It is well known that Streptococcus pneumoniae remains a leading cause of childhood mortality; in 2008, it was estimated to be responsible for over 500,000 deaths in children aged 1–59 months.1 It is also known that the pneumococcus is a cause of significant morbidity and mortality among those younger and older than the 1- to 59-month age group,2,3 although prevention of disease in these age groups has not been the target of global efforts to date. Serious diseases caused by the pneumococcus across all age groups include pneumonia, meningitis and sepsis. Developing countries, mostly in Africa and Asia, carry the highest burden of both pneumococcal disease incidence and mortality in children; this burden is measured both in terms of rates and absolute numbers of cases and deaths.1
Effective vaccines to prevent pneumococcal disease in children exist; access to these life-saving pneumococcal conjugate vaccines (PCV) has become possible in many developing countries through the focused, coordinated efforts of the GAVI Alliance, including all of the key Alliance partners. The World Health Organization, the technical partner that provides global vaccine policy recommendations, advises that PCV be a part of the routine infant vaccine schedule for all countries, but particularly those with highest infant mortality rates and death counts.4 As of December 2012, 87 countries had introduced PCV into their routine immunization schedule (including a ceremonial launch in 1 country), of which 24 were GAVI-eligible countries.5,6 An additional 27 GAVI-eligible countries are approved for introduction of PCV in 2013 and beyond. PCVs are being implemented globally, including the poorest countries of the world, at a pace exceeding that of other previously developed vaccines. These vaccines were designed to prevent pneumococcal disease in infants and toddlers, age groups for which previous pneumococcal vaccines were insufficiently protective. The introduction of PCV has been associated with a dramatic decline in pneumococcal disease caused by serotypes included in the vaccine (ie, vaccine-type disease), in children targeted for vaccination and older persons not intended for vaccination (the indirect effect of vaccination).7–16 The indirect effect on disease results from the impact of PCVs on preventing nasopharyngeal colonization.17 Reduced colonization leads to reduced spread of the pneumococcus and thus less disease in the unimmunized.18
To assure that the enormous investments being made in PCV achieve the greatest disease impact, we must move beyond gross metrics of counting the number of countries introducing the vaccines. The focus should now be on achieving optimal vaccine implementation, including but not limited to optimizing vaccine coverage, in all countries. As important as targets are for achieving timely and thorough vaccine coverage, this is an intermediate goal and not the end goal, which is disease prevention and mortality reduction. Toward that goal, we must understand the relationships between dosing schedules, measuring and understanding the magnitude of change in disease, colonization and pathogen characteristics with the intention and focus on achieving the greatest disease impact of the vaccine programs.
Vaccine delivery policy decision makers in countries, regions and globally must be able to include in their assessments evidence for how to administer these products to achieve maximal and optimal impact of the doses being administered and resources expended. This evidence should include the disease impact on both the age groups intended for vaccination and those who are not eligible for vaccination (ie, younger or older than the immunization age group). Impact in both groups contributes to the overall effect and therefore the assessment of return on investment in the health of the population as a whole. A variety of PCV immunization schedules have been assessed in controlled trials and in observational studies for a wide range of disease and pathogen outcomes. Exactly which of these schedules are preferred over others, if there is any preference to be made, has not been not fully understood and therefore recommendations on preferred schedules cannot be made to the policy decision makers.
We therefore undertook a comprehensive, systematic assessment of the absolute and relative benefit of PCV schedules to establish if there is evidence of preferred or sub-optimal schedules and to identify where essential gaps in knowledge lie so that targeted, strategic studies can be planned to assure a decisive evidence base for dosing schedule optimization. As with any vaccine, a limited number of randomized controlled trials have been conducted and these cannot answer the diverse range of biologic questions about dosing schedules. We therefore undertook this assessment putting together all of the evidence in the literature, with controlled trials and observational studies alike, aiming to be as inclusive as possible since the largest body of evidence would reveal a consistency in lessons learned even if any given trial was not the optimal study.
There are important factors to consider when weighing the benefits of different schedules. From an epidemiologic standpoint, it is important to account for differences in organism transmission dynamics in various geographic, disease burden and community settings; concluding that an introduction and dosing schedule are optimal should consider both the direct and indirect effects of the vaccine and will be influenced by the existing transmission and carriage rates in the community. From a policy standpoint, the deciding factors for a country should also take into account programmatic considerations for integrating PCV into existing vaccine schedules and the need to maximize limited financial resources. The performance of the vaccine program to deliver high coverage at each time point in the immunization schedule will likely strongly influence decision making regarding PCV schedule choice since ultimately delivering all the doses in a schedule is likely to outweigh any relative benefit of one schedule over another.
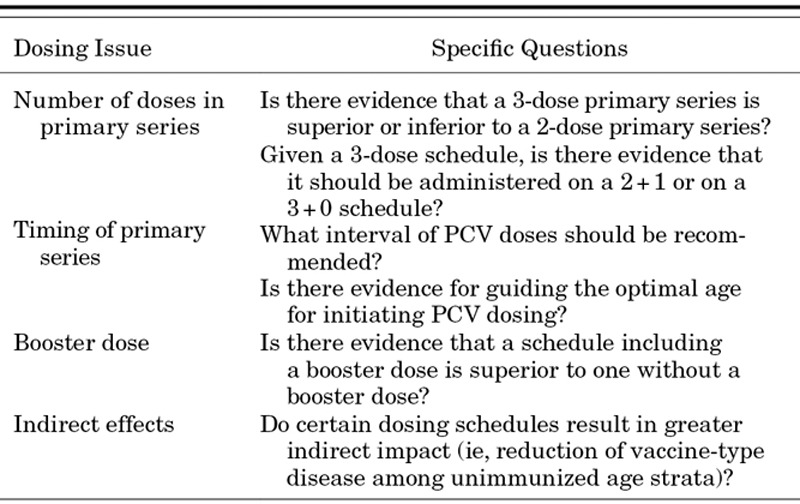
Furthermore, as infant vaccine schedules become increasingly crowded, it is essential to integrate new products into existing schedules so that parents and caretakers will not be burdened by making additional visits; this must be weighed against the acceptability of multiple injections at a single visit and against the disease epidemiology across age strata.19 In many European countries, a 2+1 schedule with doses at 3, 5 and 12 months coordinates well with existing schedules whereas a 3+1 schedule at 2, 4, 6 and 12 months fits into the current US immunization schedule.7,13,14 Most developing countries have adopted a 3+0 schedule (6, 10 and 14 weeks) as this fits well with the Expanded Programme on Immunizations that is the standard WHO platform for core vaccine delivery.20 The degree to which a preference among these schedules exists as measured by disease and colonization impact is the purpose of this systematic assessment. We approached the review with a series of policy questions (Table 1) serving as guiding principles behind the methods, analysis and conclusions. We aimed to have as much methodological consistency across the outcomes as possible while maintaining the most inclusive principles for any given analysis. Therefore, each outcome has been customized to the degree necessary to assure that the largest set of data is considered to inform the analysis and conclusions. These findings were reviewed at a meeting of experts held by WHO,21 subsequently presented to the WHO Strategic Advisory Group of Experts on Immunizations in November 2011,22 and are now provided here in this supplement.
TABLE 1.
Policy Directed Questions That Guided the PCV Dosing Landscape Analysis Project
Footnotes
Accepted for publication August 13, 2013.
D.G.’s laboratory performs contract and or collaborative research for/with Pfizer, GlaxoSmithKline, Merck, Novartis and Sanofi Pasteur. D.G. has received travel or honorarium support for participation in external expert committees for Merck, Sanofi Pasteur, Pfizer and GlaxoSmithKline. K.O.B. received grant support from Pfizer, GlaxoSmithKline and has received travel or honorarium support for participation in external expert committees for Merck, Aventis-pasteur and GlaxoSmithKline. The authors have no other funding or conflicts of interest to disclose.
Address for correspondence: Katherine L. O’Brien, MD, MPH, 855 N. Wolfe Street, Suite 600, Baltimore, MD 21205. E-mail: klobrien@jhsph.edu.
REFERENCES
- 1.O’Brien KL, Wolfson LJ, Watt JP, et al. Hib and Pneumococcal Global Burden of Disease Study Team. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009;374:893–902. doi: 10.1016/S0140-6736(09)61204-6. [DOI] [PubMed] [Google Scholar]
- 2.Jayakumar M. Global burden of neonatal invasive pneumococcal disease: a systematic review and meta-analysis. PhD Dissertation, Johns Hopkins Bloomberg School of Public Health; 2011.
- 3.Garcia CR, Johnson HL, Summers A, et al. Igaçu Falls, Brazil: March 11–15, 2012. Pneumococcal disease in older children and adults globally: results from the AGEDD project. 8th International Symposium on Pneumococci and Pneumococcal Disease; [Google Scholar]
- 4.World Health Organization. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- 5.IVAC, Johns Hopkins Bloomberg School of Public Health. Vaccine information management system (VIMS) global vaccine introduction report. December, 2012. Available at: http://www.jhsph.edu/ivac/vims.html. Accessed May 20, 2013.
- 6.GAVI Alliance. Countries approved for support. Geneva, Switzerland: GAVI Alliance. 2013. Available at: http://www.gavialliance.org/results/countries-approved-for-support. Available at May 20, 2013.
- 7.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 8.Weatherholtz R, Millar EV, Moulton LH, et al. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50:1238–1246. doi: 10.1086/651680. [DOI] [PubMed] [Google Scholar]
- 9.Bettinger JA, Scheifele DW, Kellner JD, et al. Canadian Immunization Monitoring Program, Active (IMPACT) The effect of routine vaccination on invasive pneumococcal infections in Canadian children, Immunization Monitoring Program, Active 2000–2007. Vaccine. 2010;28:2130–2136. doi: 10.1016/j.vaccine.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 10.Rodenburg GD, de Greeff SC, Jansen AG, et al. Effects of pneumococcal conjugate vaccine 2 years after its introduction, the Netherlands. Emerg Infect Dis. 2010;16:816–823. doi: 10.3201/eid1605.091223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durando P, Crovari P, Ansaldi F, et al. Collaborative Group for Pneumococcal Vaccination in Liguria. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine. 2009;27:3459–3462. doi: 10.1016/j.vaccine.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 12.Rückinger S, van der Linden M, Reinert RR, et al. Reduction in the incidence of invasive pneumococcal disease after general vaccination with 7-valent pneumococcal conjugate vaccine in Germany. Vaccine. 2009;27:4136–4141. doi: 10.1016/j.vaccine.2009.04.057. [DOI] [PubMed] [Google Scholar]
- 13.Deceuninck G, De Wals P, Boulianne N, et al. Effectiveness of pneumococcal conjugate vaccine using a 2+1 infant schedule in Quebec, Canada. Pediatr Infect Dis J. 2010;29:546–549. doi: 10.1097/INF.0b013e3181cffa2a. [DOI] [PubMed] [Google Scholar]
- 14.De Carvalho Gomes H, Muscat M, Monnet DL, Giesecke J, Lopalco PL. Use of seven-valent pneumococcal conjugate vaccine (PCV7) in Europe, 2001–2007. Euro Surveill. 2009;14:19159. [PubMed] [Google Scholar]
- 15.Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med. 2011;8:e1001017. doi: 10.1371/journal.pmed.1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenya Medical Research Institute. Pneumococcal conjugate vaccine impact study. Current disease surveillance as of April 15, 2013. Available at: http://www.kemri-wellcome.org/pcvis-current%20disease%20surveillance. Accessed May 20, 2013.
- 17.Fleming-Dutra KE, Conklin L, Loo JD, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S152–S160. doi: 10.1097/INF.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis S, Deloria-Knoll M, O’Brien KL for the PneumoCarr Consortium, and the PCV Dosing Landscape Project. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine. doi: 10.1016/j.vaccine.2013.05.005. [published online ahead of print May 16, 2013]. doi: 10.1016/j.vaccine.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Russell F, Sanderson C, Temple B, et al. Igaçu Falls, Brazil: March 11–15, 2012. Global review of the distribution of pneumococcal disease by age and region: implications for vaccination schedules. 8th International Symposium on Pneumococci and Pneumococcal Disease; [Google Scholar]
- 20.World Health Organization. Pneumococcal Vaccines, WHO position paper—2012. Wkly Epidemiol Rec. 2012;87:129–144. [PubMed] [Google Scholar]
- 21.World Health Organization. Geneva: September 12, 2011. Meeting report of PCV immunization schedules: ad hoc expert consultation. [Google Scholar]
- 22.World Health Organization. Meeting of the Strategic Advisory Group of Experts on Immunization, November 2011—conclusions and recommendations. Wkly Epidemiol Rec. 2012;87:1–16. [PubMed] [Google Scholar]


