Abstract
Whether a given patient will suffer harm from a medication or how severe that harm will be is difficult to precisely predict. As a result, many adverse drug events (ADEs) occur in patients in whom it was reasonable to believe that the drug's benefits exceeded its risks. Improving safety and reducing the burden of ADEs in older adults requires addressing this uncertainty by focusing not only on the appropriateness of the initial prescribing decision but also on detecting and mitigating adverse events once they have started to occur. Such enhanced monitoring of signs, symptoms, and laboratory parameters can determine whether an adverse event has only mild and short-term impacts or major long-term effects on morbidity and mortality. While current medication monitoring practices are often suboptimal, several strategies can be leveraged to improve the quality and outcomes of monitoring. These strategies include using health information technology to link pharmacy and laboratory data, prospective delineation of risk, and patient outreach and activation, all within a framework of team-based approaches to patient management. While many of these strategies are theoretically possible now, they are poorly utilized and will be difficult to implement without a significant restructuring of medical practice. An enhanced focus on medication monitoring will also require a new conceptual framework to re-engineer the prescribing process. In this approach, prescribing quality hinges not on static attributes of the initial prescribing decision, but entails a dynamic process in which the benefits and harms of drugs are actively monitored, managed, and reassessed over time.
Keywords: adverse drug events, drug monitoring, aged
Introduction
Mr. S. is a 78-year old man with hypertension, chronic kidney disease, and history of thromboembolic stroke. His physician, Dr. A., recently encouraged him to stop self-medicating with diphenhydramine for insomnia and conducts a careful annual review of his medications while being mindful to ensure that they are dosed properly for his renal function. In response to rising blood pressure, Dr. A. prescribes a calcium-channel blocker. Shortly after starting the drug, Mr. S. develops positional lightheadedness. At a follow-up visit 3 weeks later, the patient's blood pressure is well-controlled, but the patient's symptoms are never discussed. One month later, Mr. S. falls and fractures his hip.
The case of Mr. S. illustrates the challenges – and potentially devastating consequences – of medication prescribing in elders. By most metrics, the treating physician provided exemplary care: she stopped a drug at high risk of causing complications in elders (diphenhydramine), recognized the importance of untreated hypertension, prescribed a guideline-recommended drug, and assessed the patient's blood pressure response in a timely fashion. However, the failure to identify and address the patient's orthostatic symptoms contributed to a life-altering adverse event.
While the shortcomings in this case may appear obvious in hindsight, they represent a major gap in current approaches to pharmaceutical care for ambulatory elders. Many teaching and quality improvement programs for older adults focus largely on preempting medication problems at the time of prescribing - for example, by avoiding high-risk drugs, paying careful attention to drug dosing and drug-drug interactions, and taking a cautious approach to prescribing new drugs.1 In contrast, disproportionately less attention has been paid to medication monitoring. Research in this area is sparse, few guidelines exist, and discussion of monitoring comprises less than 5% of chapters on pharmacology and prescribing in widely-used textbooks of internal medicine and geriatrics. (When monitoring is discussed, the predominant focus is on using serum drug levels to titrate doses for medications with a narrow therapeutic range).2-4
Despite the current focus on initial prescribing decisions, intervening at the time a drug is prescribed need not be the dominant approach for reducing the burden of adverse drug events (ADEs). Paradoxically, in many cases the morbidity and mortality associated with ADEs may be more effectively reduced by promptly detecting and mitigating complications of drug therapy once they have started to arise. In other words, a key strategy for reducing the burden of ADEs, to a certain extent, requires allowing them to occur. In this paper, we describe a conceptual framework for considering a more proactive role in monitoring signs, symptoms, and laboratory parameters for adverse events and suggest approaches to help overcome current problems in monitoring practices.
Why focusing attention on the point of prescribing does not prevent most adverse drug events
Although most efforts to reduce ADEs have focused on preventing errors at the time of prescribing, it is estimated that fewer than one quarter of ADEs in the ambulatory setting are clearly preventable at this stage of the prescribing process. 5,6-8 This observation reflects the fact that most ADEs do not result from improper choices of drugs or drug doses, but instead represent known side effects of drugs that have a rightful place in the therapeutic armamentarium. For example, despite a frequent focus on reducing use of diphenhydramine, chlorpropamide, and other “drugs to avoid” in older adults, only 4% of ADEs leading to emergency room visits by elders are caused by agents cited on “drugs to avoid” lists.9 In contrast, 30% of adverse events in this population are due to warfarin or insulin, which are legitimate therapeutic options for older adults and (particularly in the case of warfarin) are often prescribed less than they should be.
Furthermore, the complex interplay of factors that determine how an individual will respond to a drug – including that person's drug receptor genotypes, cytochrome P450 system polymorphisms, environment, medication adherence, comorbidities, and concurrent drug therapies - often makes it difficult to know in advance how well that drug will work or to confidently predict the type and severity of ADEs that will occur in that individual. Thus, focusing squarely on the initial prescribing decision fails to address many of the negative consequences of multiple medication use, because many of these effects cannot be confidently predicted in advance and only emerge over time. A static approach can also contribute to underprescribing, as physicians may overly fear the risk of ADEs and thus deny patients drug therapy which is more likely to help than harm them. For example, many physicians are reluctant to prescribe warfarin to older patients with atrial fibrillation due to age-associated increases in bleeding risk, despite the fact that older patients often gain a greater absolute reduction in risk of stroke.
Stated otherwise, approaches that focus on the point of prescribing may be able to identify patients as having higher- or lower-than-average risk of ADEs, but often cannot clearly categorize a patient as having so high a risk that the potential harms of drug therapy clearly exceed the benefit, or so low a risk that monitoring is not needed. Thus, for most drugs the risk-benefit formulation must move beyond a probabilistic assessment of benefits and harms at the time a drug is first prescribed, and devote more careful attention to monitoring actual benefits and harms after the patient has been taking the drug. This dynamic approach to prescribing that embraces monitoring can reframe the unpredictability of ADEs as an opportunity rather than as a problem. In the case of warfarin, careful monitoring both increases benefit and decreases bleeding risk by increasing time spent in the therapeutic range.10 Ongoing monitoring of benefits and harms over time may allow more patients to be tried on a potentially beneficial medication with the knowledge that drug harms can be reduced by early detection and mitigation of problems that may arise.
Managing uncertainty – a conceptual framework for medication monitoring
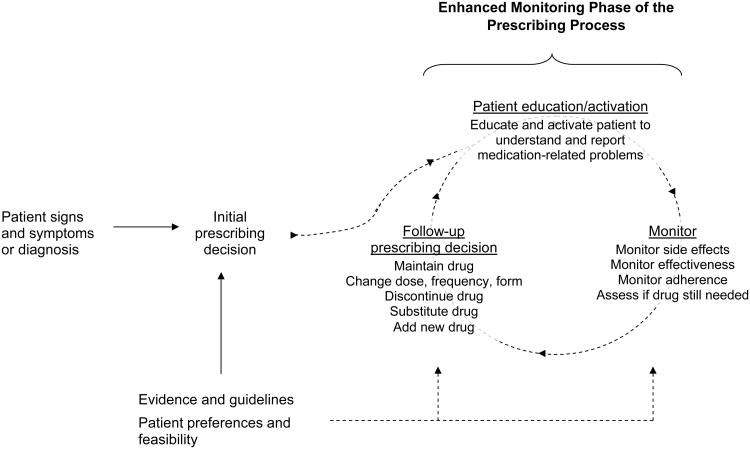
In a monitoring-focused approach, the initial prescribing decision is only the first step. After the patient is prescribed a drug, health care providers engage in an ongoing process of monitoring that comprises 3 basic steps (Figure 1). First, health care providers educate the patient about the anticipated benefits and possible problems associated with the drug, and actively engage her or him as a partner to proactively report problems should they arise. Second, the clinician regularly re-assesses drug effectiveness, adverse events, medication adherence, and whether the drug is still needed. Of note, while physicians and nurse practitioners typically make the initial prescribing decision, pharmacists, nurses, and other health professionals can play a central role in these steps. Finally, the clinician adjusts the regimen (if necessary), and the process begins anew. Just as with the initial prescribing decision, these assessments and actions should incorporate evidence and guidelines as well as patient preferences and feasibility –for example, how often to monitor, what markers to follow, how to inquire about symptoms and adherence difficulties, and what actions to take in response to problems that are detected.
Figure 1. The enhanced monitoring framework.
In the traditional approach to prescribing, the patient presents with signs and symptoms and/or a disease diagnosis. The physician consults evidence and guidelines, as well as patient preferences and feasibility, and then prescribes a medication. Quality is typically judged based on whether the physician's choice of medication is consistent with evidence and guidelines. Prevention of ADEs typically focuses on ensuring that the drug choice and dose is appropriate and consistent with the patient's renal function, other drugs, and other diseases (e.g. to avoid supratherapeutic drug levels, drug-drug interactions, and drug-disease interactions).
In the enhanced monitoring approach (shown in dotted lines), medication prescribing is viewed as an ongoing process that begins rather than ends at the initial prescribing decision. Care quality is judged in part on the quality of monitoring for drug side effects, effectiveness, adherence, and therapeutic necessity, and whether the clinician makes appropriate changes to address any problems that are detected.
Similar principles apply to monitoring drugs for effectiveness. For example, for drugs given to treat symptoms, early queries about symptomatic benefit can help establish in a timely manner whether the drug should be maintained as is, given at a different dose or schedule, or substituted altogether. The answers to these questions cannot automatically guide treatment decisions, particularly for drugs whose benefits are subtle, or in situations where observed improvement may be attributable to natural fluctuations of disease course rather than the drug. Nonetheless, they are a necessary starting place that is often overlooked in the rush of clinical practice.
The critical role of medication monitoring
There are substantial opportunities to improve care through a greater emphasis on improved medication monitoring. In the United States, 54% of ADE-related hospitalizations in older adults are attributable to drugs that require regular monitoring.11 Among community-dwelling older adults and people recently discharged from the hospital, ADEs due to errors of monitoring occur more commonly than ADEs due to errors of prescribing.6, 12 Similarly, a systematic review of 11 studies of ambulatory ADEs that resulted in hospital admission found that 45% of these ADEs were caused by inadequate monitoring and 16% by ignoring a clinical or laboratory result, which together were 1.4 times more common than ADEs resulting from prescription of an inappropriate drug, drug dose, or drug frequency.8
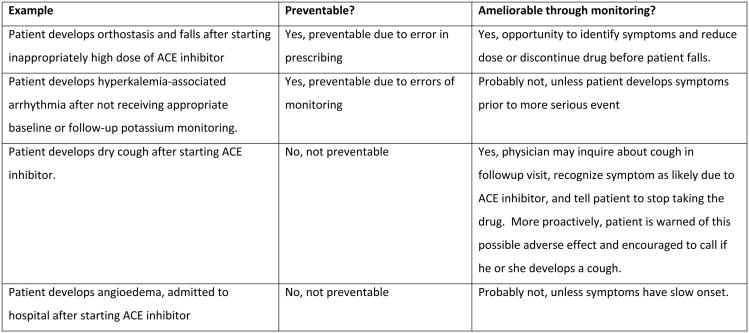
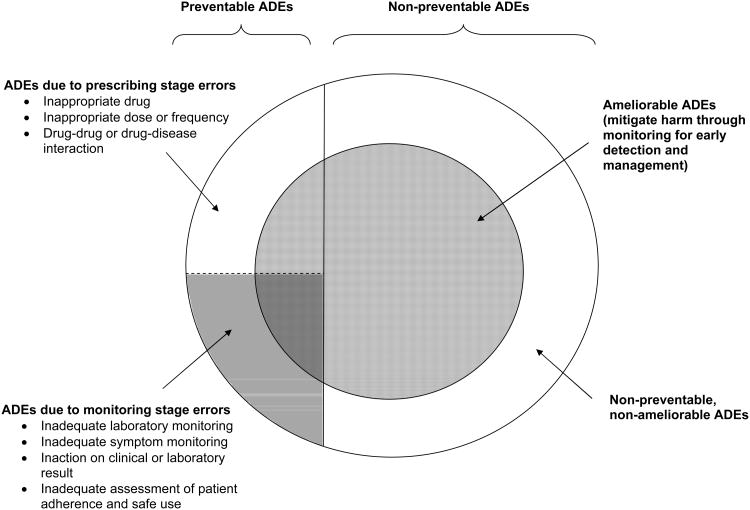
These findings highlight the importance of approaches that preemptively anticipate the emergence of a concerning laboratory result or symptom, and thus can identify and manage such problems shortly after they arise. An essential feature of this early recognition and management (known as “amelioration”) is that it can be applied regardless of whether an ADE was preventable at the time the drug was prescribed (e.g., due to a prescribing error) or not preventable (e.g., the decision to prescribe was reasonable but the patient was unlucky enough to experience bothersome or dangerous side effects). In outpatient and post-hospital discharge settings, one-quarter to one-half of ADEs can potentially be detected and mitigated at an early stage, making opportunities for amelioration up to 2.5 times more common than opportunities to prevent ADEs. 6-7, 12-14 This is demonstrated in Figure 2, which displays the relationship between preventable ADEs, ameliorable ADEs, and ADEs that occur at the prescribing and monitoring stages of drug use in rough proportion to their estimated incidence among ambulatory older adults.6-8, 13
Figure 2. Conceptual framework for preventable and ameliorable adverse drug events and their relationship to monitoring.
Different types of ADEs are shown in rough proportion to their estimated incidence among ambulatory elders.6-8, 13 Areas shaded in red are preventable or ameliorable through monitoring. Examples of preventable and ameliorable ADEs are shown in the box below, using ACE inhibitors as a case study.
The importance and impact of monitoring for ADEs varies according to the type and time course of adverse effects expected from a drug.15 For example, anaphylaxis to penicillin in a patient with no known allergy may not be amenable to monitoring strategies, since anaphylaxis is uncommon, occurs in a very short time window, is usually immediately brought to medical attention, and is easily recognizable as an ADE.16 In contrast, proactive monitoring is useful for the many ADEs that have a less rapid and dramatic onset, yet – as in the case example that began this article – can have serious implications for quality of life, function, and survival.
Current challenges to monitoring
The high frequency of ADEs related to suboptimal monitoring reflects underlying deficiencies in medication monitoring in the ambulatory setting. Many physicians report delays in tracking and reviewing test results, and 56-59% express dissatisfaction with the processes they use to manage this aspect of their practice.17-18 While systematic data about the adequacy of laboratory monitoring are limited, several studies suggest that problems are common. In studies across several types of health systems, 12-63% of patients taking ACE inhibitors had inadequate monitoring of potassium and creatinine, 37-61% of patients taking valproic acid or carbamazepine did not receive an annual CBC, and 37-55% of patients taking digoxin did not have serum digoxin concentrations checked at least once per year.19-24 Several factors likely contribute to this inadequate followup, including the fragmented nature of health care delivery; lack of team-based approaches that integrate physicians, pharmacists, nurses, and other health care professionals; use of health information technology that it neither integrated nor interoperable; conflicting recommendations and the paucity of evidence-based guidelines on how often monitoring for commonly-used drugs should occur; what threshold values should be used to generate alerts; and the specific response(s) that should be taken in the presence of an abnormal laboratory value.15, 24-26
Like problems in laboratory monitoring, assessment of troublesome symptoms caused by drugs often occurs in an ad hoc, non-systematic manner. In outpatient settings, Weingart et al found that 31% of patients' symptoms were never reported to their physicians, and Gandhi et al found that 37% of ameliorable ADEs were attributable to patients not informing physicians of their symptoms.7, 27 These findings may in part reflect limited physician attention to soliciting patient complaints and asking about medication side effects, which studies have found to be infrequently discussed during clinic visits.28-29 Moreover, missed opportunities can occur when physicians fail to recognize that a symptom or abnormal laboratory test result represents a drug side effect, or when they recognize this connection but fail to reduce the dose or discontinue the offending drug.27, 30-31 In two prominent reports, failure to respond appropriately to patient-reported symptoms accounted for 63% of ameliorable ADEs in one study, while failure to act on available clinical and laboratory information accounted for 37% of preventable ADEs in another.6-7
Integrating monitoring into clinical practice
While deficiencies in the science and practice of monitoring are considerable, they highlight substantial opportunities to improve patient care. The greatest gains are likely to be achieved through health information technology and systems redesign, risk-assessment tools, patient outreach and activation, and changes in medical education and the heuristics of prescribing (Table 1).32 A central theme uniting each of these areas is the need for team-based approaches to monitoring based on shared responsibility with pharmacists, nurses, and support staff, as exemplified by the Patient Centered Medical Home model, case management, and Geriatric Evaluation and Management programs.33-34 The emergence of accountable care organizations and other recent health system reforms such as the Health Information Technology for Economic and Clinical Health (HITECH) act creates incentives in the right direction, yet the full promise of these programs remains far off in a health care environment that provides limited support for primary care. Nevertheless, shorter-term opportunities for improvement exist, some of which are described below. For the majority of physicians still working under traditional models of care, these focus areas can guide interventions that, while less potent than those achievable with integrated health care teams, hold promise to substantially improve the practice of monitoring.
Table 1. Strategies to improve monitoring.
| Concept | Monitoring strategy | Example | Comment |
|---|---|---|---|
| Implement monitoring systems using health information technology | Laboratory tracking systems in individual physician offices17 | Locally-developed mechanisms for systematically reviewing and responding to laboratory reports | Many physicians dissatisfied with current approaches; practice management toolkits and interdisciplinary focus may support better approaches.17 |
| Automated linkages of laboratory to pharmacy36 | Electronic reminders for laboratory monitoring and real-time decision support generated by linking laboratory and pharmacy data | Most feasible in integrated delivery systems. Most effective if linked to interdisciplinary team-based patient outreach rather than reported only to physician.40 | |
| Risk-assessment tools | Individualize monitoring frequency and intensity based on an individual's predicted risk of an adverse event. | More intensive monitoring regimens for patients at higher risk of ADEs. | Typically applies only to ADEs amenable to amelioration (i.e. slow in onset, reversible with withdrawal of drug). |
| Patient outreach and activation | Patient outreach for laboratory followup43 | Automated phone calls or nurse/pharmacist outreach to remind patients to obtain labs | Studies suggest this approach is highly effective.40, 42 |
| Patient outreach for symptom assessment49 | Nurse or pharmacist contacts patient shortly after drug started to inquire about efficacy and side effects; online portals for same; targeted symptom assessments when patient checks in for physician visit | Integrates well with “medical home” model in which patient assessment and management is a shared responsibility of the health care team.50 | |
| Systems to lower patient threshold for raising questions about potential adverse effects12 | Electronic patient portals that promote patient contact with nurses, pharmacists, or physicians when problems arise. | Concerns about overwhelming clinicians with less serious symptoms unlikely to be related to medication use need to be addressed. | |
| Medication therapy management 50 | Ongoing pharmacist and/or nurse-led program for helping patient manage drugs | Requires substantial reorganization of office-based care delivery with focus on interdisciplinary care. Medication therapy management programs are covered under Medicare Part D but many patients are not eligible, and services provided are limited. | |
| Patient education and activation about identifying and reporting adverse effects45 | Clear, easy-to-read information sheets about drugs, side effects to watch for, and what to do if side effect occurs | Passive education usually has limited effect; likely to work best if coupled with patient outreach. | |
| Medical education and heuristics of prescribing | Teaching and role-modeling approaches to prescribing that use the enhanced monitoring framework | Highlight design and execution of monitoring plans by clinical preceptors of medical and pharmacy students and residents Integrate monitoring principles and practices into pharmacology courses for students |
Role-modeling and teaching monitoring in interdisciplinary settings and with a focus on systems-based practice likely to be high-yield activities. |
It is important to note that there is no single “best” solution. The evidence base is insufficiently robust to allow direct comparisons, and the effectiveness of any given strategy is highly dependent on local conditions, including the ability to piggyback monitoring interventions onto existing, broad-based programs. Thus, the choice of which strategy will yield the most value for any given office practice, institution, or health care system will depend on local circumstances, incentives, and identification of areas where there is readiness to change.
Health information technology and systems redesign
In surveys of test result management, only one-third of physicians reported having a system to detect followup tests that were overdue for completion.17, 35 By linking pharmacy and laboratory data, health information technology can close this gap through features that track and generate alerts about non-completion of laboratory tests.36 For example, the Veteran Health Administration's MUET system allows facilities to generate reports about non-completion of laboratory tests and to identify patients prescribed a given drug with laboratory values outside the recommended range (personal communication, Dana Frank, PharmD). Moreover, integrated laboratory-pharmacy linkages can provide real-time computerized decision support and integrated safety checks at the time a drug is prescribed and provide future medication monitoring recommendations. 37 Health information exchanges can further expand these possibilities by facilitating the transfer of health information across different health systems in which patients might receive care.
However, the mere presence of an electronic medical record is insufficient to improve monitoring. Many electronic medical record systems lack test-tracking capability, or may require a patient's chart to be opened for these functions to work, or such functions may simply not be used regularly or effectively. In addition, studies of electronic reminders to physicians have yielded mixed and sometimes disappointing results. A recent review found that providing information at the time of prescribing had little effect, whereas computer-based interventions that detected incomplete laboratory monitoring at some point after the patient-physician encounter had greater impact. 38 Thus, electronic linkages are likely to be of little value unless engineered in a way that accounts for clinician workflow and team-based approaches. In the meantime, simple systems to track incomplete laboratory tests, such as a spreadsheet to track laboratory tests ordered, due date, and return of test results can be useful for individual clinician offices without advanced electronic medical record capabilities. On a policy level, standards that mandate the ability to easily track and report overdue laboratory tests without having to open an individual patient's chart could be included in “meaningful use” criteria under federal programs that incentivize adoption of high-value electronic medical record systems.39
Risk-assessment tools
Risk assessment instruments including clinical prediction rules, pharmacogenomics, and drug-drug interaction checks have often been used to guide whether or not to prescribe a drug. These tools could also be deployed to better guide monitoring decisions. For example, if a risk prediction instrument identified a patient as being at lower or higher risk of suffering an ADE from a drug, the frequency and intensity of monitoring could be titrated appropriately so long as the expected ADE was ameliorable and the overall benefit-to-risk ratio favored treatment. At present, such decisions would be largely judgment-based, as few studies or guidelines offer evidence-based (or even opinion-based) assessment of monitoring strategies under various conditions. However, just as prescribing is moving toward individualization through pharmacogenomics and other such tools, evidence-based strategies to individualize monitoring offer a promising area for future inquiry.
Patient outreach and enhanced patient participation
Patient outreach using team-based approaches to monitoring is likely to yield important benefits. In several studies, pharmacist-based outreach programs and automated reminder calls to patients (either independently or teamed with physician alerts) have been found to be less costly and more effective than physician-centered approaches, increasing rates of laboratory monitoring by 6-60% over usual care.20, 38, 40-43 Collaborative practice agreements between physicians and pharmacists, in which physicians formally delegate certain test-ordering and prescribing authority to pharmacists, provides a legal and practical mechanism for collaboration.
Similarly, engaging patients as active participants in monitoring their drugs is likely to improve outcomes.44 Patients commonly know little about the indications or potential side effects of drugs prescribed to them, and this lack of awareness has been associated both with decreased adherence and higher rates of ADEs.12, 45 To remedy this, patients could be provided concise, literacy-accessible information resources that describe the purpose of the drug, side effects to watch for (and what to do should they occur), the type and frequency of laboratory monitoring required, and even a log book to help them keep track of test schedules and results.46 At follow-up visits after a new medication was started, patients could complete a brief, targeted questionnaire to screen for drug-related problems. Alternatively, automated calls several weeks after a drug was prescribed could use touchpad-based responses to assess symptoms.
Short of health systems changes that will support these interventions, physicians and nurses can encourage patients to learn more about their drugs from approachable drug information sources such as MedlinePlus (http://www.nlm.nih.gov/medlineplus/druginformation.html) and to report symptoms. Similarly, office-based clinicians can incorporate simple, validated screening questions about drug side effects and adherence as part of the patient triage assessment (for example, “since your last visit, have you noticed any side effects, unwanted reactions, or other problems from medications you were taking”).47
Education and the heuristics of prescribing
Finally, better integrating monitoring into clinical practice will require a re-thinking of how clinicians approach the prescribing process.48 Current medical norms often treat prescribing as a point-in-time, physician-centered event. The concept of “helping” the patient with a drug prescription will require teaching and role-modeling new reflexes for a longer-term, cooperative approach to anticipate and monitor for ADEs. For example, clinical pharmacology course in medical school could integrate principles and practices of drug monitoring into their curricula, and interdisciplinary training can help physicians, pharmacists, nurses, and other health professionals learn how to work together in ongoing assessments of patient care. Clinical training programs could also work with preceptors and local opinion leaders to highlight the design and execution of monitoring plans in their patient-based teaching. This same model can apply equally to monitoring for adherence and drug efficacy, and thus has broad applicability not only for preventing complications of drug use but for maximizing the quality and effectiveness of drug therapy.
Conclusions
Traditionally, medication prescribing has been approached as a single point-in-time event. The patient presents with signs of symptoms of a disease, and the clinician makes a prescribing decision. Clinicians are often judged implicitly or explicitly on whether the choice of drug is appropriate or not, and such evaluations generally stop at that point. Reducing the burden of ADEs in older patients will require a new paradigm for pharmacotherapeutic care, with monitoring of signs, symptoms, and laboratory markers firmly situated as the focal point of a comprehensive, longitudinal process. These changes will not come easily. Monitoring is a complex endeavor that requires ongoing attention and commitment and investment in new systems of care delivery. In return, a focus on monitoring may help achieve a key aim for the care of older patients: combining the best of evidence-based medicine with close and ongoing attention to the specific circumstances of each patient.
Acknowledgments
The authors thank Sneha Patil, BA and Carolyn Peterson, MA for their assistance assembling materials for this manuscript.
Funding for this work was provided by a Beeson Career Development Award from the National Institute on Aging and the American Federation for Aging Research (1K23AG030999, Dr. Steinman), by a NIH Roadmap Multidisciplinary Clinical Research Career Development Award (5KL2RR024151, Dr. Handler), by a grant from the Agency for Healthcare Research and Quality (AHRQ) (1R01HS018721-01; Dr. Handler) and another grant to the University of Illinois at Chicago Center for Education and Research in Therapeutics (CERT) (U18HS016973, Dr. Schiff), and by an award from the National Institute on Aging (1K24AG029812-02, Dr. Covinsky).
Sponsor's Role: The sponsors had no role in the conceptualization or authorship of this manuscript.
| Elements of Financial/Personal Conflicts | All authors (MAS, SMH, JHG, GDS, KEC) | |
|---|---|---|
| Yes | No | |
| Employment or Affiliation | x | |
| Grants/Funds | x | |
| Honoraria | x | |
| Speaker Forum | x | |
| Consultant | x | |
| Stocks | x | |
| Royalties | x | |
| Expert Testimony | x | |
| Board Member | x | |
| Patents | x | |
| Personal Relationship | x | |
Footnotes
Disclosure: The authors have no conflicts of interest with subjects discussed in this manuscript.
Opinions expressed in this manuscript are those of the authors and do not necessarily reflect the position of the U.S. Government or Department of Veterans Affairs.
Author contributions: Conceptualization of ideas: all authors
Initial authorship of manuscript: Steinman
Critical review and revision of manuscript: all authors
Contributor Information
Michael A. Steinman, Division of Geriatrics, University of California – San Francisco and the San Francisco VA Medical Center.
Steven M. Handler, Department of Biomedical informatics, Division of Geriatric Medicine, University of Pittsburgh and VA Pittsburgh Health Care System.
Jerry H. Gurwitz, Division of Geriatric Medicine, University of Massachusetts Medical School, and Executive Director, Meyers Primary Care Institute.
Gordon D. Schiff, Division of General Internal Medicine, Harvard Medical School and Brigham and Women's Hospital.
Kenneth E. Covinsky, Division of Geriatrics, University of California – San Francisco and the San Francisco VA Medical Center.
References
- 1.Hanlon JT, Schmader KE, Ruby CM, Weinberger M. Suboptimal prescribing in older inpatients and outpatients. J Am Geriatr Soc. 2001;49(2):200–209. doi: 10.1046/j.1532-5415.2001.49042.x. [DOI] [PubMed] [Google Scholar]
- 2.Roden DM. Chapter 5. Principles of Clinical Pharmacology. In: Fauci AS, Braunwald E, Kasper DL, et al., editors. Harrison's Online [Harrison's principles of internal medicine online edition] New York: McGraw Hill; [Google Scholar]
- 3.Hilmer SN, Ford GA. General Principles of Pharmacology. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, High KP, Asthana S, editors. Hazzard's Geriatric Medicine and Gerontology, version 6e. New York: McGraw-Hill; [Google Scholar]
- 4.Pompei P, editor. Geriatrics Review Syllabus: A Core Curriculum in Geriatric Medicine. Sixth. Fry Communications; Pharmacotherapy. [Google Scholar]
- 5.Avorn J. Improving drug use in elderly patients: getting to the next level. JAMA. 2001 Dec 12;286(22):2866–2868. doi: 10.1001/jama.286.22.2866. [DOI] [PubMed] [Google Scholar]
- 6.Gurwitz JH, Field TS, Harrold LR, et al. Incidence and preventability of adverse drug events among older persons in the ambulatory setting. JAMA. 2003 Mar 5;289(9):1107–1116. doi: 10.1001/jama.289.9.1107. [DOI] [PubMed] [Google Scholar]
- 7.Gandhi TK, Weingart SN, Borus J, et al. Adverse drug events in ambulatory care. N Engl J Med. 2003 Apr 17;348(16):1556–1564. doi: 10.1056/NEJMsa020703. [DOI] [PubMed] [Google Scholar]
- 8.Thomsen LA, Winterstein AG, Sondergaard B, Haugbolle LS, Melander A. Systematic review of the incidence and characteristics of preventable adverse drug events in ambulatory care. Ann Pharmacother. 2007 Sep;41(9):1411–1426. doi: 10.1345/aph.1H658. [DOI] [PubMed] [Google Scholar]
- 9.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann Intern Med. 2007 Dec 4;147(11):755–765. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 10.Samsa GP, Matchar DB. Relationship between test frequency and outcomes of anticoagulation: a literature review and commentary with implications for the design of randomized trials of patient self-management. J Thromb Thrombolysis. 2000 Apr;9(3):283–292. doi: 10.1023/a:1018778914477. [DOI] [PubMed] [Google Scholar]
- 11.Budnitz DS, Pollock DA, Weidenbach KN, Mendelsohn AB, Schroeder TJ, Annest JL. National surveillance of emergency department visits for outpatient adverse drug events. JAMA. 2006 Oct 18;296(15):1858–1866. doi: 10.1001/jama.296.15.1858. [DOI] [PubMed] [Google Scholar]
- 12.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. Adverse drug events occurring following hospital discharge. J Gen Intern Med. 2005 Apr;20(4):317–323. doi: 10.1111/j.1525-1497.2005.30390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forster AJ, Clark HD, Menard A, et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004 Feb 3;170(3):345–349. [PMC free article] [PubMed] [Google Scholar]
- 14.Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003 Feb 4;138(3):161–167. doi: 10.7326/0003-4819-138-3-200302040-00007. [DOI] [PubMed] [Google Scholar]
- 15.Coleman JJ, Ferner RE, Evans SJ. Monitoring for adverse drug reactions. Br J Clin Pharmacol. 2006 Apr;61(4):371–378. doi: 10.1111/j.1365-2125.2006.02596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glasziou P, Irwig L, Mant D. Monitoring in chronic disease: a rational approach. BMJ. 2005 Mar 19;330(7492):644–648. doi: 10.1136/bmj.330.7492.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poon EG, Gandhi TK, Sequist TD, Murff HJ, Karson AS, Bates DW. “I wish I had seen this test result earlier!”: Dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004 Nov 8;164(20):2223–2228. doi: 10.1001/archinte.164.20.2223. [DOI] [PubMed] [Google Scholar]
- 18.Shirts BH, Perera S, Hanlon JT, et al. Provider management of and satisfaction with laboratory testing in the nursing home setting: results of a national internet-based survey. J Am Med Dir Assoc. 2009 Mar;10(3):161–166 e163. doi: 10.1016/j.jamda.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raebel MA, McClure DL, Simon SR, et al. Laboratory monitoring of potassium and creatinine in ambulatory patients receiving angiotensin converting enzyme inhibitors and angiotensin receptor blockers. Pharmacoepidemiol Drug Saf. 2007 Jan;16(1):55–64. doi: 10.1002/pds.1217. [DOI] [PubMed] [Google Scholar]
- 20.Raebel MA, Lyons EE, Andrade SE, et al. Laboratory monitoring of drugs at initiation of therapy in ambulatory care. J Gen Intern Med. 2005 Dec;20(12):1120–1126. doi: 10.1111/j.1525-1497.2005.0257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurley JS, Roberts M, Solberg LI, et al. Laboratory safety monitoring of chronic medications in ambulatory care settings. J Gen Intern Med. 2005 Apr;20(4):331–333. doi: 10.1111/j.1525-1497.2005.40182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus SC, Olfson M, Pincus HA, Zarin DA, Kupfer DJ. Therapeutic drug monitoring of mood stabilizers in Medicaid patients with bipolar disorder. Am J Psychiatry. 1999 Jul;156(7):1014–1018. doi: 10.1176/ajp.156.7.1014. [DOI] [PubMed] [Google Scholar]
- 23.Higashi T, Shekelle PG, Solomon DH, et al. The quality of pharmacologic care for vulnerable older patients. Ann Intern Med. 2004 May 4;140(9):714–720. doi: 10.7326/0003-4819-140-9-200405040-00011. [DOI] [PubMed] [Google Scholar]
- 24.Tjia J, Field TS, Garber LD, et al. Development and pilot testing of guidelines to monitor high-risk medications in the ambulatory setting. Am J Manag Care. 2010 Jul;16(7):489–496. [PubMed] [Google Scholar]
- 25.Handler SM, Hanlon JT, Perera S, et al. Consensus list of signals to detect potential adverse drug reactions in nursing homes. J Am Geriatr Soc. 2008 May;56(5):808–815. doi: 10.1111/j.1532-5415.2008.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pirmohamed M, Ferner RE. Monitoring drug treatment. BMJ. 2003 Nov 22;327(7425):1179–1181. doi: 10.1136/bmj.327.7425.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weingart SN, Gandhi TK, Seger AC, et al. Patient-reported medication symptoms in primary care. Arch Intern Med. 2005 Jan 24;165(2):234–240. doi: 10.1001/archinte.165.2.234. [DOI] [PubMed] [Google Scholar]
- 28.Richard C, Lussier MT. Nature and frequency of exchanges on medications during primary care encounters. Patient Educ Couns. 2006 Dec;64(1-3):207–216. doi: 10.1016/j.pec.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Sleath B, Roter D, Chewning B, Svarstad B. Asking questions about medication: analysis of physician-patient interactions and physician perceptions. Med Care. 1999 Nov;37(11):1169–1173. doi: 10.1097/00005650-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Gandhi TK, Burstin HR, Cook EF, et al. Drug complications in outpatients. J Gen Intern Med. 2000 Mar;15(3):149–154. doi: 10.1046/j.1525-1497.2000.04199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rochon PA, Gurwitz JH. Optimising drug treatment for elderly people: the prescribing cascade. BMJ. 1997 Oct 25;315(7115):1096–1099. doi: 10.1136/bmj.315.7115.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Budnitz DS, Layde PM. Outpatient drug safety: new steps in an old direction. Pharmacoepidemiol Drug Saf. 2007 Feb;16(2):160–165. doi: 10.1002/pds.1242. [DOI] [PubMed] [Google Scholar]
- 33.Cohen HJ, Feussner JR, Weinberger M, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002 Mar 21;346(12):905–912. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- 34.Ma J, Berra K, Haskell WL, et al. Case management to reduce risk of cardiovascular disease in a county health care system. Arch Intern Med. 2009 Nov 23;169(21):1988–1995. doi: 10.1001/archinternmed.2009.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boohaker EA, Ward RE, Uman JE, McCarthy BD. Patient notification and follow-up of abnormal test results. A physician survey. Arch Intern Med. 1996 Feb 12;156(3):327–331. [PubMed] [Google Scholar]
- 36.Schiff GD, Klass D, Peterson J, Shah G, Bates DW. Linking laboratory and pharmacy: opportunities for reducing errors and improving care. Arch Intern Med. 2003 Apr 28;163(8):893–900. doi: 10.1001/archinte.163.8.893. [DOI] [PubMed] [Google Scholar]
- 37.Schiff GD, Aggarwal HC, Kumar S, McNutt RA. Prescribing potassium despite hyperkalemia: medication errors uncovered by linking laboratory and pharmacy information systems. Am J Med. 2000 Oct 15;109(6):494–497. doi: 10.1016/s0002-9343(00)00546-5. [DOI] [PubMed] [Google Scholar]
- 38.Hayward GL, Parnes AJ, Simon SR. Using health information technology to improve drug monitoring: a systematic review. Pharmacoepidemiol Drug Saf. 2009 Dec;18(12):1232–1237. doi: 10.1002/pds.1831. [DOI] [PubMed] [Google Scholar]
- 39.Blumenthal D, Tavenner M. The “Meaningful” Use Regulation for Electronic Health Records. N Engl J Med. 2010 Jul 13; doi: 10.1056/NEJMp1006114. [DOI] [PubMed] [Google Scholar]
- 40.Feldstein AC, Smith DH, Perrin N, et al. Improved therapeutic monitoring with several interventions: a randomized trial. Arch Intern Med. 2006 Sep 25;166(17):1848–1854. doi: 10.1001/archinte.166.17.1848. [DOI] [PubMed] [Google Scholar]
- 41.Smith DH, Feldstein AC, Perrin NA, et al. Improving laboratory monitoring of medications: an economic analysis alongside a clinical trial. Am J Manag Care. 2009 May;15(5):281–289. [PubMed] [Google Scholar]
- 42.Raebel MA, Chester EA, Newsom EE, et al. Randomized trial to improve laboratory safety monitoring of ongoing drug therapy in ambulatory patients. Pharmacotherapy. 2006 May;26(5):619–626. doi: 10.1592/phco.26.5.619. [DOI] [PubMed] [Google Scholar]
- 43.Raebel MA, Lyons EE, Chester EA, et al. Improving laboratory monitoring at initiation of drug therapy in ambulatory care: a randomized trial. Arch Intern Med. 2005 Nov 14;165(20):2395–2401. doi: 10.1001/archinte.165.20.2395. [DOI] [PubMed] [Google Scholar]
- 44.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006 Sep 25;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 45.Shrank WH, Avorn J. Educating patients about their medications: the potential and limitations of written drug information. Health Aff (Millwood) 2007 May-Jun;26(3):731–740. doi: 10.1377/hlthaff.26.3.731. [DOI] [PubMed] [Google Scholar]
- 46.Tierney WM. Adverse outpatient drug events--a problem and an opportunity. N Engl J Med. 2003 Apr 17;348(16):1587–1589. doi: 10.1056/NEJMe030026. [DOI] [PubMed] [Google Scholar]
- 47.Chrischilles EA, Segar ET, Wallace RB. Self-reported adverse drug reactions and related resource use. A study of community-dwelling persons 65 years of age and older. Ann Intern Med. 1992 Oct 15;117(8):634–640. doi: 10.7326/0003-4819-117-8-634. [DOI] [PubMed] [Google Scholar]
- 48.Schiff GD, Galanter WL. Promoting more conservative prescribing. JAMA. 2009 Feb 25;301(8):865–867. doi: 10.1001/jama.2009.195. [DOI] [PubMed] [Google Scholar]
- 49.Schnipper JL, Kirwin JL, Cotugno MC, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med. 2006 Mar 13;166(5):565–571. doi: 10.1001/archinte.166.5.565. [DOI] [PubMed] [Google Scholar]
- 50.Pindolia VK, Stebelsky L, Romain TM, Luoma L, Nowak SN, Gillanders F. Mitigation of medication mishaps via medication therapy management. Ann Pharmacother. 2009 Apr;43(4):611–620. doi: 10.1345/aph.1L591. [DOI] [PubMed] [Google Scholar]