation from benign hyperplasia. Invest Dermatol. 2006;126:869–881. doi: 10.1038/sj.jid.5700157. [DOI] [PubMed] [Google Scholar]
- 65.Saramäki OR, Porkka KP, Vessella RL, Visakorpi T. Genetic aberrations in prostate cancer by microarray analysis. Int J Cancer. 2006;119:1322–1329. doi: 10.1002/ijc.21976. [DOI] [PubMed] [Google Scholar]
- 66.Cantilena S, Pastorino F, Pezzolo A, Chayka O, Pistoia V, Ponzoni M, Sala A. Frizzled receptor 6 marks rare, highly tumourigenic stem-like cells in mouse and human neuroblastomas. Oncotarget. 2011;2:976–983. doi: 10.18632/oncotarget.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lyons JP, Mueller UW, Ji H, Everett C, Fang X, Hsieh JC, Barth AM, McCrea PD. Wnt-4 activates the canonical beta-catenin-mediated Wnt pathway and binds Frizzled-6 CRD: functional implications of Wnt/beta-catenin activity in kidney epithelial cells. Exp Cell Res. 2004;298:369–387. doi: 10.1016/j.yexcr.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 68.Heinonen KM, Vanegas JR, Lew D, Krosl J, Perreault C. Wnt4 enhances murine hematopoietic progenitor cell expansion through a planar cell polarity-like pathway. PLoS One. 2011;6:e19279. doi: 10.1371/journal.pone.0019279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Miyakoshi T, Takei M, Kajiya H, Egashira N, Takekoshi S, Teramoto A, Osamura RY. Expression of Wnt4 in human pituitary adenomas regulates activation of the beta-catenin-independent pathway. Endocr Pathol. 2008;19:261–273. doi: 10.1007/s12022-008-9048-9. [DOI] [PubMed] [Google Scholar]
- 70.Krützfeldt J, Rösch N, Hausser J, Manoharan M, Zavolan M, Stoffel M. MicroRNA-194 is a target of transcription factor 1 (Tcf1, HNF1α) in adult liver and controls expression of frizzled-6. Hepatology. 2012;55:98–107. doi: 10.1002/hep.24658. [DOI] [PubMed] [Google Scholar]
- 71.Tanaka S, Akiyoshi T, Mori M, Wands JR, Sugimachi K. A novel frizzled gene identified in human esophageal carcinoma mediates APC/beta-catenin signals. Proc Natl Acad Sci U S A. 1998;95:10164–10169. doi: 10.1073/pnas.95.17.10164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kirikoshi H, Sekihara H, Katoh M. Up-regulation of Frizzled-7 (FZD7) in human gastric cancer. Int J Oncol. 2001;19:111–115. [PubMed] [Google Scholar]
- 73.Zeng ZY, Zhou YH, Zhang WL, Xiong W, Fan SQ, Li XL, Luo XM, Wu MH, Yang YX, Huang C, Cao L, Tang K, et al. Gene expression profiling of nasopharyngeal carcinoma reveals the abnormally regulated Wnt signaling pathway. Hum Pathol. 2007;38:120–133. doi: 10.1016/j.humpath.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 74.Frierson HF, Jr, El-Naggar AK, Welsh JB, Sapinoso LM, Su AI, Cheng J, Saku T, Moskaluk CA, Hampton GM. Large scale molecular analysis identifies genes with altered expression in salivary adenoid cystic carcinoma. Am J Pathol. 2002;161:1315–1323. doi: 10.1016/S0002-9440(10)64408-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ueno K, Hiura M, Suehiro Y, Hazama S, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia. 2008;10:697–705. doi: 10.1593/neo.08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Merle P, de la Monte S, Kim M, Herrmann M, Tanaka S, Von Dem Bussche A, Kew MC, Trepo C, Wands JR. Functional consequences of frizzled-7 receptor overexpression in human hepatocellular carcinoma. Gastroenterology. 2004;127:1110–1122. doi: 10.1053/j.gastro.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 77.Merle P, Kim M, Herrmann M, Gupte A, Lefrançois L, Califano S, Trépo C, Tanaka S, Vitvitski L, de la Monte S, Wands JR. Oncogenic role of the frizzled-7/beta-catenin pathway in hepatocellular carcinoma. J Hepatol. 2005;43:854–862. doi: 10.1016/j.jhep.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 78.Bengochea A, de Souza MM, Lefrançois L, Le Roux E, Galy O, Chemin I, Kim M, Wands JR, Trepo C, Hainaut P, Scoazec JY, Vitvitski L, et al. Common dysregulation of Wnt/Frizzled receptor elements in human hepatocellular carcinoma. Br J Cancer. 2008;99:143–150. doi: 10.1038/sj.bjc.6604422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmuck R, Warneke V, Behrens HM, Simon E, Weichert W, Röcken C. Genotypic and phenotypic characterization of side population of gastric cancer cell lines. Am J Pathol. 2011;178:1792–1804. doi: 10.1016/j.ajpath.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yang L, Wu X, Wang Y, Zhang K, Wu J, Yuan YC, Deng X, Chen L, Kim CC, Lau S, Somlo G, Yen Y. FZD7 has a critical role in cell proliferation in triple negative breast cancer. Oncogene. 2011;30:4437–4446. doi: 10.1038/onc.2011.145. [DOI] [PubMed] [Google Scholar]
- 81.Kim M, Lee HC, Tsedensodnom O, Hartley R, Lim YS, Yu E, Merle P, Wands JR. Functional interaction between Wnt3 and Frizzled-7 leads to activation of the Wnt/beta-catenin signaling pathway in hepatocellular carcinoma cells. J Hepatol. 2008;48:780–791. doi: 10.1016/j.jhep.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vincan E, Darcy PK, Smyth MJ, Thompson EW, Thomas RJ, Phillips WA, Ramsay RG. Frizzled-7 receptor ectodomain expression in a colon cancer cell line induces morphological change and attenuates tumor growth. Differentiation. 2005;73:142–153. doi: 10.1111/j.1432-0436.2005.00015.x. [DOI] [PubMed] [Google Scholar]
- 83.Vincan E, Darcy PK, Farrelly CA, Faux MC, Brabletz T, Ramsay RG. Frizzled-7 dictates three-dimensional organization of colorectal cancer cell carcinoids. Oncogene. 2007;26:2340–2352. doi: 10.1038/sj.onc.1210026. [DOI] [PubMed] [Google Scholar]
- 84.Vincan E, Flanagan DJ, Pouliot N, Brabletz T, Spaderna S. Variable FZD7 expression in colorectal cancers indicates regulation by the tumour microenvironment. Dev Dyn. 2010;239:311–317. doi: 10.1002/dvdy.22045. [DOI] [PubMed] [Google Scholar]
- 85.Nishioka M, Ueno K, Hazama S, Okada T, Sakai K, Suehiro Y, Okayama N, Hirata H, Oka M, Imai K, Dahiya R, Hinoda Y. Possible involvement of Wnt11 in colorectal cancer progression. Mol Carcinog. 2011 doi: 10.1002/mc.21845. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 86.Fujii N, You L, Xu Z, Uematsu K, Shan J, He B, Mikami I, Edmondson LR, Neale G, Zheng J, Guy RK, Jablons DM. An antagonist of dishevelled protein-protein interaction suppresses beta-catenin-dependent tumor cell growth. Cancer Res. 2007;67:573–579. doi: 10.1158/0008-5472.CAN-06-2726. [DOI] [PubMed] [Google Scholar]
- 87.Nambotin SB, Lefrancois L, Sainsily X, Berthillon P, Kim M, Wands JR, Chevallier M, Jalinot P, Scoazec JY, Trepo C, Zoulim F, Merle P. Pharmacological inhibition of Frizzled-7 displays anti-tumor properties in hepatocellular carcinoma. J Hepatol. 2011;54:288–299. doi: 10.1016/j.jhep.2010.06.033. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H, Hao Y, Yang J, Zhou Y, Li J, Yin S, Sun C, Ma M, Huang Y, Xi JJ. Genome-wide functional screening of miR-23b as a pleiotropic modulator suppressing cancer metastasis. Nat Commun. 2011;2:554. doi: 10.1038/ncomms1555. [DOI] [PubMed] [Google Scholar]
- 89.Tong AW, Fulgham P, Jay C, Chen P, Khalil I, Liu S, Senzer N, Eklund AC, Han J, Nemunaitis J. MicroRNA profile analysis of human prostate cancers. Cancer Gene Ther. 2009;16:206–216. doi: 10.1038/cgt.2008.77. [DOI] [PubMed] [Google Scholar]
- 90.Salvi A, Sabelli C, Moncini S, Venturin M, Arici B, Riva P, Portolani N, Giulini SM, De Petro G, Barlati S. MicroRNA-23b mediates urokinase and c-met downmodulation and a decreased migration of human hepatocellular carcinoma cells. FEBS J. 2009;276:2966–2982. doi: 10.1111/j.1742-4658.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- 91.Saitoh T, Hirai M, Katoh M. Molecular cloning and characterization of human Frizzled-8 gene on chromosome 10p11.2. Int J Oncol. 2001;18:991–996. doi: 10.3892/ijo.18.5.991. [DOI] [PubMed] [Google Scholar]
- 92.Wang HQ, Xu ML, Ma J, Zhang Y, Xie CH. Frizzled-8 as a putative therapeutic target in human lung cancer. Biochem Biophys Res Commun. 2012;417:62–66. doi: 10.1016/j.bbrc.2011.11.055. [DOI] [PubMed] [Google Scholar]
- 93.Bourhis E, Tam C, Franke Y, Bazan JF, Ernst J, Hwang J, Costa M, Cochran AG, Hannoush RN. Reconstitution of a frizzled8.Wnt3a.LRP6 signaling complex reveals multiple Wnt and Dkk1 binding sites on LRP6. J Biol Chem. 2010;285:9172–9179. doi: 10.1074/jbc.M109.092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang YK, Samos CH, Peoples R, Pérez-Jurado LA, Nusse R, Francke U. A novel human homologue of the Drosophila frizzled wnt receptor gene binds wingless protein and is in the Williams syndrome deletion at 7q11.23. Hum Mol Genet. 1997;6:465–472. doi: 10.1093/hmg/6.3.465. [DOI] [PubMed] [Google Scholar]
- 95.Wang YK, Spörle R, Paperna T, Schughart K, Francke U. Characterization and expression pattern of the frizzled gene Fzd9, the mouse homolog of FZD9 which is deleted in Williams-Beuren syndrome. Genomics. 1999;57:235–248. doi: 10.1006/geno.1999.5773. [DOI] [PubMed] [Google Scholar]
- 96.Zhang Z, Schittenhelm J, Guo K, Bühring HJ, Trautmann K, Meyermann R, Schluesener HJ. Upregulation of frizzled 9 in astrocytomas. Neuropathol Appl Neurobiol. 2006;32:615–624. doi: 10.1111/j.1365-2990.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- 97.Martinez R, Martin-Subero JI, Rohde V, Kirsch M, Alaminos M, Fernandez AF, Ropero S, Schackert G, Esteller M. A microarray-based DNA methylation study of glioblastoma multiforme. Epigenetics. 2009;4:255–264. doi: 10.4161/epi.9130. [DOI] [PubMed] [Google Scholar]
- 98.Jiang Y, Dunbar A, Gondek LP, Mohan S, Rataul M, O'Keefe C, Sekeres M, Saunthararajah Y, Maciejewski JP. Aberrant DNA methylation is a dominant mechanism in MDS progression to AML. Blood. 2009;113:1315–1325. doi: 10.1182/blood-2008-06-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Winn RA, Marek L, Han SY, Rodriguez K, Rodriguez N, Hammond M, Van Scoyk M, Acosta H, Mirus J, Barry N, Bren-Mattison Y, Van Raay TJ, et al. Restoration of Wnt-7a expression reverses non-small cell lung cancer cellular transformation through frizzled-9-mediated growth inhibition and promotion of cell differentiation. J Biol Chem. 2005;280:19625–19634. doi: 10.1074/jbc.M409392200. [DOI] [PubMed] [Google Scholar]
- 100.Winn RA, Van Scoyk M, Hammond M, Rodriguez K, Crossno JT, Jr, Heasley LE, Nemenoff RA. Antitumorigenic effect of Wnt 7a and Fzd 9 in non-small cell lung cancer cells is mediated through ERK-5-dependent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 2006;281:26943–26950. doi: 10.1074/jbc.M604145200. [DOI] [PubMed] [Google Scholar]
- 101.Tennis MA, Van Scoyk MM, Freeman SV, Vandervest KM, Nemenoff RA, Winn RA. Sprouty-4 inhibits transformed cell growth, migration and invasion, and epithelial-mesenchymal transition, and is regulated by Wnt7A through PPARgamma in non-small cell lung cancer. Mol Cancer Res. 2010;8:833–843. doi: 10.1158/1541-7786.MCR-09-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fujimoto T, Tomizawa M, Yokosuka O. SiRNA of frizzled-9 suppresses proliferation and motility of hepatoma cells. Int J Oncol. 2009;35:861–866. doi: 10.3892/ijo_00000400. [DOI] [PubMed] [Google Scholar]
- 103.Koike J, Takagi A, Miwa T, Hirai M, Terada M, Katoh M. Molecular cloning of Frizzled-10, a novel member of the Frizzled gene family. Biochem Biophys Res Commun. 1999;262:39–43. doi: 10.1006/bbrc.1999.1161. [DOI] [PubMed] [Google Scholar]
- 104.Terasaki H, Saitoh T, Shiokawa K, Katoh M. Frizzled-10, up-regulated in primary colorectal cancer, is a positive regulator of the WNT - beta-catenin - TCF signaling pathway. Int J Mol Med. 2002;9:107–112. [PubMed] [Google Scholar]
- 105.Nagayama S, Yamada E, Kohno Y, Aoyama T, Fukukawa C, Kubo H, Watanabe G, Katagiri T, Nakamura Y, Sakai Y, Toguchida J. Inverse correlation of the up-regulation of FZD10 expression and the activation of beta-catenin in synchronous colorectal tumors. Cancer Sci. 2009;100:405–412. doi: 10.1111/j.1349-7006.2008.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gugger M, White R, Song S, Waser B, Cescato R, Rivière P, Reubi JC. GPR87 is an overexpressed G-protein coupled receptor in squamous cell carcinoma of the lung. Dis Markers. 2008;24:41–50. doi: 10.1155/2008/857474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nagayama S, Fukukawa C, Katagiri T, Okamoto T, Aoyama T, Oyaizu N, Imamura M, Toguchida J, Nakamura Y. Therapeutic potential of antibodies against FZD 10, a cell-surface protein, for synovial sarcomas. Oncogene. 2005;24:6201–6212. doi: 10.1038/sj.onc.1208780. [DOI] [PubMed] [Google Scholar]
- 108.Togashi A, Katagiri T, Ashida S, Fujioka T, Maruyama O, Wakumoto Y, Sakamoto Y, Fujime M, Kawachi Y, Shuin T, Nakamura Y. Hypoxia-inducible protein 2 (HIG2), a novel diagnostic marker for renal cell carcinoma and potential target for molecular therapy. Cancer Res. 2005;65:4817–4826. doi: 10.1158/0008-5472.CAN-05-0120. [DOI] [PubMed] [Google Scholar]
- 109.Fukukawa C, Hanaoka H, Nagayama S, Tsunoda T, Toguchida J, Endo K, Nakamura Y, Katagiri T. Radioimmunotherapy of human synovial sarcoma using a monoclonal antibody against FZD10. Cancer Sci. 2008;99:432–440. doi: 10.1111/j.1349-7006.2007.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kikuchi A, Yamamoto H, Kishida S. Multiplicity of the interactions of Wnt proteins and their receptors. Cell Signal. 2007;19:659–671. doi: 10.1016/j.cellsig.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 111.Schulte G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol Rev. 2010;62:632–667. doi: 10.1124/pr.110.002931. [DOI] [PubMed] [Google Scholar]
- 112.Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- 113.Liu G, Bafico A, Harris VK, Aaronson SA. A novel mechanism for Wnt activation of canonical signaling through the LRP6 receptor. Mol Cell Biol. 2003;23:5825–5835. doi: 10.1128/MCB.23.16.5825-5835.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schweizer L, Varmus H. Wnt/Wingless signaling through beta-catenin requires the function of both LRP/Arrow and frizzled classes of receptors. BMC Cell Biol. 2003;4:4. doi: 10.1186/1471-2121-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cong F, Schweizer L, Varmus H. Wnt signals across the plasma membrane to activate the beta-catenin pathway by forming oligomers containing its receptors, Frizzled and LRP. Development. 2004;131:5103–5115. doi: 10.1242/dev.01318. [DOI] [PubMed] [Google Scholar]
- 116.Lindvall C, Evans NC, Zylstra CR, Li Y, Alexander CM, Williams BO. The Wnt signaling receptor Lrp5 is required for mammary ductal stem cell activity and Wnt1-induced tumorigenesis. J Biol Chem. 2006;281:35081–35087. doi: 10.1074/jbc.M607571200. [DOI] [PubMed] [Google Scholar]
- 117.Li Y, Lu W, He X, Schwartz AL, Bu G. LRP6 expression promotes cancer cell proliferation and tumorigenesis by altering beta-catenin subcellular distribution. Oncogene. 2004;23:9129–9135. doi: 10.1038/sj.onc.1208123. [DOI] [PubMed] [Google Scholar]
- 118.Björklund P, Akerström G, Westin G. An LRP5 receptor with internal deletion in hyperparathyroid tumors with implications for deregulated WNT/beta-catenin signaling. PLoS Med. 2007;4:e328. doi: 10.1371/journal.pmed.0040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer. 2004;109:106–111. doi: 10.1002/ijc.11677. [DOI] [PubMed] [Google Scholar]
- 120.Liu CC, Prior J, Piwnica-Worms D, Bu G. LRP6 overexpression defines a class of breast cancer subtype and is a target for therapy. Proc Natl Acad Sci U S A. 2010;107:5136–5141. doi: 10.1073/pnas.0911220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Björklund P, Svedlund J, Olsson AK, Akerström G, Westin G. The internally truncated LRP5 receptor presents a therapeutic target in breast cancer. PLoS One. 2009;4:e4243. doi: 10.1371/journal.pone.0004243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.DiMeo TA, Anderson K, Phadke P, Fan C, Perou CM, Naber S, Kuperwasser C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ettenberg SA, Charlat O, Daley MP, Liu S, Vincent KJ, Stuart DD, Schuller AG, Yuan J, Ospina B, Green J, Yu Q, Walsh R, et al. Inhibition of tumorigenesis driven by different Wnt proteins requires blockade of distinct ligand-binding regions by LRP6 antibodies. Proc Natl Acad Sci U S A. 2010;107:15473–15478. doi: 10.1073/pnas.1007428107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gong Y, Bourhis E, Chiu C, Stawicki S, DeAlmeida VI, Liu BY, Phamluong K, Cao TC, Carano RA, Ernst JA, Solloway M, Rubinfeld B, et al. Wnt isoform-specific interactions with coreceptor specify inhibition or potentiation of signaling by LRP6 antibodies. PLoS One. 2010;5:e12682. doi: 10.1371/journal.pone.0012682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Berndt JD, Aoyagi A, Yang P, Anastas JN, Tang L, Moon RT. Mindbomb 1, an E3 ubiquitin ligase, forms a complex with RYK to activate Wnt/β-catenin signaling. J Cell Biol. 2011;194:737–750. doi: 10.1083/jcb.201107021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hovens CM, Stacker SA, Andres AC, Harpur AG, Ziemiecki A, Wilks AF. RYK, a receptor tyrosine kinase-related molecule with unusual kinase domain motifs. Proc Natl Acad Sci U S A. 1992;89:11818–11822. doi: 10.1073/pnas.89.24.11818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu W, Yamamoto V, Ortega B, Baltimore D. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell. 2004;119:97–108. doi: 10.1016/j.cell.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 128.Kim GH, Her JH, Han JK. Ryk cooperates with Frizzled 7 to promote Wnt11-mediated endocytosis and is essential for Xenopus laevis convergent extension movements. J Cell Biol. 2008;182:1073–1082. doi: 10.1083/jcb.200710188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang XC, Katso R, Butler R, Hanby AM, Poulsom R, Jones T, Sheer D, Ganesan TS. H-RYK, an unusual receptor kinase: isolation and analysis of expression in ovarian cancer. Mol Med. 1996;2:189–203. [PMC free article] [PubMed] [Google Scholar]
- 130.Katso RM, Manek S, Ganjavi H, Biddolph S, Charnock MF, Bradburn M, Wells M, Ganesan TS. Overexpression of H-Ryk in epithelial ovarian cancer: prognostic significance of receptor expression. Clin Cancer Res. 2000;6:3271–3281. [PubMed] [Google Scholar]
- 131.Micci F, Panagopoulos I, Haugom L, Andersen HK, Tjønnfjord GE, Beiske K, Heim S. t(3;21)(q22;q22) leading to truncation of the RYK gene in atypical chronic myeloid leukemia. Cancer Lett. 2009;277:205–211. doi: 10.1016/j.canlet.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 132.Masiakowski P, Carroll RD. A novel family of cell surface receptors with tyrosine kinase-like domain. J Biol Chem. 1992;267:26181–26190. [PubMed] [Google Scholar]
- 133.Li C, Chen H, Hu L, Xing Y, Sasaki T, Villosis MF, Li J, Nishita M, Minami Y, Minoo P. Ror2 modulates the canonical Wnt signaling in lung epithelial cells through cooperation with Fzd2. BMC Mol Biol. 2008;9:11. doi: 10.1186/1471-2199-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Enomoto M, Hayakawa S, Itsukushima S, Ren DY, Matsuo M, Tamada K, Oneyama C, Okada M, Takumi T, Nishita M, Minami Y. Autonomous regulation of osteosarcoma cell invasiveness by Wnt5a/Ror2 signaling. Oncogene. 2009;28:3197–3208. doi: 10.1038/onc.2009.175. [DOI] [PubMed] [Google Scholar]
- 135.Wright TM, Brannon AR, Gordan JD, Mikels AJ, Mitchell C, Chen S, Espinosa I, van de Rijn M, Pruthi R, Wallen E, Edwards L, Nusse R, et al. Ror2, a developmentally regulated kinase, promotes tumor growth potential in renal cell carcinoma. Oncogene. 2009;28:2513–2523. doi: 10.1038/onc.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamamoto H, Oue N, Sato A, Hasegawa Y, Yamamoto H, Matsubara A, Yasui W, Kikuchi A. Wnt5a signaling is involved in the aggressiveness of prostate cancer and expression of metalloproteinase. Oncogene. 2010;29:2036–2046. doi: 10.1038/onc.2009.496. [DOI] [PubMed] [Google Scholar]
- 137.O'Connell MP, Fiori JL, Xu M, Carter AD, Frank BP, Camilli TC, French AD, Dissanayake SK, Indig FE, Bernier M, Taub DD, Hewitt SM, et al. The orphan tyrosine kinase receptor, ROR2, mediates Wnt5A signaling in metastatic melanoma. Oncogene. 2010;29:34–44. doi: 10.1038/onc.2009.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Edris B, Espinosa I, Mühlenberg T, Mikels A, Lee CH, Steigen SE, Zhu S, Montgomery KD, Lazar AJ, Lev D, Fletcher JA, Beck AH, et al. ROR2 is a novel prognostic biomarker and a potential therapeutic target in leiomyosarcoma and gastrointestinal stromal tumour. J Pathol. 2012;227:223–233. doi: 10.1002/path.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kawano Y, Kypta R. Secreted antagonists of the Wnt signalling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 140.Roth W, Wild-Bode C, Platten M, Grimmel C, Melkonyan HS, Dichgans J, Weller M. Secreted Frizzled-related proteins inhibit motility and promote growth of human malignant glioma cells. Oncogene. 2000;19:4210–4220. doi: 10.1038/sj.onc.1203783. [DOI] [PubMed] [Google Scholar]
- 141.Suzuki H, Watkins DN, Jair KW, Schuebel KE, Markowitz SD, Chen WD, Pretlow TP, Yang B, Akiyama Y, Van Engeland M, Toyota M, Tokino T, et al. Epigenetic inactivation of SFRP genes allows constitutive WNT signaling in colorectal cancer. Nat Genet. 2004;36:417–422. doi: 10.1038/ng1330. [DOI] [PubMed] [Google Scholar]
- 142.Nozaki I, Tsuji T, Iijima O, Ohmura Y, Andou A, Miyazaki M, Shimizu N, Namba M. Reduced expression of REIC/Dkk-3 gene in non-small cell lung cancer. Int J Oncol. 2001;19:117–121. doi: 10.3892/ijo.19.1.117. [DOI] [PubMed] [Google Scholar]
- 143.Kobayashi K, Ouchida M, Tsuji T, Hanafusa H, Miyazaki M, Namba M, Shimizu N, Shimizu K. Reduced expression of the REIC/Dkk-3 gene by promoter-hypermethylation in human tumor cells. Gene. 2002;282:151–158. doi: 10.1016/s0378-1119(01)00838-1. [DOI] [PubMed] [Google Scholar]
- 144.Wissmann C, Wild PJ, Kaiser S, Roepcke S, Stoehr R, Woenckhaus M, Kristiansen G, Hsieh JC, Hofstaedter F, Hartmann A, Knuechel R, Rosenthal A, et al. WIF1, a component of the Wnt pathway, is down-regulated in prostate, breast, lung, and bladder cancer. J Pathol. 2003;201:204–212. doi: 10.1002/path.1449. [DOI] [PubMed] [Google Scholar]
- 145.Mazieres J, He B, You L, Xu Z, Lee AY, Mikami I, Reguart N, Rosell R, McCormick F, Jablons DM. Wnt inhibitory factor-1 is silenced by promoter hypermethylation in human lung cancer. Cancer Res. 2004;64:4717–4720. doi: 10.1158/0008-5472.CAN-04-1389. [DOI] [PubMed] [Google Scholar]
- 146.Ilyas M, Tomlinson IP. The interactions of APC, E-cadherin and beta-catenin in tumour development and progression. J Pathol. 1997;182:128–137. doi: 10.1002/(SICI)1096-9896(199706)182:2<128::AID-PATH839>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 147.Diaz MO, Ziemin S, Le Beau MM, Pitha P, Smith SD, Chilcote RR, Rowley JD. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci U S A. 1988;85:5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Laï JL, Fenaux P, Pollet JP, Estienne MH, Savary JB, Huart JJ, Deminatti M. Acute lymphocytic leukemia with 9p anomalies. A report of four additional cases and review of the literature. Cancer Genet Cytogenet. 1988;33:99–109. doi: 10.1016/0165-4608(88)90055-6. [DOI] [PubMed] [Google Scholar]
- 149.ountain JW, Karayiorgou M, Ernstoff MS, Kirkwood JM, Vlock DR, Titus-Ernstoff L, Bouchard B, Vijayasaradhi S, Houghton AN, Lahti J, Kidd VJ, Housman DE, et al. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992;89:10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Miyao N, Tsai YC, Lerner SP, Olumi AF, Spruck CH, 3rd, Gonzalez-Zulueta M, Nichols PW, Skinner DG, Jones PA. Role of chromosome 9 in human bladder cancer. Cancer Res. 1993;53:4066–4070. [PubMed] [Google Scholar]
- 151.Taguchi T, Jhanwar SC, Siegfried JM, Keller SM, Testa JR. Recurrent deletions of specific chromosomal sites in 1p, 3p, 6q, and 9p in human malignant mesothelioma. Cancer Res. 1993;53:4349–4355. [PubMed] [Google Scholar]
- 152.Osborne RJ, Leech V. Polymerase chain reaction allelotyping of human ovarian cancer. Br J Cancer. 1994;69:429–438. doi: 10.1038/bjc.1994.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fujino T, Risinger JI, Collins NK, Liu FS, Nishii H, Takahashi H, Westphal EM, Barrett JC, Sasaki H, Kohler MF. Andrew Berchuck, and Jeff Boyd. Allelotype of endometrial carcinoma. Cancer Res. 1994;54:4294–4298. [PubMed] [Google Scholar]
- 154.Hanaoka H, Katagiri T, Fukukawa C, Yoshioka H, Yamamoto S, Iida Y, Higuchi T, Oriuchi N, Paudyal B, Paudyal P, Nakamura Y, Endo K. Radioimmunotherapy of solid tumors targeting a cell-surface protein, FZD10: therapeutic efficacy largely depends on radiosensitivity. Ann Nucl Med. 2009;23:479–485. doi: 10.1007/s12149-009-0265-1. [DOI] [PubMed] [Google Scholar]
- 155.Pode-Shakked N, Harari-Steinberg O, Haberman-Ziv Y, Rom-Gross E, Bahar S, Omer D, Metsuyanim S, Buzhor E, Jacob-Hirsch J, Goldstein RS, Mark-Danieli M, Dekel B. Resistance or sensitivity of Wilms' tumor to anti-FZD7 antibody highlights the Wnt pathway as a possible therapeutic target. Oncogene. 2011;30:1664–1680. doi: 10.1038/onc.2010.549. [DOI] [PubMed] [Google Scholar]
- 156.Wang Z, Rao DD, Senzer N, Nemunaitis J. RNA interference and cancer therapy. Pharm Res. 2011;28:2983–2995. doi: 10.1007/s11095-011-0604-5. [DOI] [PubMed] [Google Scholar]
- 157.Henry JC, Azevedo-Pouly AC, Schmittgen TD. MicroRNA replacement therapy for cancer. Pharm Res. 2011;28:3030–3042. doi: 10.1007/s11095-011-0548-9. [DOI] [PubMed] [Google Scholar]
- 158.Yeung ML, Jeang KT. MicroRNAs and cancer therapeutics. Pharm Res. 2011;28:3043–3049. doi: 10.1007/s11095-011-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kasinski AL, Slack FJ. Epigenetics and genetics. MicroRNAs en route to the clinic: progress in validating and targeting microRNAs for cancer therapy. Nat Rev Cancer. 2011;11:849–864. doi: 10.1038/nrc3166. [DOI] [PMC free article] [PubMed] [Google Scholar]