Abstract
Background:
Despite the breadth of studies demonstrating benefits of pneumococcal conjugate vaccine (PCV), uncertainty remains regarding the optimal PCV dosing schedule in infants.
Methods:
We conducted a systematic literature review of PCV immunogenicity published from 1994 to 2010 (supplemented post hoc with studies from 2011). Studies included for analysis evaluated ≥2 doses of 7-valent or higher product (excluding Aventis-Pasteur PCV11) administered to nonhigh-risk infants ≤6 months of age. Impact of PCV schedule on geometric mean antibody concentration (GMC) and proportion of subjects over 0.35 mcg/mL were assessed at various time points; the GMC 1 month postdose 3 (for various dosing regimens) for serotypes 1, 5, 6B, 14, 19F and 23F was assessed in detail using random effects linear regression, adjusted for product, acellular diphtheria-tetanus-pertussis/whole-cell diphtheria- tetanus-pertussis coadministration, laboratory method, age at first dose and geographic region.
Results:
From 61 studies, we evaluated 13 two-dose (2+0) and 65 three-dose primary schedules (3+0) without a booster dose, 11 “2+1” (2 primary plus booster) and 42 “3+1” schedules. The GMC after the primary series was higher following 3-dose schedules compared with 2-dose schedules for all serotypes except for serotype 1. Pre- and postbooster GMCs were generally similar regardless of whether 2 or 3 primary doses were given. GMCs were significantly higher for all serotypes when dose 3 was administered in the second year (2+1) compared with ≤6 months of age (3+0).
Conclusions:
While giving the third dose in the second year of life produces a higher antibody response than when given as part of the primary series in the first 6 months, the lower GMC between the 2-dose primary series and booster may result in less disease protection for infants in that interval than those who completed the 3-dose primary series. Theoretical advantages of higher antibodies induced by giving the third dose in the second year of life, such as increased protection against serotype 1 disease, longer duration of protection or more rapid induction of herd effects, need to be evaluated in practice.
Keywords: pneumococcal conjugate vaccine, immunogenicity, immunization schedule
Over 25% of the 7.6 million deaths occurring in children <5 years of age worldwide in 2010 were due to pneumonia, sepsis and meningitis.1 Streptococcus pneumoniae is a leading cause of these diseases, estimated by the World Health Organization (WHO) to kill over 500,000 children in 20082; over 90% of these deaths occur in developing countries. Three licensed pneumococcal conjugate vaccines (PCVs) include antigens from 7, 10 or 13 of the >91 known pneumococcal serotypes (PCV7, PCV10 and PCV13), which account for many severe pneumococcal disease (PD) episodes worldwide.3
PCVs are being introduced rapidly into an increasing number of countries, but the optimal dosing schedule(s) is unclear. The first widespread introduction was with PCV7 in the United States using a 4-dose schedule (3+1 administered at 2, 4, 6 and 12–15 months of age); with this schedule, there has been virtual elimination of vaccine-type invasive pneumococcal disease (VT-IPD) in children <5 years of age.4 However, not all countries use this schedule or these ages for vaccine administration. Other PCV schedules used around the world include 2 primary doses plus a booster (2+1) and 3 primary doses without a booster (3+0); for example, the United Kingdom schedule is at 2, 4 and 13 months of age and Australia uses a 2-, 4- and 6-month schedule. Numerous studies have been conducted showing direct and indirect PCV7 efficacy and impact on disease given at various dosing regimens (reviewed in this supplement)5–8; similar studies are now being conducted on the more recently licensed PCV10 and PCV13 products. However, much is still unknown regarding an optimal schedule, which may vary by serotype, epidemiologic setting (ie, child mortality rate, community HIV prevalence or pneumococcal burden) and immunization program characteristics (ie, vaccine coverage, timeliness). Furthermore, the impact of catch-up campaigns as part of PCV introduction on disease control is not characterized or fully understood in its relationship to dosing schedule choices.
PCV regimens in use vary by number of doses, age at dosing, interval between doses, use of a booster dose, PCV product and booster product [PCV vs. 23-valent pneumococcal polysaccharide vaccine (PPV23)]. The optimum PCV schedule for a particular setting may depend not only on immunogenicity but also on the routine immunization program, expected coverage rates and ages at actual vaccination.
The scientific community does not have consensus on which PCV schedules are optimal for a given epidemiologic setting. Furthermore, there is no consensus on what gaps remain in the evidence base that would assist with policy development. Consequently, we conducted a comprehensive, systematic review of available data evaluating the effect of PCV dosing schedules on immunogenicity, nasopharyngeal carriage, IPD, pneumonia and indirect effects. The aim of this work was to provide the evidence base for a strategic analysis of key information gaps required to guide PCV policy development in relation to the WHO’s Expanded Programme for Immunization schedule. Results for the clinical outcomes are presented elsewhere.5–8. In this report, we assessed the effects on immunogenicity of the number of PCV doses, interval between doses, age at dosing, timing of a third dose and impact of a booster dose.
METHODS
Literature Search
This analysis is part of a larger project describing the impact of PCV dosing schedules on IPD, immunogenicity, nasopharyngeal carriage, pneumonia and indirect effects.5–8 Details on the literature search terms and methods used in this systematic review are described in the Methods Appendix.9 In brief, a systematic literature review was performed to collect all available English language data published from January 1994 to September 2010 (supplemented post hoc with studies from 2011) on the effect of various PCV vaccination schedules among immunized children on immunogenicity, NP colonization, IPD, pneumonia and on indirect effects among unvaccinated populations. Articles published in 14 databases, from ad hoc unpublished sources and abstracts from meetings of the International Symposium on Pneumococci and Pneumococcal Disease (1998–2010) and the Interscience Conference on Antimicrobial Agents and Chemotherapeutics (1994–2010), were searched. We included all randomized-controlled clinical trials, nonrandomized trials, surveillance database analyses and observational studies of any PCV schedule on 1 or more outcomes of interest. Studies were included for abstraction if PPV was used as a booster dose, but not as a primary dose. Titles and abstracts were reviewed twice and those with relevant content on 1 of the 5 outcomes (immunogenicity, carriage, invasive disease, pneumonia and indirect effects) underwent full review using a standardized data collection instrument. Details on the search methods are provided in the Methods Appendix.9
Data Abstraction
Citations recovered through the literature search went through several stages of independent review to determine their eligibility, as described earlier. Citations meeting inclusion criteria were categorized on an outcome-specific basis into “study families,” where each family included abstracts or publications generated from a single protocol, population, surveillance system or other data collection system relevant to that outcome. Investigators identified primary data from the individual studies making up each study family for inclusion in the analysis. The primary data were selected as the most current and complete data available for that study family. In some cases, these data were drawn from more than one publication within a family. We also defined “study arms” as a group of children distinguished by immunization schedule or PCV product.
We abstracted core information on the following: number of children in a “study arm”; PCV manufacturer, valency and conjugate protein; coadministered vaccines; country; age at each dose and date of study and publication. Additional data abstracted for the immunogenicity outcome included antibody levels; age at each dose and blood draw and antibody assay methods. Geometric mean antibody concentration (GMC) with confidence interval was abstracted for immunoglobulin G (IgG) antibody determined by enzyme-linked immunoabsorbent assay (ELISA). We also abstracted the percentage of children with serotype-specific IgG concentration >0.35 µg/mL [or >0.2 µg/mL if the GlaxoSmithKline ELISA method was used (GSK, Middlesex, United Kingdom)], defined previously as the correlate of observed efficacy for VT-IPD across the randomized trials.10–13 Results of other assays were abstracted if performed, such as opsonophagocytic assay (OPA) and avidity. If age at vaccination for PCV product or coadministered vaccines were not described, recommended ages for receiving doses from that country’s national immunization plan for the year when the study was conducted were used.
Inclusion and Exclusion Criteria
Study arms meeting the following criteria were included in analyses: subjects immunized with at least 2 doses of PCV, with the first dose ≤4 months and last primary dose ≤6 months of age; licensed PCV with 7 or more antigens, or unlicensed PCV but sufficiently similar to a licensed PCV, and ELISA IgG GMC or percentage >0.35 µg/mL (or >0.2 µg/mL if GSK ELISA method used) provided for any of the 6 serotypes of interest (1, 5, 6B, 14, 19F and 23F). Data following immunization with PPV23 were excluded. Results from populations at high risk for PD were excluded (ie, HIV-infected, sickle cell disease, those with chronic illness and indigenous populations). Antibody responses to serotypes 1 and 5 were excluded from analyses for PCVs not containing these serotypes (eg, PCV7, PCV8). Antibody responses to the Aventis PCV11 were substantially higher than those of other PCV products and studies of this product were limited to a 3-dose primary series schedule; therefore, we excluded studies of Aventis PCV11 because they obscured our ability to assess the effect of dosing schedule on the antibody response.
Pneumococcal Vaccine Dosing Schedules
Any study arm with immunogenicity data after a second primary dose, including study arms eventually receiving a third primary dose, was defined as “2 primary doses”; “3 primary doses” was defined as any study arm with immunogenicity data after a third primary dose, whether a booster dose was given. Schedules “2+0” and “2+1” refer to 2 primary doses without and with 1 PCV booster dose, respectively; “3+0” and “3+1” schedules refer to 3 primary doses without and with 1 PCV booster dose, respectively. A booster dose was defined as immunization with PCV between 9 and 18 months of age in infants who had completed a 2-dose or 3-dose primary series. Mean age at immunization, if available, defined age at each dose; otherwise, scheduled age was used. To collapse into schedules, age was rounded to the nearest 2 weeks. Interval between doses was determined by number of months between first and second primary dose; all but 1 study retained the same interval between first and second as between second and third primary doses.
Data Analysis
Because most studies did not directly compare 1 PCV dosing schedule to another, we followed an ecological regression approach to compare dosing schedules across studies. We fitted random effects meta-regression models of log-transformed GMC levels by serotype, weighting by the inverse of their variances, to account for intrastudy variation for studies assessing more than one study arm. We calculated robust standard errors to account for multiple arms within studies.14,15 We adjusted for study-specific covariates: age at first dose, geographic region, PCV product, type of coadministered diphtheria-tetanus-pertussis (DTP) vaccine [ie, whole-cell (DTwP) vs. acellular (DTaP) pertussis], and ELISA method. For comparisons of immunogenicity at different time points, such as postprimary versus postboost and preboost versus postboost, only studies with data for both time points were included in the analyses. For studies that did not report the variance of the GMC (between 10% and 18% among analyses), we assigned the median of the variances reported by studies with the same region, coadministered DTP vaccine and age at first dose. To investigate the effect of this simple imputation approach, we conducted analyses with and without the studies missing variance; there was no association between absence of variance estimates and GMC.
To calculate 95% confidence intervals for the proportion of children with antibody concentrations above the cut-off, we used the normal approximation interval with proportion (p)=(X+2)/(n+4), a near approximation to the Wilson interval method, which is valid for extreme values (ie, p = 100%).16,17
Statistical significance was defined as P < 0.05; there was no adjustment for multiple testing. SAS version 9.2 (SAS Institute Inc., Cary, NC) was used for all analyses.
RESULTS
Of 12,980 citations reviewed, we identified 113 with immunogenicity data, of which 61 had data relevant for analysis.18–78 More studies evaluated 3-dose primary schedules than 2-dose primary schedules (Table 1); every geographic region evaluated a 3-dose primary schedule, whereas there were no 2-dose primary schedules evaluated in the Latin America/Caribbean region. Only Europe evaluated 1 booster dose following 2 primary doses (2+1), whereas all but Oceania evaluated 3+1 schedules. Most studies (74%) evaluated PCV7 and few (21%) evaluated PCVs with serotypes 1 and 5. The GSK ELISA method was used to evaluate postprimary GMCs in all GSK PCV10 study arms, 4 of 7 GSK PCV11 study arms, 11 of 72 PCV7 study arms and no (0/54) study arms of other products. Very few studies reported avidity or functional OPA responses to evaluate the effect of number of doses on those outcomes. Immunogenicity varied by region, PCV product, coadministration with DTaP (ST14 only) and ELISA method.80 Schedule effects were adjusted for these covariates in all analyses.
TABLE 1.
Characteristics of Studies and Study Arms by Number of Primary Doses and Schedule
2-dose Versus 3-dose Priming Schedules
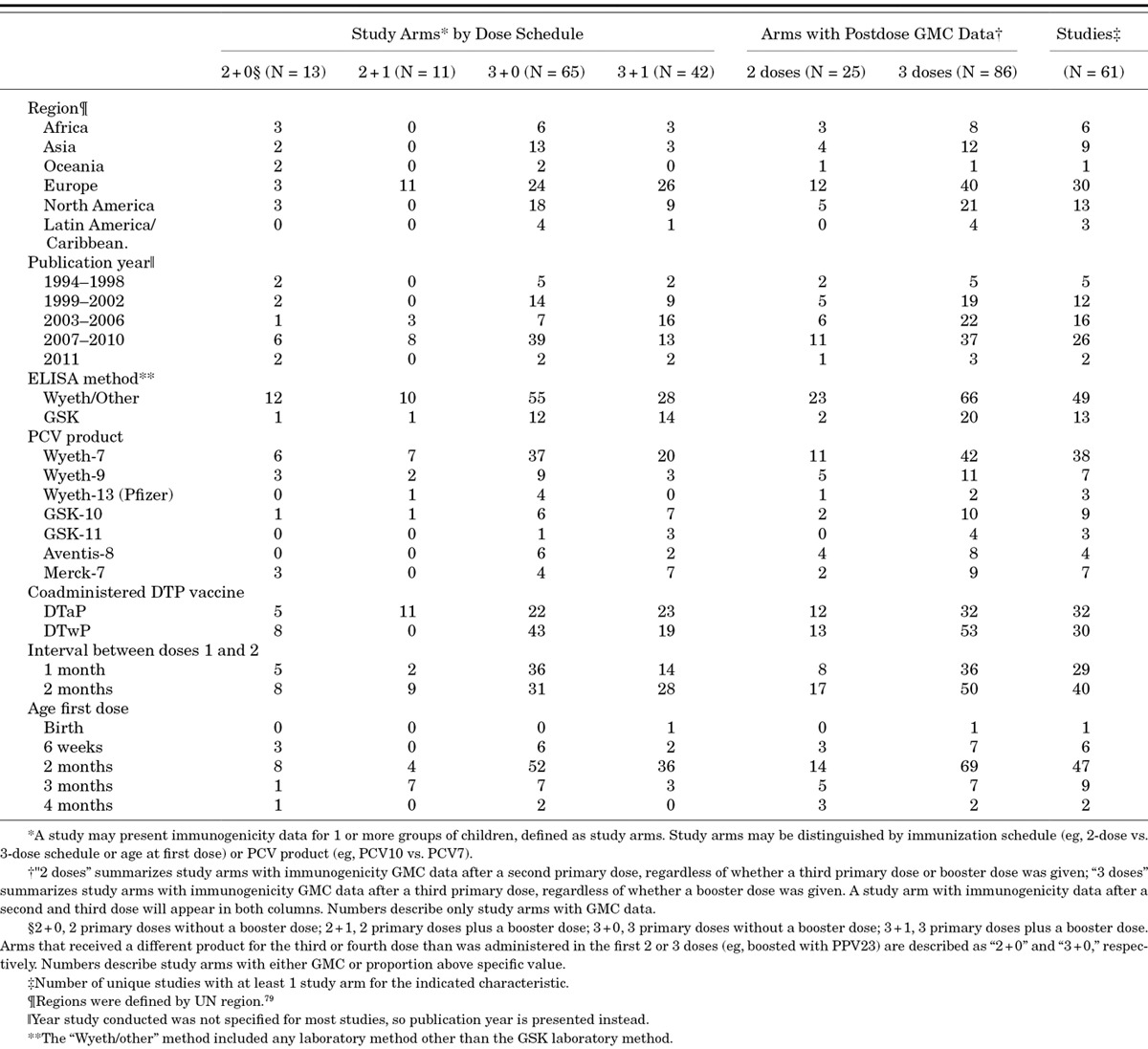
Of 61 studies included in the 2-dose versus 3-dose primary series analysis, 6 directly compared these different schedules and 9 additional studies provided after dose 2 results for 13 study arms eventually receiving a third primary dose. GMC results were available for 25 two-dose and 86 three-dose study arms (Table 1). The 3-dose primary schedule produced significantly higher postprimary GMC antibody response than the 2-dose schedule for all except serotype 1 (Fig. 1A,B). However, the number of primary doses did not meaningfully affect GMCs measured in the second year of life [ie, prebooster (Fig. 1B) or postbooster GMCs (Fig. 1C)], except the postboost response to serotype 6B, which was significantly higher in children who received 3 primary doses.
FIGURE 1.
Effect of primary PCV dosing schedule on GMC by serotype. A) 2-dose versus 3-dose primary schedule on postprimary (~7 months) GMC; B) 2-dose versus 3-dose primary schedule on preboost (~12 months) GMC; C) effect of 2-dose versus 3-dose primary schedule on postboost (~13 months) GMC; D) effect of delaying age at first dose by 1 month on postprimary (~7 months) GMC; E) effect of increasing the interval between doses from 1 to 2 months on postprimary (~7 months) GMC and F) effect of delaying age at last dose by 1 month on postprimary (~7 months) GMC. Adjusted for age at first dose, geographic region, PCV product, coadministration of DTaP versus DTwP and laboratory method (GSK vs. Wyeth/other). N is the number of study arms. Asterick indicates that the significant ST1 finding in Figure 1B is due to 1 study where the two 2-dose arms had lower GMCs than the two 3-dose arms.33 Otherwise, when looking at other studies, there is no difference.
FIGURE 2.
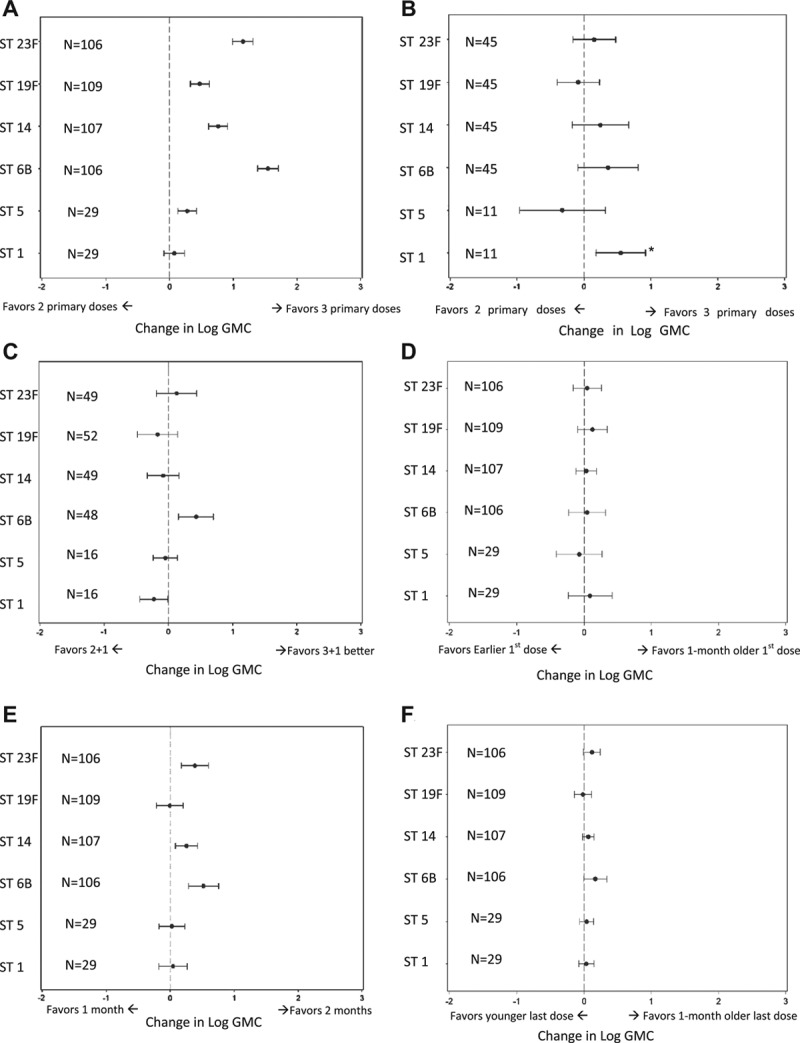
Log GMC by serotype, geographic region and number of primary series PCV doses. PCV product is indicated by manufacturer [W, Wyeth (Pfizer); G, GSK; A, Aventis; M, Merck] and valency (ie, number of serotypes it contains) on the x-axis, and by (o) for licensed or precursor or (+) for unlicensed product.
Timing of Primary Schedule
We evaluated the age at first dose, interval between doses and age at last dose, while adjusting for whether 2 or 3 primary doses were administered; albeit, each is a factor of the other 3 so they cannot be disentangled entirely. Administering the first dose at 1 month of age (eg, at 3 months vs. 2 months of age) did not meaningfully affect the postprimary GMCs (Fig. 1D). However, increasing the interval between doses from 1 to 2 months significantly increased the postprimary GMCs for serotypes 6B, 14 and 23F (Fig. 1E). Increasing the age at last dose had a similar effect to increasing months between doses although the effect size was smaller and did not reach statistical significance (Fig. 1F). Among the studies in the meta-analysis, only one directly compared interval of dosing while holding age at first dose constant, and it did so in a randomized trial setting.33 That study’s findings support the meta-analysis results; it showed that children randomly assigned to a 2-month interval between 2 primary doses had significantly higher postprimary GMCs for serotypes 6B, 14 and 23F and preboost GMCs at 1 year of age for serotypes 4, 6B and 23F compared with children who received their 2 primary doses with 1-month interval.
Booster Dose Effect
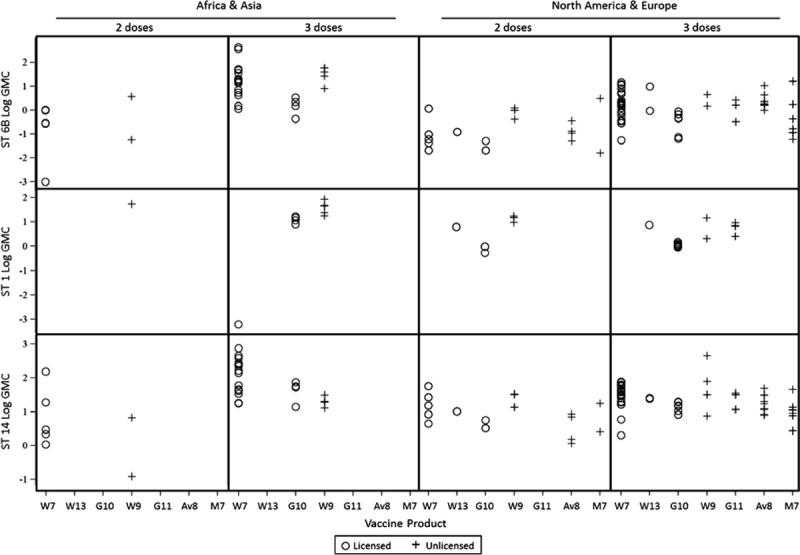
Of 53 study arms evaluating a PCV booster dose (generally in the second year of life), 11 (21%) had received a 2-dose primary schedule and 42 (79%) a 3-dose primary schedule. The booster dose increased GMCs from pre- to postbooster for all serotypes regardless of the priming schedule (Fig. 3A). Postbooster GMCs (mean age 13.5 months) were also significantly higher than postprimary GMC (mean age 7.2 months) for all serotypes regardless of the priming schedule (Fig. 3B).
FIGURE 3.
Effect of PCV booster dose in second year of life on GMC by serotype. A) Change in GMC pre- to postbooster; B) Change in GMC from postprimary (~7 months) to postbooster (~13 months).
3+0 Versus 2+1 Schedules
There were 58 studies with immunogenicity data on 2+1 or 3+0 schedules; GMC results were available for 86 “3+0” and 11 “2+1” study arms. The unadjusted median postbooster GMC response (in µg/mL) of 2+1 schedules (median age at blood draw 12.5 months) compared with the postprimary response of 3+0 schedules (median age at blood draw 7.0 months) was 5.1 versus 2.5, 3.4 versus 3.0, 7.4 versus 1.4, 12.0 versus 4.7, 7.7 versus 3.6, 4.5 versus 1.7 for serotypes 1, 5, 6B, 14, 19F and 23F, respectively. After adjusting for geographic region, age at first dose, coadministration of DTaP versus DTwP, PCV product and ELISA laboratory method, the mean 2+1 postbooster antibody response (median age at blood draw 12.5 months) was significantly higher than the 3+0 postprimary response (median age at blood draw 7.0 months) for all serotypes (Figs. 4 and 5), although the covariates are tightly correlated and so confounding might not be entirely controlled for.
FIGURE 4.
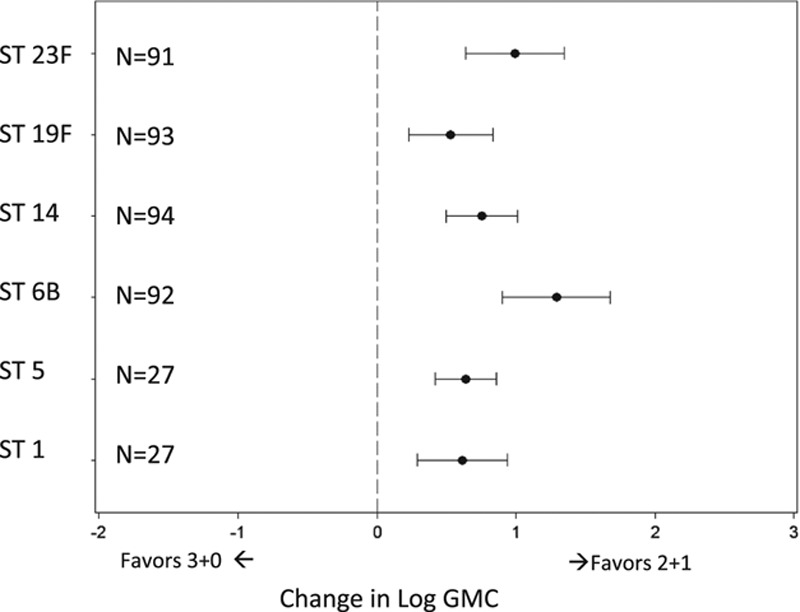
Difference in post-third dose GMC when changing from 3+0 (GMC at 6 months) to 2+1 (GMC at 15 months) PCV schedule.
FIGURE 5.
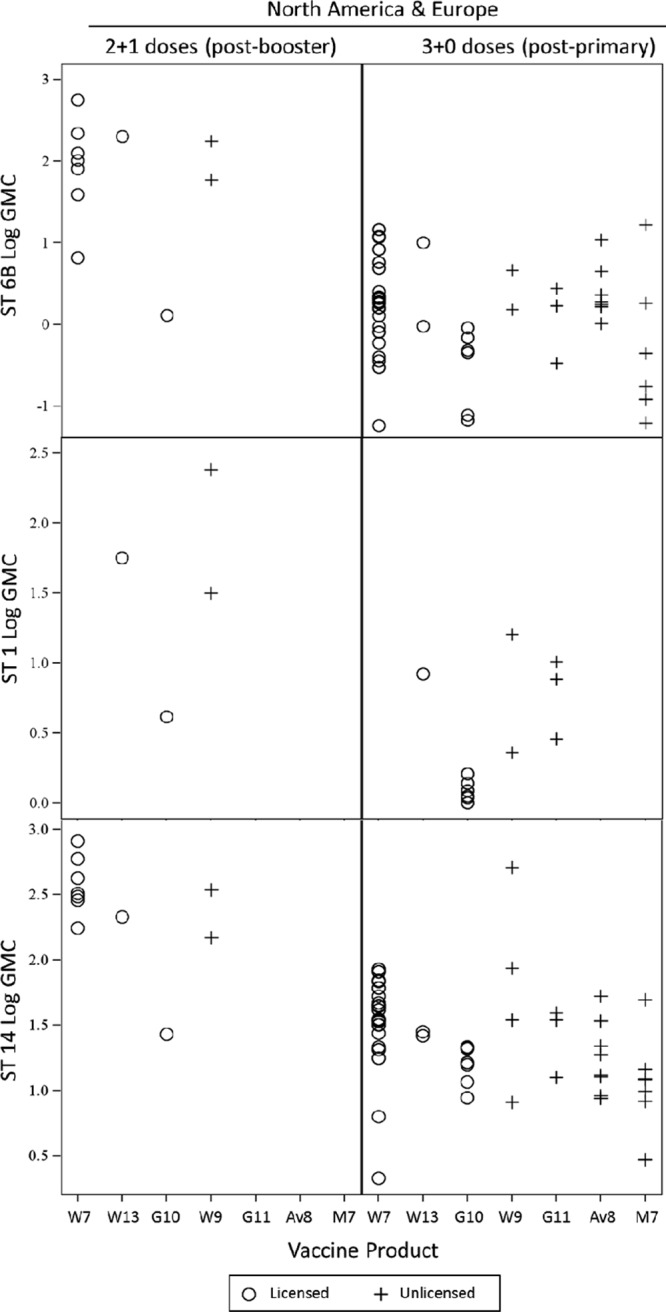
After postdose-3 log GMC, by serotype and PCV schedule: 2+1 (postbooster) versus 3+0 (postprimary). PCV product is indicated by manufacturer and valency (ie, number of serotypes it contains). W, Wyeth (Pfizer); G, GSK; A, Aventis; M, Merck.
Proportion Achieving IPD Correlate of Protection Cut-off
The percentage of children with IgG antibody concentrations >0.35 µg/mL (or >0.20 µg/mL for GSK ELISA) was high and comparable for both 2-dose and 3-dose primary schedules for all serotypes except for 6B and 23F (Fig. 6). For these serotypes, the proportion of children above the threshold was higher for those receiving a 3-dose than a 2-dose primary schedule; 17/24 (70.8%) 3-dose study arms reported over 80% of subjects above the cut-off for serotype 6B compared with 3/13 (23.1%) 2-dose study arms. For serotype 23F, results were 21/24 (87.5%) compared with 4/11 (36.4%), respectively.
FIGURE 6.
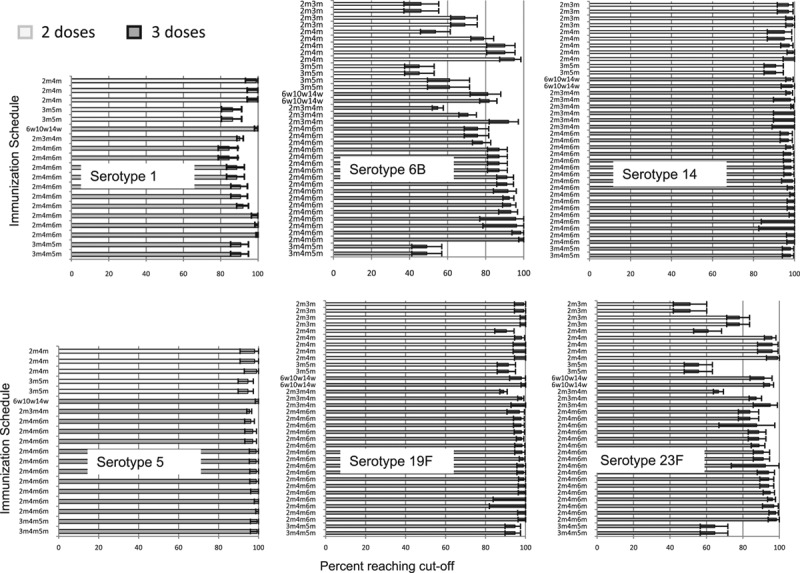
Proportion of children achieving >0.35 μg/mL (or >0.2 μg/mL if GSK ELISA used), by serotype, number of doses in primary series and immunization schedule. Each bar represents results from one study arm. Schedule is noted on the y-axis in months (m) or weeks (w). Error bars are 95% confidence intervals.
Only one 2+1 study presented percentage response data following the booster dose, which showed >96% response (IgG antibody concentrations ≥0.2 µg/mL) using the GSK ELISA for all serotypes except for 6B which had 88% response; response was also high (>87% for all serotypes) using the 0.35 µg/mL cut point.72
DISCUSSION
In general, a 3-dose primary schedule induced higher antibody than a 2-dose schedule for most serotypes assessed and a booster dose induced higher antibodies over those following the primary dose schedule for all serotypes. The degree to which higher antibody concentrations are important for protecting against serious disease is not established; there may be a threshold above which higher circulating antibody concentrations are not meaningfully more protective for an individual. While the aggregate, population-based correlate of protection used to license new PCV vaccines is 0.35 µg/mL, higher IgG levels may be important in protecting against NP colonization, conferring herd immunity, prolonging individual protection and, up to a point, may correlate at the individual level with disease protection. It is likely that the true threshold will vary by both serotype and disease syndrome, with higher concentrations probably required for mucosal infection like nonbacteremic pneumonia compared with systemic infection like invasive pneumococcal sepsis.
While both 2+1 and 3+0 schedules show evidence of robust immunogenicity for the serotypes evaluated (1, 5, 6B, 14, 19F and 23F), determining which schedule produced a superior response for serotypes 1 and 5 was not possible because data were limited, especially for 2+1 regimens (N = 4 study arms). In addition, the analyses for serotypes 1 and 5 included mostly studies of unlicensed formulations. Restricting analyses for serotypes 1 and 5 to only licensed products (ie, GSK PCV10 and Wyeth PCV13) provided very few studies for analysis: there was only one 2+1 study for each GSK PCV10 and Wyeth PCV13, and 6 and four 3+0 studies, respectively).
Considering options for a 3-dose schedule, which is recommended by the WHO and for which GAVI Alliance funding is available for resource-limited countries, administering the third dose as a booster in the second year of life (2+1) induced higher antibodies than using the third dose to complete a primary series (3+0). However, for many epidemiologic settings, the first year of life is a time of high disease burden, so the advantage of delaying the third dose until the age of 1 year to achieve higher responses may be offset by the risk of leaving infants relatively at risk with lower antibodies after 2 primary doses.
While the use of meta-analysis allowed us to incorporate data from a large number of studies, controlling for study differences in covariates and confounding factors is a challenge in meta-analyses. Head-to-head studies that directly compare different schedules are more robust since they inherently control for potential confounders by keeping all covariates the same except the schedule, ideally through randomization. There were only 6 head-to-head randomized controlled trials that evaluated 2-dose versus 3-dose primary schedules. These were systematically reviewed in detail by investigators at the University of Berne who found that 3 primary doses provided higher antibody concentrations compared with 2 primary doses.81 While there was representation across regions and a variety of schedules evaluated, their analysis was limited to just these 6 trials and all but 1 evaluated 3-dose schedules with a 1-month interval between doses compared with 2-dose schedules with a 2-month interval between doses (ie, age at dosing was 2 and 4 vs. 2, 3, and 4 months); dosing interval, therefore, confounds the relationship between antibody response and number of doses administered in that analysis. Our analyses included an additional 13 study arms where the antibody response after dose 2 was compared with that after dose 3 in the same individual. We also included a wealth of immunogenicity data available from studies evaluating only one schedule. Our more inclusive analyses reinforce the findings from the University of Berne analysis of head-to-head trials that not only 3 primary doses provide improved immunogenicity compared with 2 primary doses for most serotypes, but also enabled assessment of schedule effects in a broader variety of settings and combinations and enabled assessment of the timing of doses with respect to age at administration and months between doses.
Because most of the analysis was based on between-study comparisons rather than direct comparisons within a study, controlling for potential confounders of immunogenicity is essential when drawing inferences about dosing schedule effects. For some analyses, covariates such as coadministration of DTaP versus DTwP or the interval between doses (eg, 6, 10 and 14 weeks vs. 2, 4 and 6 months) could not be completely adjusted for because these factors are region specific and therefore linked. We could only compare 2+1 and 3+0 schedules within Europe because there were no eligible 2+1 schedule studies from other regions. Disentangling the effect of number of doses from the effect of age at each immunization or interval between primary doses is nearly impossible because as one factor changes necessarily at least one more also has to change.
Serum IgG antibody concentration ≥0.35 μg/mL (or ≥0.20 μg/mL for studies using the GSK ELISA) after the primary series is considered a correlate for licensure of PCV products as they predict aggregate vaccine efficacy against vaccine serotype IPD among the immunized population.13 We observed the same trend in the percentage meeting this cutoff for the 2-dose and 3-dose priming schedules as with GMCs but with less differentiation between these 2 schedules. It is our assessment that GMC is a more finely differentiating metric of the various dosing schedules than the proportion above the licensure threshold; however, it is not known which measure is more meaningful and predictive of clinical impact. We emphasize GMC values for several reasons. First, the population level protection effects are dependent on NP colonization. Preventing pneumococcal colonization is critical for population benefits of a pneumococcal vaccination program, since protection against colonization will necessarily mean protection against disease as well as reduced transmission in the community. Studies evaluating the correlation of antibody and protection against NP colonization have shown that antibody concentrations in the range of 4–5 µg/mL correlate with protection, although this is likely a marker of immune response and not the direct effector of protection.82–84 Second, the prevention of pneumococcal pneumonia is the primary syndrome of concern for PCV programs, since most pneumococcal deaths that occur in young children globally are due to pneumonia, and the threshold for prevention of pneumococcal pneumonia is estimated to be significantly > 0.35 μg/mL (K.L.O.B. and G.D., unpublished data). Third, given the mucosal nature of pneumococcal pneumonia (when compared with IPD syndromes), it is not entirely clear whether circulating antibody or mucosal cells are the more important effector molecule for its prevention, although passive antibody studies (eg, studies evaluating administration of bacterial polysaccharide immunoglobulin) would argue for a role of circulating pneumococcal serum antibody.85 Functional OPA may be a better predictor of protection than serum IgG; however, few studies assessed functional responses, so we were restricted to evaluating dosing schedule impact on serum IgG.
This, the largest such analysis of existing PCV immunogenicity data conducted to date, contributed to the WHO Strategic Advisory Group of Experts statement regarding optimizing PCV dosing schedules86 and will help to guide PCV policy development in relation to the WHO’s Expanded Programme for Immunization schedule. We have shown improved immunogenicity for 3 doses compared with 2 doses for most serotypes when serum IgG GMC is used as the metric for comparison. As expected, the 2+1 schedule leads to significantly higher antibody concentrations following the third dose, because it is administered remote from the primary series and acts as a booster dose when compared with the 3+0 schedule where the third dose is administered in the primary series. The tradeoff, therefore, is fundamentally between higher antibody concentrations in the second year of life following the booster with a 2+1 schedule and higher antibody concentrations in the first year of life from a 3-dose instead of a 2-dose primary schedule. While the relative merits of these 2 approaches will likely vary by serotype, disease syndrome and vaccine program, in practice, the herd effects induced by a successful vaccination program may minimize the impact on disease burden caused by schedule-related differences in immune response.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the tremendous support for abstracting data from the following: Becky Roberts, Karrie-Ann Toews and Carolyn Wright from the Centers for Disease Control and Prevention, Respiratory Diseases Branch; Catherine Bozio, Rose Chang, Jamie Felzer, Amy Fothergill, Sara Gelb, Kristen Hake, Sydney Hubbard, Grace Hunte and Shuling Liu from Emory University Rollins School of Public Health; and Bethany Baer, Stephanie Davis, Sylvia Kauffman, Min Joo Kwak, Paulami Naik and Meena Ramakrishnan from The Johns Hopkins Bloomberg School of Public Health.
Footnotes
Accepted for publication August 13, 2013.
Support for this project was provided by Program for Appropriate Technology in Health (PATH) through funding from the Global Alliance for Vaccines and Immunisation (GAVI). The views expressed by the authors do not necessarily reflect the views of GAVI and/or PATH. M.D.K. has received support from Novartis for participation on a Data and Safety Monitoring Board, meeting travel reimbursement from Pfizer and grant support from Merck. D.G.’s laboratory performs contract and or collaborative research for/with Pfizer, GlaxoSmithKline, Merck, Novartis and Sanofi Pasteur. D.G. has received travel or honorarium support for participation in external expert committees for Merck, Sanofi Pasteur, Pfizer and GlaxoSmithKline. K.O.B. received grant support from Pfizer, GlaxoSmithKline and has received travel or honorarium support for participation in external expert committees for Merck, Aventis-pasteur and GlaxoSmithKline. The authors have no other funding or conflicts of interest to declare.
Address for correspondence: Katherine L. O'Brien, MD, MPH, Johns Hopkins Bloomberg School of Public Health, Center for American Indian Health, 621 N. Washington St., Baltimore, MD 21205. E-mail: klobrien@jhsph.edu.
REFERENCES
- 1.Liu L, Johnson HL, Cousens S, et al. Child Health Epidemiology Reference Group of WHO and UNICEF. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization and UNICEF. Global immunization data. October 2012 Available at: http://www.who.int/immunization_monitoring/Global_Immunization_Data.pdf. p. 2. Accessed February 4, 2013. [Google Scholar]
- 3.Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pilishvili T, Lexau C, Farley MM, et al. Active Bacterial Core Surveillance/Emerging Infections Program Network. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J Infect Dis. 2010;201:32–41. doi: 10.1086/648593. [DOI] [PubMed] [Google Scholar]
- 5.Conklin L, Loo JD, Kirk J, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type invasive pneumococcal disease among young children. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S109–S118. doi: 10.1097/INF.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleming-Dutra KE, Conklin L, Loo JD, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S152–S160. doi: 10.1097/INF.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Loo JD, Conklin L, Fleming-Dutra KE, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on prevention of pneumonia. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S140–S151. doi: 10.1097/INF.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis S, Deloria Knoll M, O’Brien KL; for the Pneumococcal Carriage Group (PneumoCarr), and the PCV Dosing Landscape Project. Impact of pneumococcal conjugate vaccines on nasopharyngeal and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine. doi: 10.1016/j.vaccine.2013.05.005. [published online ahead of print May 16, 2013]. doi: 10.1016/j.vaccine.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 9.Loo JD, Conklin L, Deloria Knoll M, et al. Methods for a systematic review of pneumococcal conjugate vaccine dosing schedules. Pediatr Infect Dis J. 2014;33(Suppl 2):S182–S187. doi: 10.1097/INF.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Recommendations to assure the quality, safety and efficacy of pneumococcal conjugate vaccines: proposed replacement of TRS 927, Annex 2, ECBS. October 2009. Available at: http://www.who.int/biologicals/publications/trs/areas/vaccines/pneumo/en/index.html. Accessed February 4, 2013. [Google Scholar]
- 11.World Health Organization. Recommendations for production and control of pneumococcal conjugate vaccines. WHO Technical Report Series 927, Annex 2. 2005 Available at: http://www.who.int/biologicals/publications/trs/areas/vaccines/pneumo/ANNEX%202%20PneumococcalP64-98.pdf. Accessed February 4, 2013. [Google Scholar]
- 12.Henckaerts I, Goldblatt D, Ashton L, et al. Critical differences between pneumococcal polysaccharide enzyme-linked immunosorbent assays with and without 22F inhibition at low antibody concentrations in pediatric sera. Clin Vaccine Immunol. 2006;13:356–360. doi: 10.1128/CVI.13.3.356-360.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jódar L, Butler J, Carlone G, et al. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine. 2003;21:3265–3272. doi: 10.1016/s0264-410x(03)00230-5. [DOI] [PubMed] [Google Scholar]
- 14.Jackson D, White IR, Thompson SG. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med. 2010;29:1282–1297. doi: 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med. 1998;17:857–872. doi: 10.1002/(sici)1097-0258(19980430)17:8<857::aid-sim777>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Wilson EB. Probable inference, the law of succession, and statistical inference. JASA. 1927;22:209–212. [Google Scholar]
- 18.Anderson EL, Kennedy DJ, Geldmacher KM, et al. Immunogenicity of heptavalent pneumococcal conjugate vaccine in infants. J Pediatr. 1996;128(5 pt 1):649–653. doi: 10.1016/s0022-3476(96)80130-2. [DOI] [PubMed] [Google Scholar]
- 19.Anttila M, Eskola J, Ahman H, et al. Differences in the avidity of antibodies evoked by four different pneumococcal conjugate vaccines in early childhood. Vaccine. 1999;17:1970–1977. doi: 10.1016/s0264-410x(98)00458-7. [DOI] [PubMed] [Google Scholar]
- 20.Bermal N, Szenborn L, Chrobot A, et al. The 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) coadministered with DTPw-HBV/Hib and poliovirus vaccines: assessment of immunogenicity. Pediatr Infect Dis J. 2009;28(4 suppl):S89–S96. doi: 10.1097/INF.0b013e318199f901. [DOI] [PubMed] [Google Scholar]
- 21.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Block SL, Hendrick JA, Smith RA, et al. American Society for Microbiology. Abstracts of the 37th Interscience Conference on Antimicrobial Agents & Chemotherapy (ICAAC) Canada: Toronto: Ontario; 1997. Pneumococcal conjugate vaccine (PCV) vs. pneumococcal polysaccharide 23 (PN23) in 6 month old infants after 2 prior doses of PCV. p. 208. p. [Google Scholar]
- 23.Bryant KA, Block SL, Baker SA, et al. PCV13 Infant Study Group. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010;125:866–875. doi: 10.1542/peds.2009-1405. [DOI] [PubMed] [Google Scholar]
- 24.Buttery JP, Riddell A, McVernon J, et al. Immunogenicity and safety of a combination pneumococcal-meningococcal vaccine in infants: a randomized controlled trial. JAMA. 2005;293:1751–1758. doi: 10.1001/jama.293.14.1751. [DOI] [PubMed] [Google Scholar]
- 25.Choo S, Seymour L, Morris R, et al. Immunogenicity and reactogenicity of a pneumococcal conjugate vaccine administered combined with a haemophilus influenzae type B conjugate vaccine in United Kingdom infants. Pediatr Infect Dis J. 2000;19:854–862. doi: 10.1097/00006454-200009000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Dicko A, Odusanya OO, Diallo AI, et al. Primary vaccination with the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in infants in Mali and Nigeria: a randomized controlled trial. BMC Public Health. 2011;11:882. doi: 10.1186/1471-2458-11-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durando P, Crovari P, Ansaldi F, et al. Collaborative Group for Pneumococcal Vaccination in Liguria. Universal childhood immunisation against Streptococcus pneumoniae: the five-year experience of Liguria Region, Italy. Vaccine. 2009;27:3459–3462. doi: 10.1016/j.vaccine.2009.01.052. [DOI] [PubMed] [Google Scholar]
- 28.Eick A, Croll J, Weatherholtz R, et al. Safety and immunogenicity of two octavalent pneumococcal conjugate vaccines in American Indian infants. Vaccine. 2004;22:1260–1264. doi: 10.1016/j.vaccine.2003.09.026. [DOI] [PubMed] [Google Scholar]
- 29.Ekström N, Ahman H, Verho J, et al. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect Immun. 2005;73:369–377. doi: 10.1128/IAI.73.1.369-377.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esposito S, Pugni L, Bosis S, et al. Immunogenicity, safety and tolerability of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months post-natally to pre- and full-term infants. Vaccine. 2005;23:1703–1708. doi: 10.1016/j.vaccine.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 31.Esposito S, Tansey S, Thompson A, et al. Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine compared to those of a 7-valent pneumococcal conjugate vaccine given as a three-dose series with routine vaccines in healthy infants and toddlers. Clin Vaccine Immunol. 2010;17:1017–1026. doi: 10.1128/CVI.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Givon-Lavi N, Greenberg D, Dagan R. Immunogenicity of alternative regimens of the conjugated 7-valent pneumococcal vaccine: a randomized controlled trial. Pediatr Infect Dis J. 2010;29:756–762. doi: 10.1097/INF.0b013e3181d99345. [DOI] [PubMed] [Google Scholar]
- 33.Goldblatt D, Southern J, Ashton L, et al. Immunogenicity of a reduced schedule of pneumococcal conjugate vaccine in healthy infants and correlates of protection for serotype 6B in the United Kingdom. Pediatr Infect Dis J. 2010;29:401–405. doi: 10.1097/INF.0b013e3181c67f04. [DOI] [PubMed] [Google Scholar]
- 34.Goldblatt D, Southern J, Ashton L, et al. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 2006;25:312–319. doi: 10.1097/01.inf.0000207483.60267.e7. [DOI] [PubMed] [Google Scholar]
- 35.Huebner RE, Mbelle N, Forrest B, et al. Immunogenicity after one, two or three doses and impact on the antibody response to coadministered antigens of a nonavalent pneumococcal conjugate vaccine in infants of Soweto, South Africa. Pediatr Infect Dis J. 2002;21:1004–1007. doi: 10.1097/00006454-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Jonsdottir I, Sigurdardottir ST, Gudnason T, et al. Sun City, South Africa: March 19–23, 2000. Concomitant administration of octavalent pneumococcal polysaccharide conjugate vaccine, PncD, and Haemophilus influenzae conjugate vaccine, PRP-D, sharing the carrier DT, may induce interference in infants. Presented at the second International Symposium on Pneumococci and Pneumococcal Diseases; Abstract P39. [Google Scholar]
- 37.Käyhty H, Ahman H, Eriksson K, et al. Immunogenicity and tolerability of a heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 12 months of age. Pediatr Infect Dis J. 2005;24:108–114. doi: 10.1097/01.inf.0000151022.92222.be. [DOI] [PubMed] [Google Scholar]
- 38.Kieninger DM, Kueper K, Steul K, et al. 006 study group. Safety, tolerability, and immunologic noninferiority of a 13-valent pneumococcal conjugate vaccine compared to a 7-valent pneumococcal conjugate vaccine given with routine pediatric vaccinations in Germany. Vaccine. 2010;28:4192–4203. doi: 10.1016/j.vaccine.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Kim NH, Lee J, Lee SJ, et al. Immunogenicity and safety of pneumococcal 7-valent conjugate vaccine (diphtheria CRM(197) protein conjugate; Prevenar) in Korean infants: differences that are found in Asian children. Vaccine. 2007;25:7858–7865. doi: 10.1016/j.vaccine.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 40.Kim CH, Kim JS, Cha SH, et al. Response to primary and booster vaccination with 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine in Korean infants. Pediatr Infect Dis J. 2011;30:e235–e243. doi: 10.1097/INF.0b013e31822a8541. [DOI] [PubMed] [Google Scholar]
- 41.Knuf M, Habermehl P, Cimino C, et al. Immunogenicity, reactogenicity and safety of a 7-valent pneumococcal conjugate vaccine (PCV7) concurrently administered with a DTPa-HBV-IPV/Hib combination vaccine in healthy infants. Vaccine. 2006;24:4727–4736. doi: 10.1016/j.vaccine.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 42.Lagos R, Munoz A, Levine MM, et al. Immunology of combining CRM(197) conjugates for Streptococcus pneumoniae, Neisseria meningitis and Haemophilus influenzae in Chilean infants. Vaccine. 2009;27:2299–2305. doi: 10.1016/j.vaccine.2009.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Lagos RE, Muñoz AE, Levine MM, et al. Safety and immunogenicity of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Chilean children. Hum Vaccine. 2011;7:511–522. doi: 10.4161/hv.7.5.14634. [DOI] [PubMed] [Google Scholar]
- 44.Lee H, Nahm MH, Burton R, et al. Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol. 2009;16:376–381. doi: 10.1128/CVI.00344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li RC, Li FX, Li YP, et al. Safety and immunogenicity of a 7-valent pneumococcal conjugate vaccine (Prevenar): primary dosing series in healthy Chinese infants. Vaccine. 2008;26:2260–2269. doi: 10.1016/j.vaccine.2008.02.029. [DOI] [PubMed] [Google Scholar]
- 46.Lin TY, Lu CY, Chang LY, et al. Immunogenicity and safety of 10-valent pneumococcal non-typeable Haemophilus influenzae protein D-conjugate vaccine (PHiD-CV) co-administered with routine childhood vaccines in Taiwan. J Formos Med Assoc. 2012;111:495–503. doi: 10.1016/j.jfma.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 47.McNeely TB, Arena DE, Kniskern PJ, et al. Opsonic antibody responses to a three dose Pn-OMPC conjugate vaccination with a fourth dose boost of Pn-OMPC conjugate or PNEUMOVAXa 23. Abstr Intersci Conf Antimicrob Agents Chemother. 1997:209. [Google Scholar]
- 48.Miernyk KM, Parkinson AJ, Rudolph KM, et al. Immunogenicity of a heptavalent pneumococcal conjugate vaccine in Apache and Navajo Indian, Alaska native, and non-native American children aged <2 years. Clin Infect Dis. 2000;31:34–41. doi: 10.1086/313907. [DOI] [PubMed] [Google Scholar]
- 49.Moss SJ, Fenton AC, Toomey J, et al. Immunogenicity of a heptavalent conjugate pneumococcal vaccine administered concurrently with a combination diphtheria, tetanus, five-component acellular pertussis, inactivated polio, and Haemophilus influenzae type B vaccine and a meningococcal group C conjugate vaccine at 2, 3, and 4 months of age. Clin Vaccine Immunol. 2010;17:311–316. doi: 10.1128/CVI.00315-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nurkka A, Ahman H, Korkeila M, et al. Serum and salivary anti-capsular antibodies in infants and children immunized with the heptavalent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2001;20:25–33. doi: 10.1097/00006454-200101000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Nurkka A, Ahman H, Yaich M, et al. Serum and salivary anti-capsular antibodies in infants and children vaccinated with octavalent pneumococcal conjugate vaccines, PncD and PncT. Vaccine. 2001;20:194–201. doi: 10.1016/s0264-410x(01)00250-x. [DOI] [PubMed] [Google Scholar]
- 52.Nurkka A, Joensuu J, Henckaerts I, et al. Immunogenicity and safety of the eleven valent pneumococcal polysaccharide-protein D conjugate vaccine in infants. Pediatr Infect Dis J. 2004;23:1008–1014. doi: 10.1097/01.inf.0000143640.03214.18. [DOI] [PubMed] [Google Scholar]
- 53.O’Brien KL, Swift AJ, Winkelstein JA, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM(197) among infants with sickle cell disease. Pneumococcal Conjugate Vaccine Study Group. Pediatrics. 2000;106:965–972. doi: 10.1542/peds.106.5.965. [DOI] [PubMed] [Google Scholar]
- 54.Obaro SK, Adegbola RA, Chang I, et al. Safety and immunogenicity of a nonavalent pneumococcal vaccine conjugated to CRM197 administered simultaneously but in a separate syringe with diphtheria, tetanus and pertussis vaccines in Gambian infants. Pediatr Infect Dis J. 2000;19:463–469. doi: 10.1097/00006454-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 55.Obaro SK, Enwere GC, Deloria M, et al. Safety and immunogenicity of pneumococcal conjugate vaccine in combination with diphtheria, tetanus toxoid, pertussis and Haemophilus influenzae type b conjugate vaccine. Pediatr Infect Dis J. 2002;21:940–947. doi: 10.1097/00006454-200210000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Olivier C, Belohradsky BH, Stojanov S, et al. Immunogenicity, reactogenicity, and safety of a seven-valent pneumococcal conjugate vaccine (PCV7) concurrently administered with a fully liquid DTPa-IPV-HBV-Hib combination vaccine in healthy infants. Vaccine. 2008;26:3142–3152. doi: 10.1016/j.vaccine.2007.11.096. [DOI] [PubMed] [Google Scholar]
- 57.Osendarp SJ, Prabhakar H, Fuchs GJ, et al. Immunization with the heptavalent pneumococcal conjugate vaccine in Bangladeshi infants and effects of zinc supplementation. Vaccine. 2007;25:3347–3354. doi: 10.1016/j.vaccine.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 58.Ota MO, Akinsola A, Townend J, et al. The immunogenicity and impact on nasopharyngeal carriage of fewer doses of conjugate pneumococcal vaccine immunization schedule. Vaccine. 2011;29:2999–3007. doi: 10.1016/j.vaccine.2011.01.098. [DOI] [PubMed] [Google Scholar]
- 59.Pichichero ME, Bernstein H, Blatter MM, et al. 085 Study Investigators. Immunogenicity and safety of a combination diphtheria, tetanus toxoid, acellular pertussis, hepatitis B, and inactivated poliovirus vaccine coadministered with a 7-valent pneumococcal conjugate vaccine and a Haemophilus influenzae type b conjugate vaccine. J Pediatr. 2007;151:43–49, 49.e1. doi: 10.1016/j.jpeds.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006;367:740–748. doi: 10.1016/S0140-6736(06)68304-9. [DOI] [PubMed] [Google Scholar]
- 61.Reinert P, Guy M, Girier B, et al. [The safety and immunogenicity of an heptavalent pneumococcal polysaccharide conjugate vaccine (Prevenar) administered in association with a whole-cell pertussis-based pediatric combination vaccine (DTP-IPV/PRP-T) to French infants with a two-, three-, and four-month schedule]. Arch Pediatr. 2003;10:1048–1055. doi: 10.1016/j.arcped.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 62.Rennels MB, Edwards KM, Keyserling HL, et al. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics. 1998;101(4 pt 1):604–611. doi: 10.1542/peds.101.4.604. [DOI] [PubMed] [Google Scholar]
- 63.Rodenburg GD, van Gils EJ, Veenhoven RH, et al. Comparability of antibody response to a booster dose of 7-valent pneumococcal conjugate vaccine in infants primed with either 2 or 3 doses. Vaccine. 2010;28:1391–1396. doi: 10.1016/j.vaccine.2009.10.151. [DOI] [PubMed] [Google Scholar]
- 64.Ruggeberg JU, Collins C, Clarke P, et al. Immunogenicity and induction of immunological memory of the heptavalent pneumococcal conjugate vaccine in preterm UK infants. Vaccine. 2007;25:264–271. doi: 10.1016/j.vaccine.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 65.Ruiz-Palacios GM, Guerrero ML, Hernández-Delgado L, et al. Immunogenicity, reactogenicity and safety of the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) in Mexican infants. Hum Vaccine. 2011;7:1137–1145. doi: 10.4161/hv.7.11.17984. [DOI] [PubMed] [Google Scholar]
- 66.Russell FM, Balloch A, Tang ML, et al. Immunogenicity following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2009;27:5685–5691. doi: 10.1016/j.vaccine.2009.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmitt HJ, Faber J, Lorenz I, et al. The safety, reactogenicity and immunogenicity of a 7-valent pneumococcal conjugate vaccine (7VPnC) concurrently administered with a combination DTaP-IPV-Hib vaccine. Vaccine. 2003;21:3653–3662. doi: 10.1016/s0264-410x(03)00389-x. [DOI] [PubMed] [Google Scholar]
- 68.Scott JA, Ojal J, Ashton L, et al. Pneumococcal conjugate vaccine given shortly after birth stimulates effective antibody concentrations and primes immunological memory for sustained infant protection. Clin Infect Dis. 2011;53:663–670. doi: 10.1093/cid/cir444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shao PL, Lu CY, Chang LY, et al. Safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in Taiwanese infants. J Formos Med Assoc. 2004;103:613–617. [PubMed] [Google Scholar]
- 70.Shapiro ED, Kaplan SL, Steinhoff MC, et al. Immunogenicity of two pneumococcal conjugate vaccines. Pediatr Res. 1997;41:130. [Google Scholar]
- 71.Shinefield HR, Black S, Ray P, et al. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 1999;18:757–763. doi: 10.1097/00006454-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 72.Sigurdardottir ST, Davidsdottir K, Arason VA, et al. Safety and immunogenicity of CRM197-conjugated pneumococcal-meningococcal C combination vaccine (9vPnC-MnCC) whether given in two or three primary doses. Vaccine. 2008;26:4178–4186. doi: 10.1016/j.vaccine.2008.05.072. [DOI] [PubMed] [Google Scholar]
- 73.Silfverdal SA, Hogh B, Bergsaker MR, et al. Immunogenicity of a 2-dose priming and booster vaccination with the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine. Pediatr Infect Dis J. 2009;28:e276–e282. doi: 10.1097/INF.0b013e3181b48ca3. [DOI] [PubMed] [Google Scholar]
- 74.Soininen A, Lehtonen H, Lahdenkari M, et al. Alice Springs, Australia: April 2–6, 2006. Functional activity of antibodies against serotype 19F evoked by pneumococcal conjugate vaccines. 5th International Symposium on Pneumococci and Pneumococcal Diseases; Abstract 249. [Google Scholar]
- 75.Tichmann-Schumann I, Soemantri P, Behre U, et al. Immunogenicity and reactogenicity of four doses of diphtheria-tetanus-three-component acellular pertussis-hepatitis B-inactivated polio virus-Haemophilus influenzae type b vaccine coadministered with 7-valent pneumococcal conjugate Vaccine. Pediatr Infect Dis J. 2005;24:70–77. doi: 10.1097/01.inf.0000148923.46453.48. [DOI] [PubMed] [Google Scholar]
- 76.Vesikari T, Wysocki J, Chevallier B, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J. 2009;28(4 suppl):S66–S76. doi: 10.1097/INF.0b013e318199f8ef. [DOI] [PubMed] [Google Scholar]
- 77.Wysocki J, Tejedor JC, Grunert D, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) when coadministered with different neisseria meningitidis serogroup C conjugate vaccines. Pediatr Infect Dis J. 2009;28(4 suppl):S77–S88. doi: 10.1097/INF.0b013e318199f609. [DOI] [PubMed] [Google Scholar]
- 78.Zangwill KM, Greenberg DP, Chiu CY, et al. Safety and immunogenicity of a heptavalent pneumococcal conjugate vaccine in infants. Vaccine. 2003;21:1894–1900. doi: 10.1016/s0264-410x(03)00013-6. [DOI] [PubMed] [Google Scholar]
- 79.United Nations. Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. Available at: http://unstats.un.org/unsd/methods/m49/m49regin.htm. Accessed August 13, 2013.
- 80.Park DE, Deloria Knoll M, Johnson TS, et al. The differential impact of coadministered vaccines, geographic region, vaccine product and other covariates on pneumococcal conjugate vaccine immunogenicity. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S130–S139. doi: 10.1097/INF.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scott P, Rutjes AW, Bermetz L, et al. Comparing pneumococcal conjugate vaccine schedules based on 3 and 2 primary doses: systematic review and meta-analysis. Vaccine. 2011;29:9711–9721. doi: 10.1016/j.vaccine.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 82.Goldblatt D, Hussain M, Andrews N, et al. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J Infect Dis. 2005;192:387–393. doi: 10.1086/431524. [DOI] [PubMed] [Google Scholar]
- 83.Dagan R, Givon-Lavi N, Fraser D, et al. Serum serotype-specific pneumococcal anticapsular immunoglobulin g concentrations after immunization with a 9-valent conjugate pneumococcal vaccine correlate with nasopharyngeal acquisition of pneumococcus. J Infect Dis. 2005;192:367–376. doi: 10.1086/431679. [DOI] [PubMed] [Google Scholar]
- 84.Millar EV, O’Brien KL, Bronsdon MA, et al. Anticapsular serum antibody concentration and protection against pneumococcal colonization among children vaccinated with 7-valent pneumococcal conjugate vaccine. Clin Infect Dis. 2007;44:1173–1179. doi: 10.1086/513199. [DOI] [PubMed] [Google Scholar]
- 85.Santosham M, Reid R, Chandran A, et al. Contributions of Native Americans to the global control of infectious diseases. Vaccine. 2007;25:2366–2374. doi: 10.1016/j.vaccine.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 86.Optimizing pneumococcal conjugate vaccine (PCV) schedules. Wkly Epidemiol Rec. 2012;87:13–15. [Google Scholar]


