Abstract
Since second generation pneumococcal conjugate vaccines (PCVs) targeting 10 and 13 serotypes became available in 2010, the number of national policy makers considering these vaccines has steadily increased. An important consideration for a national immunization program is the timing and number of doses—the schedule—that will best prevent disease in the population. Data on disease epidemiology and the efficacy or effectiveness of PCV schedules are typically considered when choosing a schedule. Practical concerns, such as the existing vaccine schedule, and vaccine program performance are also important. In low-income countries, pneumococcal disease and deaths typically peak well before the end of the first year of life, making a schedule that provides PCV doses early in life (eg, a 6-, 10- and 14-week schedule) potentially the best option. In other settings, a schedule including a booster dose may address disease that peaks in the second year of life or may be seen to enhance a schedule already in place. A large and growing body of evidence from immunogenicity studies, as well as clinical trials and observational studies of carriage, pneumonia and invasive disease, has been systematically reviewed; these data indicate that schedules of 3 or 4 doses all work well, and that the differences between these regimens are subtle, especially in a mature program in which coverage is high and indirect (herd) effects help enhance protection provided directly by a vaccine schedule. The recent World Health Organization policy statement on PCVs endorsed a schedule of 3 primary doses without a booster or, as a new alternative, 2 primary doses with a booster dose. While 1 schedule may be preferred in a particular setting based on local epidemiology or practical considerations, achieving high coverage with 3 doses is likely more important than the specific timing of doses.
Keywords: pneumococcal vaccine, policy, children, disease prevention
The first pneumococcal conjugate vaccine (PCV) was licensed and used as part of a national immunization program in 2000.1 Since 2004, the number of countries adopting PCVs has steadily increased. Initially, most introductions occurred in high-income settings. The licensure of new conjugate vaccines including 10 (PCV10) and 13 (PCV13) serotypes along with financing support beginning in 2009 from the GAVI Alliance2 has meant that lower income countries are now introducing PCVs. Because of the important contribution of pneumococcal disease to illness and deaths in young children,3 the increasing availability of these vaccines around the world holds promise for dramatically improving child health in all settings.
Health officials have used a variety of schedules as they worked to fit PCVs into their existing national immunization programs (Fig. 1). In the United States, the first country to use PCV in 2000, PCV7 was licensed and introduced on a schedule that matched the schedule of other routine infant immunizations (3 primary doses at 2, 4, 6 and a booster at 12–15 months), and later PCV13 was licensed on that same “3+1” schedule.4 This schedule was supported by high efficacy demonstrated by 2 large US-based clinical trials.5,6 In 2007, the World Health Organization (WHO) recommended that PCV be included in the routine schedule of all countries and that PCV could be administered as 3 primary doses without a booster.7 The 3+0 schedule was supported by clinical trial evidence from Africa and matched most Expanded Programme Immunization (EPI) schedules.8 Other countries have since adopted PCVs on schedules of either 3 or 4 total doses; the WHO recently updated their PCV policy to support use of 3 doses as either 3+0 or 2+1 schedules.9
FIGURE 1.
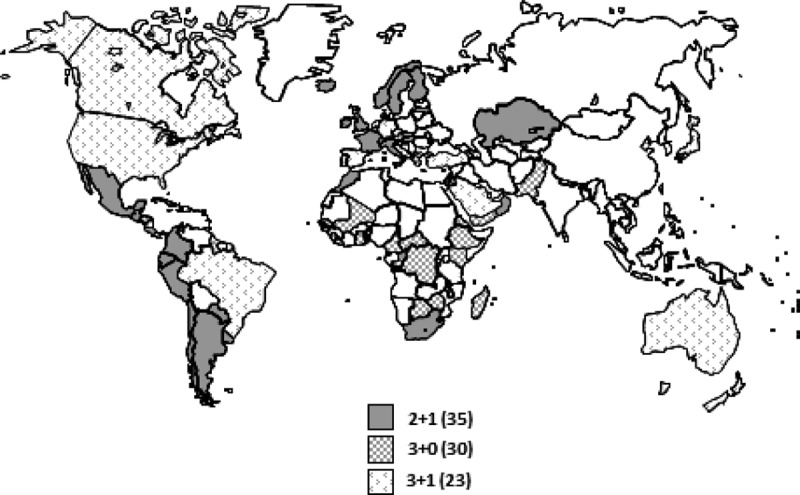
Countries routinely using PCVs by schedule.71,72 Note that 2 countries use >1 schedule: Australia (3+0 and 3+1) and Canada (2+1 and 3+1).
In general, policy makers consider disease epidemiology (ie, “Who is most affected?”), evidence of vaccine efficacy or effectiveness (ie, “What schedule will prevent the most disease or deaths?”) and practical concerns (ie, “What schedule fits best with our current immunization program?”) when choosing a particular schedule. Here, we aim to synthesize data that are relevant for policy makers looking to introduce PCV or revise their PCV schedule. No single schedule is clearly optimal for every setting; each schedule offers both benefits and limitations and the relative importance of these should be considered. First, we review aspects of pneumococcal epidemiology, in particular the timing for acquisition of carriage and peaks of pneumococcal disease that a vaccination program will aim to prevent. Next, we summarize the knowledge about how well different schedules have been shown to reduce carriage and disease from our systematic review of PCV dosing reported in this supplement and other sources. Finally, we discuss practical considerations for implementation that might impact schedule choice.
EPIDEMIOLOGY
While anyone can develop pneumococcal disease, disease most often strikes the vulnerable: infants, the elderly and persons of any age who have underlying medical conditions that might weaken their immune systems. Persons with sickle cell disease or AIDS have disease rates up to 100 times than those seen in healthy persons of the same age. As reviewed later in this article, policy makers in some countries recommend a PCV schedule with 4 doses (eg, 3+1) for selected groups of children at highest risk of pneumococcal disease, while using a 3-dose schedule for the general population of children.
In wealthy populations, nearly all deaths from pneumococcal disease occur in the elderly.10 In low-income settings, however, pneumococcal disease also leads to death in a large number of young children. Worldwide, an estimated 541,000 deaths attributed to pneumococcal disease occurred in 2008 among children <5 years of age, nearly all of these in low-income countries.11 The timing of disease onset in children also differs between low-income and high-income settings. In developing countries, most pneumococcal disease and deaths among children <5 years of age occur in the first year of life, with a peak in disease incidence before 6 months of age; in wealthy countries, disease peaks closer to 12 months of age with about half of the episodes occurring by 18 months (Fig. 2). This timing of disease and mortality is a critical factor in deciding on a vaccine schedule. Delaying doses in settings with high mortality or high disease burden among children <1 year of age, even for a schedule that may confer advantages for disease prevention in the second year of life and later in childhood, means that the earliest doses are not delivered at the time when protection is most needed.
FIGURE 2.
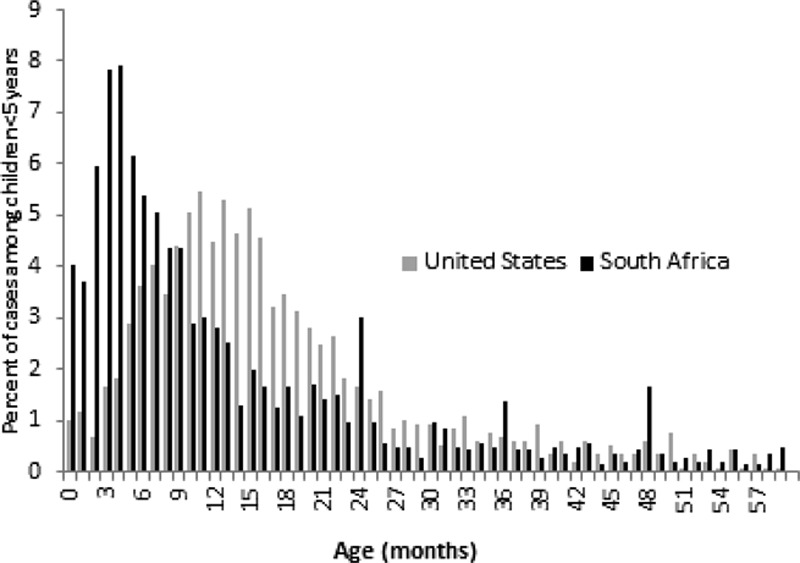
Distribution of cases of invasive pneumococcal disease for children <5 years, by month of age for children in a developing country (South Africa) and in an industrialized country (United States) [Data are unpublished and from the Group for Enteric, Respiratory and Meningeal Disease Surveillance in South Africa (GERM-SA), courtesy of Anne Von Gottberg and the US Active Bacterial Core surveillance/Emerging Infections Program Network, courtesy of Matthew Moore.].
Recommended vaccination schedules differ somewhat in response to the disease epidemiology seen in different settings. The routine EPI schedule recommended by the WHO and used in most low-income countries in Africa and Asia consists of 3 doses given at 6, 10 and 14 weeks of age; this schedule starts earlier and has shorter intervals between doses than what is often used in high-income settings (eg, 2, 4 and 6 months for the primary series).9 The relatively compressed EPI schedule is designed to quickly build immunity in children whose disease risk begins very early in life. In some settings, the ages of actual vaccine administration are delayed from the intended schedule; if so, policy makers should take this delay into account before recommending a drawn-out dosing schedule.
The serotypes predominantly causing pneumococcal disease also vary with age, disease syndrome and, to some degree, setting; serotype epidemiology may be a consideration for PCV schedule choice. Over 90 pneumococcal serotypes have been identified based on differences in carbohydrate structure of the capsule, and the number is growing as molecular methods to serotype strains are expanded.12 Fortunately, a relatively small number of serotypes cause most disease among children <2 years of age worldwide. The first conjugate vaccine, PCV7, included the 7 most common serotypes causing disease among children <2 years of age in the United States.10 A global systematic review showed that the serotypes in PCV7 caused at least 70% of infections among young children in North America, Europe and Australia, but 50–60% among young children in Africa, Asia and Latin America. New formulations, PCV10 and PCV13, are estimated to include at least 70% of disease-causing serotypes in all regions of the world.13
PCV10 and PCV13 provided substantially better serotype coverage in Africa, Asia and Latin America than PCV7 because these vaccine formulations include serotypes 1 and 5. These serotypes may appear in epidemics, causing a lot of disease in some years and little in others, and are more likely to be associated with pneumonia than other syndromes.14 Furthermore, children >2 years and healthy adults remain at risk for disease caused by these serotypes, even though rates of disease for the common pediatric serotypes (eg, 6B, 14) are generally lower in these age groups than in the youngest children14; as a result, the age distribution for serotypes 1 and 5 is shifted to older ages than the age distribution for the other serotypes commonly causing disease in infants and children. If prevention of disease caused by serotype 1 is a high priority for a PCV immunization program, a schedule that optimizes immunogenicity for this serotype and is thought to provide an extended duration of direct protection should be considered. While no data definitively indicate which schedule is best at preventing disease caused by serotype 1, it has been hypothesized that a schedule that includes a booster dose late in the first year or early in the second year of life might be needed, given the age distribution of cases.15
Pneumococcal transmission dynamics may also be an important consideration for immunization schedule choice. The ability of a PCV immunization program to prevent disease in a household and throughout a community by reducing spread of pneumococci from vaccinated children to nonvaccinated persons has been shown to be a powerful driver of the cost-effectiveness of the program.16 While colonization with pneumococci is common in children up to age 5 years and beyond in some high transmission settings, colonization with serotypes contained in PCVs is less common in adults than children. In some but not all settings, this reduced risk of vaccine serotype colonization can be seen as children age from infancy through older childhood.17,18 Catch-up programs targeting toddlers have been used in addition to routine infant schedules to quickly reduce transmission of vaccine-type pneumococci in a community as well as prevent disease in an age group still at relatively high risk for disease.
PERFORMANCE OF DIFFERENT PCV SCHEDULES
A growing body of literature has evaluated different PCV schedules on specific outcomes, such as immunogenicity, invasive pneumococcal disease, pneumonia or carriage. While a large number of studies have looked at how well a single vaccine schedule works against no vaccination, relatively few were designed to compare the effects of various schedules in a head-to-head fashion. Most head-to-head studies of vaccine schedules evaluated PCV immunogenicity, and more studies have been published on immunogenicity of PCVs than other outcomes. In this section, we will synthesize what these studies tell us about PCV schedules, in particular evaluating the number and timing of doses in the primary series, what a booster dose may add and what we have learned from evaluations of full schedules. Finally, what little is available on performance in high-risk groups and duration of protection is reviewed.
Number and Timing of Doses in a Primary Series
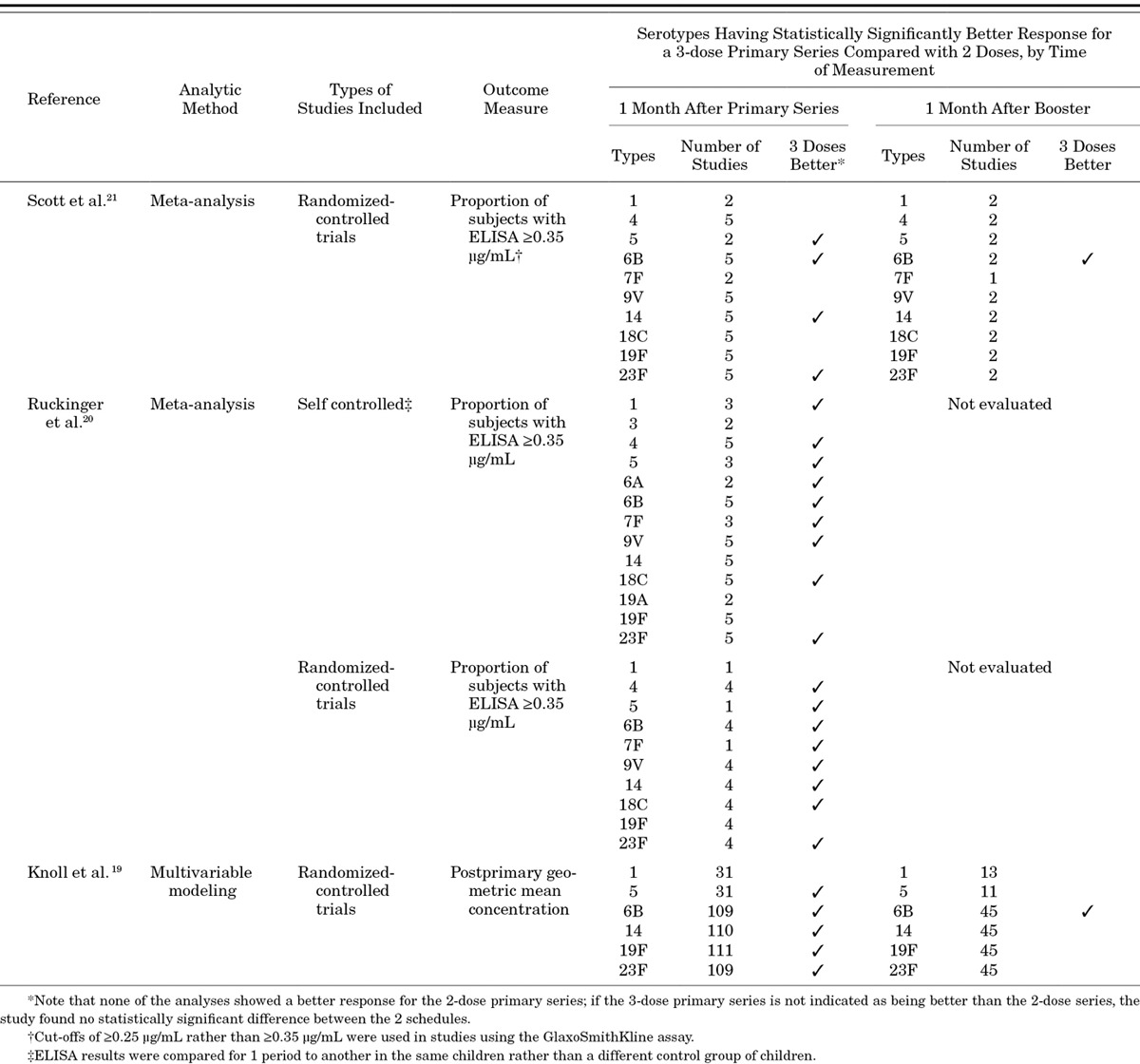
More than 60 publications address the immune response to PCV7 or higher valency PCVs.19 In addition, 3 different groups have conducted systematic reviews of the effect of different PCV dosing schedules on immune response, including 2 meta-analyses of head-to-head trials of different schedules20,21 and 1 analysis that used modeling to compare across studies.19 The 3 reviews all found that a primary series of 3 doses results in a better immune response than 2 doses for most serotypes, especially when measured right after the primary series, at about 6–7 months of age (Table 1). The differences are largest for serotypes 6B and 23F and relatively small for the other serotypes evaluated. For example, in the meta-analysis by Scott et al,21 which included 5 randomized-controlled, head-to-head trials of PCV schedules, the proportion of children achieving antibody levels ≥0.35 µg/mL (the correlate predicting efficacy and used for licensure purposes) for 3 primary doses was 21% higher compared with 2 primary doses for serotype 6B and 17% higher for serotype 23F, but no more than 7% higher for the other serotypes examined. When measured at about 1 year of age, before a booster dose was given, little difference has been seen in antibody concentrations in children who had received either 2 or 3 primary doses within the first 6 months of life.19,22,23
TABLE 1.
Summary of Systematic Reviews Evaluating Immunogenicity After the Primary Series and Booster Dose for 2- and 3-dose Primary Series
Studies of the effects of different PCV schedules on pneumococcal colonization in general correlate with findings of immunogenicity studies, indicating that 3 primary doses reduce colonization better than 2 primary doses, at least when measured shortly after finishing the series. Results from these carriage studies are summarized in a systematic review.24 The review identified 3 head-to-head clinical trials, conducted in Fiji, The Gambia and Israel, comparing colonization after the primary series among children who had received 2 doses to colonization among children receiving a 3-dose series.25–28 In these studies, children who had received 3 primary doses had less colonization than those receiving 2 doses when measured a few months after completing the series. In Israel, the difference in colonization rates was driven by reductions in colonization with serotypes 6B and 6A in the group that had received 3 primary doses.25,26 The systematic review also identified 10 clinical trials of various PCV formulations that compared prevalence of colonization in the first year of life following either a 2- or 3-dose primary series compared with no vaccination.24 In all except 1 study of an investigational product, both 2- and 3-dose primary series reduced colonization of vaccine types compared with no vaccination.
Only 1 study has attempted to directly look for a difference in effectiveness between schedules of 2 or 3 primary doses for preventing a clinical disease endpoint, finding that any differences may be short lived.29 In this study, the investigators evaluated the impact of 2 versus 3 primary PCV7 doses against incidence of lower respiratory tract infections in a general pediatric population in the United States. This propensity-score-matched case-cohort study found that children who received 3 primary doses had fewer hospitalizations for lower respiratory tract infections up to the time of receipt of a booster dose (9.5 admissions per 1000 children) than those who only received 2 primary doses (17.3 admissions per 1000 children). No difference was seen in the number of hospitalizations after the booster dose. In addition, this difference in admissions was only seen among children born in 2002; the authors hypothesized that by 2003, 3 years after introduction of PCV7, herd effects lessened the difference in risk between the 2 groups. A US study conducted shortly after PCV7 introduction suggested that cases of invasive disease caused by serotype 6A were overrepresented among children who had received only 1 or 2 doses compared with those who had received 3 or 4 doses.30
In addition to the number of doses, the interval between doses in the primary series may be an important consideration. A trial by Goldblatt et al31 that evaluated timing between doses found that an interval of 2 months resulted in significantly better immune responses compared with a 1-month interval when starting vaccination at 2 months of age. In an analysis using multivariable modeling that included results from 145 study arms from 63 different immunogenicity studies, IgG geometric mean concentrations were generally higher for schedules using a 2-month interval compared with 1-month interval, but the difference was not significant when controlling for other factors.32 The age at first dose did not appear to be an important predictor of immune response in this model, and even doses given at birth have been shown in a clinical trial to be immunogenic and prime for doses given later.33 These studies, taken together, suggest that if a 2-dose primary series is used in a PCV immunization program, an interval of 2 months rather than 1 month may help to maximize immune response to vaccination.
Use of a Booster Dose
A booster dose is a powerful addition to a PCV schedule, in particular in terms of measured immune response. Giving a dose at about 1 year of age to children who have received a primary series results in a vigorous antibody response for most serotypes, and while immune responses following the primary series differ between those who received 2 or 3 primary doses, such differences disappear following a booster dose. Two systematic reviews have assessed antibody level concentrations after the booster dose.19,21 In these, children who received 3 primary doses had, compared with children who received 2 primary doses, significantly better immune responses after the booster dose only for serotype 6B (Table 1); this was true whether measured by the proportion of children with antibody levels above the 0.35 µg/mL threshold for predicting efficacy21 or by geometric mean antibody concentrations.19 In addition, a booster dose generally results in higher antibody concentrations than does a primary series, regardless of whether antibody concentrations are measured approximately 1 month after a booster would be given (eg, 13 months) or when comparing results 1 month after the booster to those 1 month after the primary series.19 Antibody levels following a third dose of vaccine are higher when those doses are given as a 2+1 schedule than when given as a 3+0 schedule.19
While the benefits of a booster dose are clear when looking at immunological endpoints, few studies have explored the additional benefits a booster dose might provide for reducing carriage or clinical disease caused by vaccine serotypes. The best information comes from carriage studies, which suggest a benefit at least in the short term for a booster dose—but only if the primary series contained 2 doses rather than 3. In a study from Israel, differences in vaccine serotype colonization between those getting 2 primary doses compared with 3 primary doses (ie, measured after the primary series) were no longer seen after children in both the 2-dose and 3-dose arms received a booster dose with PCV7.25,34 Of note, the study in Israel found that among those receiving a 3-dose primary schedule, there was no additional impact of a booster dose for further reducing VT colonization.26 Another clinical trial, conducted in the Netherlands, demonstrated that a booster dose added to a 2-dose primary schedule increased the reduction in vaccine serotype colonization.35 In that study, children who received PCV7 at 2 and 4 months (2+0) were compared to those getting doses at 2, 4 and 11 months (2+1) and unvaccinated controls. At 12, 18 and 24 months of age, children in both the 2+0 and 2+1 groups had significantly less vaccine-type carriage than unvaccinated controls. Children in the 2+1 group also had a significantly lower prevalence of vaccine-type carriage at 18 months (16%) than the 2+0 group (24%, P = 0.01); no difference was found at 24 months.
Taken together, these head-to-head studies of the effect of different schedules on colonization show that 3 primary doses reduce colonization for some serotypes (eg, 6B) better than 2 primary doses up until the time of a booster dose and that a booster dose provides additional protection against vaccine serotype carriage but only for children who have received a 2-dose primary series and not for children who already received 3 doses. These findings agree with the observational study discussed earlier of lower respiratory tract infections, which indicated that after a booster dose, no difference was found in hospitalizations for lower respiratory tract infections for children who had received either 2 or 3 primary doses.29 These findings, however, are inconsistent with the 1 study of invasive disease that assessed whether a booster dose provided additional benefit; in this case-control study, a schedule of 3 primary doses plus a booster was significantly more effective than 3 primary doses alone.36 Overall, booster doses are clearly beneficial for programs that use only 2 primary doses, but the clinical benefit of a booster dose remains uncertain for programs that achieve high coverage with 3 primary doses.
Evaluations of Complete Schedules (2+1, 3+0 and 3+1)
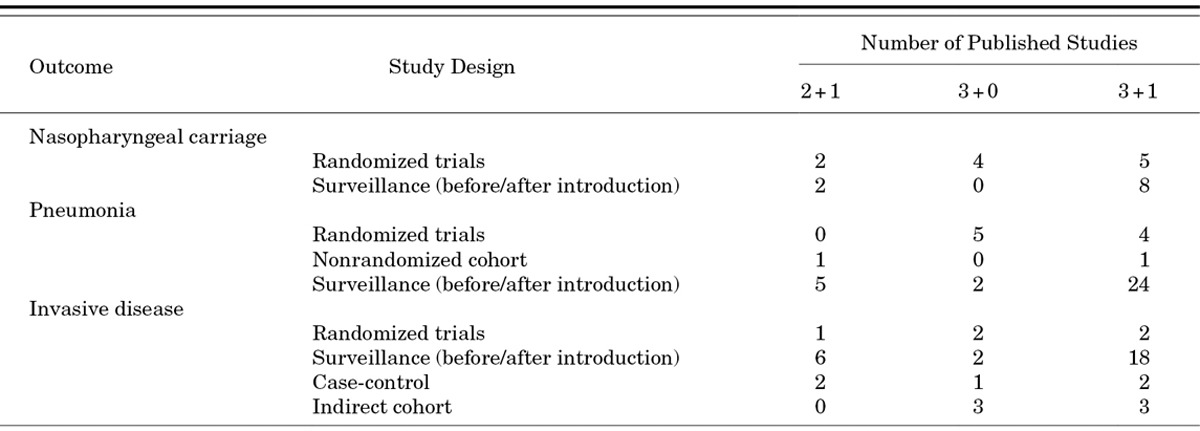
While examining the effects of the primary series and booster dose separately is useful for understanding differences between the biologic effects of various schedules, policy makers generally want to review the evidence in support of a complete schedule when making vaccine recommendations. The 3+1 schedule has more published evidence documenting its efficacy and effectiveness than the other schedules (Table 2), primarily because PCV7 was first used in the United States, a country that uses a 3+1 schedule. However, the body of evidence from clinical trials and observational studies supporting 3+0 and 2+1 schedules continues to grow. At this point, clinical trials have studied the 3+0 schedule more thoroughly than the 2+1 schedule, but more observational data are available for the 2+1 schedule than the 3+0 schedule. With few exceptions, studies show that all of these schedules are protective against carriage, pneumonia and invasive disease. Unfortunately, the different study methods do not readily allow direct comparison of the efficacy or effectiveness of 1 schedule to another.
TABLE 2.
Number of Studies in Young Children Evaluating PCV Efficacy, Effectiveness or Impact After Vaccine Introduction by Outcome, Study Design and Schedule; Studies Using PPV23 Booster Doses are Not Included24,43,45
Clinical trials using PCV7 were conducted in the United States and assessed a 3+1 schedule, with doses at 2, 4, 6 and 12–15 months.5,6 Efficacy against invasive disease in these 2 prelicensure clinical trials ranged from 83% to 94%. The similar 9-valent vaccine formulation was evaluated in South Africa and The Gambia using 3+0 schedules with doses given at 6, 10 and 14 weeks; efficacy against invasive disease in these studies ranged from 71% to 83% among the general population of children and was a little lower (65%) among children with HIV in South Africa.8,37 While reports of clinical trials for 3+1 and 3+0 schedules were published between 2000 and 2005, the first randomized trial demonstrating efficacy for the 2+1 schedule was not published until 2012. This cluster-randomized trial in Finland38 evaluated both 2+1 schedule and 3+1 schedule for PCV10; the 2 schedules were each compared to a control group that received hepatitis B vaccine. Compared with 12 cases of vaccine-type invasive disease that occurred in the control group, no cases occurred among children receiving the 3+1 schedule (vaccine effectiveness 100%, 95% confidence interval (CI): 83–100%) and only 1 case occurred among children receiving the 2+1 schedule (vaccine effectiveness 92%, 95% CI: 58–100%). Note that even in the control arm, no children <1 year of age developed invasive disease, suggesting little opportunity to evaluate the potential for breakthrough disease before the booster dose in the 2+1 arm and emphasizing the low disease risk in this population.
Similar to the literature on invasive disease, clinical trials evaluating pneumonia prevention using different PCV schedules show that 3+0, 2+1 and 3+1 schedules all provide some protection. Randomized clinical trials of 3+0 schedules from the Philippines, South Africa and The Gambia have found reductions of 23–37% for chest X-ray confirmed pneumonia among vaccinated children.8,37,39 In the United States, efficacy of a 3+1 schedule for PCV7 was 30% among children in Northern California40 although efficacy was not demonstrated for hospitalized pneumonia episodes in a cluster-randomized trial among Navajo children.41 Preliminary data for PCV10 from Latin America suggest good efficacy for the 3+1 schedule (23%).42 A systematic review identified several trials that used clinical diagnosis of pneumonia rather than X-ray confirmed pneumonia for both 3+0 and 3+1 schedules; results for this less-specific endpoint were mixed.43 Only 1 nonrandomized cohort study has evaluated efficacy of a 2+1 schedule, finding 65% (95% CI: 47–78%) less X-ray confirmed pneumonia among children whose parents chose PCV compared with unvaccinated children.44
Observational studies conducted in settings where PCVs are routinely used also demonstrated PCV effectiveness against vaccine-type invasive disease and pneumonia for the 3- and 4-dose schedules. A systematic review of invasive disease studies identified 3 case-control studies and 3 indirect cohort analyses that evaluated PCV7 effectiveness (Table 2).45 Five of 6 studies were done in settings using 3+1 schedules, although most of the studies were able to include information on children with incomplete schedules and evaluate effectiveness for schedules with fewer doses. Effectiveness estimates for schedules using 3 or more doses (76–100%) were similar to efficacy measured in clinical trials evaluating an invasive disease endpoint.
Surveillance studies have also shown benefits of PCV by documenting drops in disease rates or reported cases of invasive disease and pneumonia following PCV introduction (Table 2). In general, these studies show disease reductions at least as good as those demonstrated in clinical trials and results are often better because of indirect effects. A systematic review identified 26 such studies of invasive disease.45 In nearly all studies regardless of schedule, rates of vaccine-type invasive disease dropped quickly after vaccine introduction. While few data are available from developing countries, preliminary data from Kilifi, Kenya indicate promising reductions in invasive disease using 3 doses at 6, 10 and 14 weeks with catch-up provided for children up to age 5 years.46 To date, 33 surveillance-type studies have assessed a pneumonia or empyema endpoint after introduction into a routine immunization schedule.43 While results of surveillance studies of pneumonia endpoints are less consistent than those evaluating invasive disease, in general, the findings suggest 2+1, 3+0 and 3+1 schedules are all effective.
High-risk Populations
Children with chronic illnesses such as HIV or sickle cell disease can have disease rates >20 times than those seen in healthy children.1 While PCVs have been shown to provide protection for children with HIV,8 sickle cell disease47 and chronic illnesses as a group,36 some evidence suggests that PCVs may be somewhat less effective in children with chronic illnesses than in healthy children. In a large randomized-controlled clinical trial in South Africa, PCV9 used on a 3+0 schedule was shown to be efficacious against invasive pneumococcal disease caused by vaccine serotypes in both children with HIV and healthy children, although the point estimates were lower for HIV-infected children (65% vs. 83%).8 For the endpoint of radiographically confirmed pneumonia, point estimates of efficacy were 13% and 20% for HIV-infected and uninfected children, respectively, and the estimate was only statistically significant for HIV-uninfected children. In a case-control evaluation that was conducted in the United States, vaccine effectiveness for PCV7 serotypes was significantly lower among children with any chronic illness (as a group) than in healthy children (81% vs. 96%).36 The recommended schedule in the United States was 3 primary doses plus a booster, although at the time of the study many children were missing doses because of a national shortage.
In Australia and North America, some populations of indigenous children have documented rates of pneumococcal disease up to 20 times higher than nonindigenous children.48–51 PCV7 has been shown to be effective in these groups. Efficacy among the Navajo and Apache in the United States in a community–randomized-controlled clinical trial evaluating a 4-dose schedule of PCV7 was 82.6% against invasive disease,6 and invasive disease rates, nasopharyngeal carriage and pneumococcal transmission to adults decreased among Navajo and Alaska Natives after routine vaccination with PCV7 began.52,53 In Australia, where a schedule of 3 primary doses of PCV7 was used with a booster of 23-valent pneumococcal polysaccharide vaccine (PPV23), vaccination did not reduce pneumonia rates among aboriginal children in the Northern Territories.54
Whether using a schedule of 4 PCV doses will provide better protection than a schedule with 3 doses has not been evaluated for aboriginal children or for children with chronic illnesses. In spite of the lack of evidence, policy makers in some, but not all, countries that recommend either 3+0 or 2+1 schedules for the general population of children recommend 4-dose schedules for aboriginal or chronically ill children because of the higher risk of severe disease and complications compared with healthy children as well as concern over poor vaccine response.55 While a schedule consisting of 3 primary doses of PCV and a booster dose of PPV23 may seem attractive for high-risk children because of the potential for preventing more serotypes, this schedule should be avoided because of the lack of effectiveness in aboriginal children in Australia54 and the potential for inducing hyporesponsiveness.56
Duration of Protection
Ideally, a PCV schedule should provide long-term protection through at least the period of childhood during which disease burden is significant. To date, however, few studies have attempted to assess the duration of protection that PCV provides. The best information comes from South Africa, where investigators continued to follow subjects from the randomized clinical trial to assess duration of efficacy of PCV9.57 In this population, where PCV9 was given at 6, 10 and 14 weeks without a booster dose, efficacy against invasive disease caused by vaccine serotypes was similar at 6.2 years of follow-up (78%) compared with 2.3 years (83%) for HIV-uninfected children, but efficacy fell among HIV infected [65% at 2.3 years vs. 39% (95% CI: −7.8% to 65%) at 6.2 years]. The evidence from South Africa provides some reassurance that a schedule without a booster can provide long-term protection against disease, at least in healthy children. However, because of the reduction in effectiveness over time among HIV-infected children, policy makers in South Africa adopted a novel 3-dose schedule (doses at 6 and 14 weeks and 9 months), putting the last dose late in the first year of life in an attempt to improve longer term protection for these HIV-infected children.56 More recently, South Africa recommended a 3+1 schedule for HIV-infected children, as data from a case-control study suggested the 2-dose primary series was not adequately protective (C. Cohen et al, unpublished data).
Concern over duration of protection of a 3-dose primary series without a booster comes in part from experience with Haemophilus influenzae type b (Hib) vaccine. In the United Kingdom, Hib vaccine was introduced on a 3+0 schedule with catch-up vaccination for all children <5 years of age, resulting in dramatic reductions in disease. However, several years into the program, the rate of Hib meningitis among older children and adults began to rise, likely as a result of a complex interplay between reductions in population level Hib colonization, lower immunogenicity of Hib in combination vaccines that included acellular instead of whole cell pertussis antigens and the Hib dosing regimen; in response, a booster dose was added to the schedule and rates subsequently decreased.59 In the United States, where PCV7 has been used on a 4-dose schedule (2, 4, 6 and 12–15 months) since 2000, no increase in disease caused by vaccine serotypes has been noted in older children through 2012.60
Duration of protection might be more of a concern for serotype 1 than for other PCV types, both because of the serotype’s tendency to cause more disease in older children and adults and because some data suggest that any protection provided by a primary series of this vaccine antigen may be short lived. In an analysis combining data from the clinical trials in South Africa and The Gambia, both of which used PCV9 at 6, 10 and 14 weeks, investigators found that PCV9 did not provide significant protection against serotype 1 invasive disease.15 Serotype 1 cases that occurred in the PCV9 group all occurred after 12 months of age, whereas several cases of serotype 1 disease occurred before 12 months of age in the control arms. While the authors of this study speculated that a booster dose may be needed for control of serotype 1 disease, results from ongoing surveillance studies in the United Kingdom and Kenya, expected soon, should provide more definitive information.
PRACTICAL CONSIDERATIONS
Practical considerations about effectively delivering PCV as part of a national program, such as cost and coverage, are often as important as scientific considerations like disease epidemiology, vaccine safety and the vaccine’s likely benefits against disease. The licensed schedule is 1 such practical consideration. As new vaccines become available, data supporting their safety and efficacy are carefully considered by licensing bodies before the vaccines can be sold. The data reviewed may be from studies that used 1 particular schedule or may include studies using a variety of schedules, and the license granted may be limited to the data that was presented to the agency for review. The schedule used in the license is often what is adopted by policy makers. For example, in the United States, the Food and Drug Administration only reviewed data for PCV7 and PCV13 from studies that used schedules of 3 primary doses given at 2, 4 and 6 months followed by a booster at 12–15 months.61 Because these vaccines were then licensed in the United States on this schedule, US vaccine policy as directed by the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices recommended this schedule for PCV administration.4 Elsewhere, policy makers may decide to use a schedule different than the licensed schedule or vaccine licenses may provide flexibility. In Europe, the European Medicines Agency has licensed both PCV10 and PCV13 with labels that provide the option of using either a 3+1 schedule or a 2+1 schedule, if the latter schedule is used as part of a routine immunization program.62,63 The WHO has “prequalified” both PCV10 and PCV13, performing a review similar to that done for licensing purposes. Prequalification allows purchase of these vaccines by UNICEF and other United Nations agencies. The package inserts used for PCVs purchased by UNICEF specify use of either a 3+1 or 2+1 schedule but also recommend taking into account local vaccination policy when determining the schedule.64 This latter language allows flexibility for PCV to be given on a 3+0 schedule, if that is the local policy, and still be in accordance with labeling.
Another important driver for choice of PCV schedule is the schedule already in use for other vaccines. Clinical trials demonstrated efficacy of PCVs when given along with other routine infant vaccines, and PCVs are licensed to be administered concomitantly with other vaccines. Providing PCV at the same healthcare visit as other routine vaccines is often easier for both parents and immunization providers than requiring separate visits, and consolidating vaccinations into the fewest number of visits improves immunization coverage rates.65
Conversely, the choice of schedule could affect the immunization coverage rates ultimately achieved for PCV. In some settings, vaccine doses given early in infancy have higher uptake than doses given later. For example, in Burkina Faso, coverage for a measles-containing vaccine (scheduled for administration at 9 months of age) in 2011 as reported by the WHO was only 63%, but coverage for the third dose of diphtheria-tetanus-pertussis (DTP3) vaccine (scheduled for 14 weeks of age) was 91%.66 The difference in coverage rates suggest that compliance is worse for vaccination visits later in the first year of life compared with earlier visits. In settings where coverage for later doses is significantly lower than for earlier doses, a 3+0 schedule is a better choice than a schedule requiring a booster dose.
Cost is also a practical consideration for policy makers, especially for those deciding between 3-dose and 4-dose schedules. Our review of the literature on PCV dosing schedules found only small differences in protection between 3- and 4-dose schedules, in particular for schedules using a 2-dose primary series compared with those using 3 primary doses in the months before the booster dose; those differences may not result in measurably different reductions in disease burden especially in settings where vaccine coverage is high enough for herd effects to provide additional protection. Cost-effectiveness analyses from the Netherlands and the United States suggest that a 3+1 series was not cost-effective compared with a 2+1 series, assuming that vaccine price was the same for the 2 schedules67 (CDC unpublished data). Note that while a 2-dose infant schedule has been proposed as potentially a better use of resources than a 3-dose series in low-income settings,68 a 2-dose infant schedule has not been tested in a clinical trial nor recommended routinely in any country because of concerns that it may be significantly less effective than 3- or 4-dose schedules.
Many policy makers who have introduced PCVs have done so using a catch-up campaign in their countries rather than restricting PCV to only those children born after the initiation of the PCV program. One objective of catch-up campaigns is usually to provide direct protection to infants and children targeted to receive the catch-up doses. The ability to conduct a catch-up vaccination program and the age group included in the program depends on local epidemiology and available vaccine supply and resources. When PCV7 was first introduced in the United States, vaccination was recommended for all children <2 years of age and a subset of children at higher risk of disease (eg, those with certain medical conditions), who were 2–4 years of age.1 The recommendation was based on the high rates of pneumococcal disease among children <2 years of age (Fig. 2). In other settings where disease peaks well before age 1 (see South Africa data in Fig. 2), a catch-up program limited to children <1 year of age would quickly protect those in the highest risk group, although the absolute risk of children beyond 1 year of age may still be important, especially relative to that risk in more developed countries. Others have provided vaccination to toddlers and older children < 5 years of age, not only to protect them but also to quickly produce “herd protection” by reducing transmission of vaccine serotype pneumococci in the community. In Kilifi, Kenya, 2 doses of PCV10 were offered to all children <5 years of age in addition to the primary schedule of 3 doses given at 6, 10 and 14 weeks, and early results from an intensive surveillance program suggest a rapid reduction in disease rates following vaccine implementation.46 While introduction of PCV for infants along with a catch-up program for older children may protect a population more quickly than introduction without a catch-up program, the added benefit that a catch-up program provides has not been directly measured. An assessment of catch-up policies is underway and will evaluate disease reduction, cost-effectiveness and financing of such programs.
How to best change from 1 vaccine formulation to another has become a question. Since licensure of PCV10 and PCV13, programs that began with PCV7 are now switching to these newer formulations, and policy makers question whether children who started their series with 1 vaccine can finish with another. Only a few studies have evaluated schedules comprised of >1 pneumococcal vaccine. Schedules using all PCV7 doses and those starting with PCV7 and finishing with PCV13 (products made by the same manufacturer, Pfizer, and using the same carrier protein, CRM197) result in comparable antibody levels for the 7 serotypes in common between the 2 vaccines, but somewhat lower antibody levels for the 6 additional serotypes in PCV13.4 In the 1 study in which children received 3 primary doses with PCV7 and were then boosted with either PCV7 or PCV10 (a product made by a different manufacturer using a different carrier protein), antibody levels were higher for children receiving all PCV7 doses, especially for serotypes 6B and 23F, although functional activities measured by opsonophagocytic assays were similar.69 In practice, programs that were using PCV7 often simply have children receive PCV13 or PCV10 at their next scheduled dose. Programs that switched from PCV7 to PCV10 and use a schedule with a booster dose have sometimes opted to use PCV7 to complete the primary series and boost with PCV10.
PPV23 has been used in the past as a booster dose in some research studies and routinely among aboriginal children in Australia, but this practice is no longer recommended in any setting, to our knowledge. In Australia, the PPV23 booster did not seem to help with disease prevention.54 In a trial in Fiji, use of PPV23 as a booster dose at 12 months of age in children who had received a primary series with PCV7 resulted in good antibody responses 1 month after the booster dose, but responses were significantly blunted to a partial dose of PPV23 given at age 18 months compared with children who had not received PPV23 at 12 months, suggesting that use of PPV23 in this age group could result in immune hyporesponsiveness.56,70
CONCLUSION
With the increasing supply of 10- and 13-valent vaccines and financing as well as programmatic support from the GAVI Alliance, a large number of low-income countries are introducing PCV into their routine immunization schedules. Introducing PCV into an existing national immunization program can be complicated because of resource concerns and the need to expand the cold chain and other delivery resources. Choosing the optimal schedule, however, can be straightforward in many settings, especially if the 3- or 4-dose schedule in place for other routine infant immunizations can accommodate an additional vaccine and the epidemiology of disease will be addressed by the proposed timing of doses. As summarized earlier, the literature on PCV schedules has identified few differences between 3- and 4-dose schedules, and a large body of evidence suggests that 3+0, 2+1 and 3+1 schedules are all highly effective compared with no vaccination. The differences that do exist between schedules are likely only relevant early in the course of a PCV program; once coverage is sufficient to induce herd effects such differences would likely be minimized and difficult to measure.
The WHO’s recent guidance on PCV now allows for flexibility in the timing of doses. In October 2011, the WHO pulled together a meeting of epidemiologists, immunologists and other experts in the field to review and discuss available evidence supporting use of different vaccination schedules for PCV. The information was later summarized and presented to WHO’s Strategic Advisory Group of Experts (SAGE), an international group of vaccine experts who assist WHO with vaccine policy formation. After this review, WHO published updated SAGE recommendations for PCV10 and PCV13 that replaced an earlier statement on PCV7. The new statement, in addition to again recommending a schedule of 3 primary doses, recommended as an alternate schedule 2 primary doses plus a booster at around 1 year of age (Table 3).9 According to the statement, giving 3 primary doses may be preferred in settings in which disease rates peak well before the end of the first year of life or in which coverage is low for vaccines given late in the first year (eg, measles vaccine); use of a 2+1 schedule may be preferred in settings in which duration of protection may be a concern, especially for ongoing protection against serotype 1, but whether such a schedule provides longer protection than a 3+0 schedule has not been proven.
TABLE 3.
Summary of 2012 WHO SAGE Recommendations for Use of PCV10 and PCV139

Some questions about vaccine schedules remain. In particular, some antigens in the new conjugate formulations, such as those targeting serotypes 1, 3 and 5, may prevent disease better in schedules using a booster dose; compared with other serotypes targeted by the vaccine, disease caused by these serotypes often occurs among older children. Whether the duration of protection is improved by added a booster dose, however, is unclear. In addition, the benefit of catch-up vaccination programs, compared with programs solely providing PCV to infants, has also not been measured. While these and other questions remain about how to fully optimize a PCV schedule, a large and growing body of evidence indicates that currently available PCVs are highly effective on a range of schedules, allowing flexibility for policy makers to incorporate PCV into existing vaccination programs.
ACKNOWLEDGMENT
The authors thank Jennifer Loo for help with preparation of this article.
Footnotes
Accepted for publication August 13, 2013.
Support for this project was provided by Program for Appropriate Technology in Health PATH) through funding from the GAVI. The views expressed by the authors do not necessarily reflect the views of the Centers for Disease Control and Prevention, GAVI and/or PATH. D.G.’s laboratory performs contract and or collaborative research for/with Pfizer, GlaxoSmithKline, Merck, Novartis and Sanofi Pasteur. D.G. has received travel or honorarium support for participation in external expert committees for Merck, Sanofi Pasteur, Pfizer and GlaxoSmithKline. K.O.B. received grant support from Pfizer and GlaxoSmithKline and has received travel or honorarium support for participation in external expert committees for Merck, Aventis-pasteur and GlaxoSmithKline. The authors have no other funding or conflicts of interest to declare.
Address for correspondence: Cynthia G. Whitney, MD, MPH, Centers for Disease Control and Prevention, Mailstop C25, 1600 Clifton Road NE, Atlanta, GA 30333. E-mail: cwhitney@cdc.gov.
REFERENCES
- 1.Centers for Disease Control and Prevention. Preventing pneumococcal disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep. 2000;49(RR-9):1–35. [PubMed] [Google Scholar]
- 2.GAVI Alliance. GAVI Alliance. 2012. Available at: http://www.gavialliance.org/. Accessed December 6, 2012.
- 3.World Health Organization, United Nations Childrens Fund. Global Action Plan for Prevention and Control of Pneumonia (GAPP) Geneva, Switzerland: WHO Press; 2009. [Google Scholar]
- 4.Centers for Disease Control and Prevention. Prevention of pneumococcal disease among infants and children—use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). Morb Mortal Wkly Rep. 2010;59(RR11):1–18. [Google Scholar]
- 5.Black S, Shinefield H, Fireman B, et al. Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Northern California Kaiser Permanente Vaccine Study Center Group. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 6.O’Brien KL, Moulton LH, Reid R, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet. 2003;362:355–361. doi: 10.1016/S0140-6736(03)14022-6. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Pneumococcal conjugate vaccine for childhood immunization—WHO position paper. Wkly Epidemiol Rec. 2007;82:93–104. [PubMed] [Google Scholar]
- 8.Klugman KP, Madhi SA, Huebner RE, et al. Vaccine Trialists Group. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Pneumococcal vaccines WHO position paper—2012. Wkly Epidemiol Rec. 2012;87:129–44. [PubMed] [Google Scholar]
- 10.Robinson KA, Baughman W, Rothrock G, et al. Active Bacterial Core Surveillance (ABCs)/Emerging Infections Program Network. Epidemiology of invasive Streptococcus pneumoniae infections in the United States, 1995–1998: opportunities for prevention in the conjugate vaccine era. JAMA. 2001;285:1729–1735. doi: 10.1001/jama.285.13.1729. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization, UNICEF. Global immunization data. October 2012 Available at: http://www.who.int/immunization_monitoring/Global_Immunization_Data.pdf. Accessed April 9, 2013. [Google Scholar]
- 12.McEllistrem MC, Nahm MH. Novel pneumococcal serotypes 6C and 6D: anomaly or harbinger. Clin Infect Dis. 2012;55:1379–1386. doi: 10.1093/cid/cis691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson HL, Deloria-Knoll M, Levine OS, et al. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 2010;7:e1000348. doi: 10.1371/journal.pmed.1000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausdorff WP, Feikin DR, Klugman KP. Epidemiological differences among pneumococcal serotypes. Lancet Infect Dis. 2005;5:83–93. doi: 10.1016/S1473-3099(05)01280-6. [DOI] [PubMed] [Google Scholar]
- 15.Klugman KP, Madhi SA, Adegbola RA, et al. Timing of serotype 1 pneumococcal disease suggests the need for evaluation of a booster dose. Vaccine. 2011;29:3372–3373. doi: 10.1016/j.vaccine.2011.02.089. [DOI] [PubMed] [Google Scholar]
- 16.Ray GT, Whitney CG, Fireman BH, et al. Cost-effectiveness of pneumococcal conjugate vaccine: evidence from the first 5 years of use in the United States incorporating herd effects. Pediatr Infect Dis J. 2006;25:494–501. doi: 10.1097/01.inf.0000222403.42974.8b. [DOI] [PubMed] [Google Scholar]
- 17.Abdullahi O, Karani A, Tigoi CC, et al. The prevalence and risk factors for pneumococcal colonization of the nasopharynx among children in Kilifi District, Kenya. PLoS One. 2012;7:e30787. doi: 10.1371/journal.pone.0030787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien KL, Millar EV, Zell ER, et al. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J Infect Dis. 2007;196:1211–1220. doi: 10.1086/521833. [DOI] [PubMed] [Google Scholar]
- 19.Deloria Knoll M, Park D, Johnson TS, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on immunogenicity. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S119–S129. doi: 10.1097/INF.0000000000000079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rückinger S, Dagan R, Albers L, et al. Immunogenicity of pneumococcal conjugate vaccines in infants after two or three primary vaccinations: a systematic review and meta-analysis. Vaccine. 2011;29:9600–9606. doi: 10.1016/j.vaccine.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Scott P, Rutjes AW, Bermetz L, et al. Comparing pneumococcal conjugate vaccine schedules based on 3 and 2 primary doses: systematic review and meta-analysis. Vaccine. 2011;29:9711–9721. doi: 10.1016/j.vaccine.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 22.Russell FM, Balloch A, Tang ML, et al. Immunogenicity following one, two, or three doses of the 7-valent pneumococcal conjugate vaccine. Vaccine. 2009;27:5685–5691. doi: 10.1016/j.vaccine.2009.06.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silfverdal SA, Hogh B, Bergsaker MR, et al. Immunogenicity of a 2-dose priming and booster vaccination with the 10-valent pneumococcal nontypeable Haemophilus influenzae protein D conjugate vaccine. Pediatr Infect Dis J. 2009;28:e276–e282. doi: 10.1097/INF.0b013e3181b48ca3. [DOI] [PubMed] [Google Scholar]
- 24.Fleming-Dutra KE, Conklin L, Loo JD, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on vaccine-type nasopharyngeal carriage. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S152–S160. doi: 10.1097/INF.0000000000000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagan R, Givon-Lavi N, Porat N, et al. The effect of an alternative reduced-dose infant schedule and a second year catch-up schedule with 7-valent pneumococcal conjugate vaccine on pneumococcal carriage: a randomized controlled trial. Vaccine. 2012;30:5132–5140. doi: 10.1016/j.vaccine.2012.05.059. [DOI] [PubMed] [Google Scholar]
- 26.Klugman KP, Black S, Dagan R, et al. Pneumococcal conjugate vaccine and pneumococcal common protein vaccines. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 6th ed. Amsterdam: Elsevier Saunders; 2012. [Google Scholar]
- 27.Ota MO, Akinsola A, Townend J, et al. The immunogenicity and impact on nasopharyngeal carriage of fewer doses of conjugate pneumococcal vaccine immunization schedule. Vaccine. 2011;29:2999–3007. doi: 10.1016/j.vaccine.2011.01.098. [DOI] [PubMed] [Google Scholar]
- 28.Russell FM, Carapetis JR, Satzke C, et al. Pneumococcal nasopharyngeal carriage following reduced doses of a 7-valent pneumococcal conjugate vaccine and a 23-valent pneumococcal polysaccharide vaccine booster. Clin Vaccine Immunol. 2010;17:1970–1976. doi: 10.1128/CVI.00117-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pelton SI, Weycker D, Klein JO, et al. 7-Valent pneumococcal conjugate vaccine and lower respiratory tract infections: effectiveness of a 2-dose versus 3-dose primary series. Vaccine. 2010;28:1575–1582. doi: 10.1016/j.vaccine.2009.11.053. [DOI] [PubMed] [Google Scholar]
- 30.Park SY, Van Beneden CA, Pilishvili T, et al. Active Bacterial Core surveillance team. Invasive pneumococcal infections among vaccinated children in the United States. J Pediatr. 2010;156:478–483.e2. doi: 10.1016/j.jpeds.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Goldblatt D, Southern J, Ashton L, et al. Immunogenicity of a reduced schedule of pneumococcal conjugate vaccine in healthy infants and correlates of protection for serotype 6B in the United Kingdom. Pediatr Infect Dis J. 2010;29:401–405. doi: 10.1097/INF.0b013e3181c67f04. [DOI] [PubMed] [Google Scholar]
- 32.Park DE, Johnson TS, Nonyane BAS, et al. The differential impact of co-administered vaccines, geographic region, vaccine product and other covariates on pneumococcal conjugate vaccine immunogenicity. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S130–S139. doi: 10.1097/INF.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott JA, Ojal J, Ashton L, et al. Pneumococcal conjugate vaccine given shortly after birth stimulates effective antibody concentrations and primes immunological memory for sustained infant protection. Clin Infect Dis. 2011;53:663–670. doi: 10.1093/cid/cir444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Givon-Lavi N, Greenberg D, Dagan R. Immunogenicity of alternative regimens of the conjugated 7-valent pneumococcal vaccine: a randomized controlled trial. Pediatr Infect Dis J. 2010;29:756–762. doi: 10.1097/INF.0b013e3181d99345. [DOI] [PubMed] [Google Scholar]
- 35.van Gils EJ, Veenhoven RH, Hak E, et al. Effect of reduced-dose schedules with 7-valent pneumococcal conjugate vaccine on nasopharyngeal pneumococcal carriage in children: a randomized controlled trial. JAMA. 2009;302:159–167. doi: 10.1001/jama.2009.975. [DOI] [PubMed] [Google Scholar]
- 36.Whitney CG, Pilishvili T, Farley MM, et al. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet. 2006;368:1495–1502. doi: 10.1016/S0140-6736(06)69637-2. [DOI] [PubMed] [Google Scholar]
- 37.Cutts FT, Zaman SM, Enwere G, et al. Gambian Pneumococcal Vaccine Trial Group. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet. 2005;365:1139–1146. doi: 10.1016/S0140-6736(05)71876-6. [DOI] [PubMed] [Google Scholar]
- 38.Palmu AA, Jokinen J, Borys D, et al. Effectiveness of the ten-valent pneumococcal Haemophilus influenzae protein D conjugate vaccine (PHiD-CV10) against invasive pneumococcal disease: a cluster randomised trial. Lancet. 2013;381:214–222. doi: 10.1016/S0140-6736(12)61854-6. [DOI] [PubMed] [Google Scholar]
- 39.Lucero MG, Nohynek H, Williams G, et al. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. Pediatr Infect Dis J. 2009;28:455–462. doi: 10.1097/INF.0b013e31819637af. [DOI] [PubMed] [Google Scholar]
- 40.Hansen J, Black S, Shinefield H, et al. Effectiveness of heptavalent pneumococcal conjugate vaccine in children younger than 5 years of age for prevention of pneumonia: updated analysis using World Health Organization standardized interpretation of chest radiographs. Pediatr Infect Dis J. 2006;25:779–781. doi: 10.1097/01.inf.0000232706.35674.2f. [DOI] [PubMed] [Google Scholar]
- 41.O’Brien KL. Helsinki, Finland: 2002. The effect of conjugate pneumococcal vaccine on pneumonia and otitis media among Navajo and White Mountain Apache children. 3rd International Symposium on Pneumococci and Pneumococcal Diseases; [Google Scholar]
- 42.Tregnaghi MW, Sáez-Llorens X, López P, et al. The Hague, Netherlands: June 7–11, 2011. Evaluating the efficacy of 10-valent pneumococcal non-typeable Haemophilus influenzae protein-D conjugate vaccine (PHiDCV) against community-acquired pneumonia in Latin America. European Society of Pediatric Infectious Disease; [Google Scholar]
- 43.Loo JD, Conklin L, Fleming-Dutra KE, et al. Systematic review of the effect of pneumococcal conjugate vaccine dosing schedules on prevention of pneumonia. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S140–S151. doi: 10.1097/INF.0000000000000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Esposito S, Lizioli A, Lastrico A, et al. Impact on respiratory tract infections of heptavalent pneumococcal conjugate vaccine administered at 3, 5 and 11 months of age. Respir Res. 2007;8:12. doi: 10.1186/1465-9921-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conklin L, Loo JD, Kirk J, et al. Systematic review of pneumococcal conjugate vaccine dosing schedules’ effect on vaccine-type invasive pneumococcal disease among young children. Pediatr Infect Dis J. 2014;;33 (Suppl 2)::S109–S118. doi: 10.1097/INF.0000000000000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.KEMRI/Wellcome Trust. The Pneumococcal Conjugate Vaccine Impact Study (PCVIS). Available at: http://www.kemri-wellcome.org/index.php/en/studies_inner/75. Accessed October 15, 2013. [Google Scholar]
- 47.Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008;121:562–569. doi: 10.1542/peds.2007-0018. [DOI] [PubMed] [Google Scholar]
- 48.O’Brien KL, Shaw J, Weatherholtz R, et al. Epidemiology of invasive Streptococcus pneumoniae among Navajo children in the era before use of conjugate pneumococcal vaccines, 1989-1996. Am J Epidemiol. 2004;160:270–278. doi: 10.1093/aje/kwh191. [DOI] [PubMed] [Google Scholar]
- 49.Lacapa R, Bliss SJ, Larzelere-Hinton F, et al. Changing epidemiology of invasive pneumococcal disease among White Mountain Apache persons in the era of the pneumococcal conjugate vaccine. Clin Infect Dis. 2008;47:476–484. doi: 10.1086/590001. [DOI] [PubMed] [Google Scholar]
- 50.Davidson M, Parkinson AJ, Bulkow LR, et al. The epidemiology of invasive pneumococcal disease in Alaska, 1986–1990—ethnic differences and opportunities for prevention. J Infect Dis. 1994;170:368–376. doi: 10.1093/infdis/170.2.368. [DOI] [PubMed] [Google Scholar]
- 51.Torzillo PJ, Hanna JN, Morey F, et al. Invasive pneumococcal disease in central Australia. Med J Aust. 1995;162:182–186. doi: 10.5694/j.1326-5377.1995.tb126016a.x. [DOI] [PubMed] [Google Scholar]
- 52.Weatherholtz R, Millar EV, Moulton LH, et al. Invasive pneumococcal disease a decade after pneumococcal conjugate vaccine use in an American Indian population at high risk for disease. Clin Infect Dis. 2010;50:1238–1246. doi: 10.1086/651680. [DOI] [PubMed] [Google Scholar]
- 53.Wenger JD, Zulz T, Bruden D, et al. Invasive pneumococcal disease in Alaskan children: impact of the seven-valent pneumococcal conjugate vaccine and the role of water supply. Pediatr Infect Dis J. 2010;29:251–256. doi: 10.1097/INF.0b013e3181bdbed5. [DOI] [PubMed] [Google Scholar]
- 54.O’Grady KA, Lee KJ, Carlin JB, et al. Increased risk of hospitalization for acute lower respiratory tract infection among Australian indigenous infants 5-23 months of age following pneumococcal vaccination: a cohort study. Clin Infect Dis. 2010;50:970–978. doi: 10.1086/651079. [DOI] [PubMed] [Google Scholar]
- 55.Australian Government Department of Health and Aging. National immunisation program schedule. March 7, 2013 Available at: http://www.health.gov.au/internet/immunise/publishing.nsf/Content/nips-1. Acccessed April 29 2013. [Google Scholar]
- 56.Russell FM, Carapetis JR, Balloch A, et al. Hyporesponsiveness to re-challenge dose following pneumococcal polysaccharide vaccine at 12 months of age, a randomized controlled trial. Vaccine. 2010;28:3341–3349. doi: 10.1016/j.vaccine.2010.02.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madhi SA, Adrian P, Kuwanda L, et al. Long-term immunogenicity and efficacy of a 9-valent conjugate pneumococcal vaccine in human immunodeficient virus infected and non-infected children in the absence of a booster dose of vaccine. Vaccine. 2007;25:2451–2457. doi: 10.1016/j.vaccine.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 58.Madhi S, Cohen C, von Gottberg A. Introduction of pneumococcal conjugate vaccine into the public immunization program in South Africa: Translating research into policy. Vaccine. 2012;30(s(uppl 3):C23–C27. doi: 10.1016/j.vaccine.2012.05.055. [DOI] [PubMed] [Google Scholar]
- 59.Goldblatt D, Assari T. Module 9: Haemophilus influenzae type b. World Health Organisation Immunological Basis of Immunization Series. Geneva: World Health Organization; 2007. [Google Scholar]
- 60.Stoecker C, Hampton LM, Link-Gelles R, Messonnier ML, Zhou F, Moore MR. Cost-effectiveness of using 2 vs 3 primary doses of 13-valent pneumococcal conjugate vaccine. Pediatrics. 2013;132:e324–32. doi: 10.1542/peds.2012-3350. doi: 10.1542/peds.2012-3350. Epub 2013 Jul 1. [DOI] [PubMed] [Google Scholar]
- 61.U.S. Food and Drug Administration. Vaccines, blood & biologics: Prevnar 13. September 17, 2012 Available at: http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm201667.htm. Accepted December 6, 2012. [Google Scholar]
- 62.European Medicines Agency. Prevenar 13: pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed) September 5, 2012 Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001104/human_med_001220.jsp&mid=WC0b01ac058001d124. Accessed December 4, 2012. [Google Scholar]
- 63.European Medicines Agency. Synflorix: pneumococcal polysaccharide conjugate vaccine (adsorbed) February 20, 2012 Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000973/human_med_001071.jsp&mid=WC0b01ac058001d124. Accessed December 4, 2012. [Google Scholar]
- 64.World Health Organization. WHO prequalified vaccines: filterable search for prequalified vaccines with product details. 2011 Available at: http://www.who.int/immunization_standards/vaccine_quality/PQ_vaccine_list_en/en/index.html. Accessed December 4, 2012. [Google Scholar]
- 65.Marshall GS, Happe LE, Lunacsek OE, et al. Use of combination vaccines is associated with improved coverage rates. Pediatr Infect Dis J. 2007;26:496–500. doi: 10.1097/INF.0b013e31805d7f17. [DOI] [PubMed] [Google Scholar]
- 66.World Health Organization, Unicef. Estimated coverage by country, year, and vaccine. July 14, 2012 Available at: http://apps.who.int/immunization_monitoring/en/globalsummary/timeseries/tswucoveragedtp3.htm. Accessed December 7, 2012. [Google Scholar]
- 67.Rozenbaum M, Sanders E, van Hoek A, et al. Cost effectiveness of pneumococcal vaccination among Dutch infants: an economic analysis of the seven valent pneumococcal conjugated vaccine and forecast for the 10 valent and 13 valent vaccines. Brit Med J. 2010;340 doi: 10.1136/bmj.c2509. [DOI] [PubMed] [Google Scholar]
- 68.Daniels N, Valencia-Mendoza A, Gelpi A, et al. The art of public health: pneumococcal vaccine coverage in Mexico. Lancet. 2010;375:114–115. doi: 10.1016/S0140-6736(10)60037-2. [DOI] [PubMed] [Google Scholar]
- 69.Vesikari T, Wysocki J, Chevallier B, et al. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J. 2009;28(4 suppl):S66–S76. doi: 10.1097/INF.0b013e318199f8ef. [DOI] [PubMed] [Google Scholar]
- 70.O’Brien KL, Hochman M, Goldblatt D. Combined schedules of pneumococcal conjugate and polysaccharide vaccines: is hyporesponsiveness an issue? Lancet Infect Dis. 2007;7:597–606. doi: 10.1016/S1473-3099(07)70210-4. [DOI] [PubMed] [Google Scholar]
- 71.Johns Hopkins Bloomberg School of Public Health International Vaccine Access Center (IVAC) VIMS Report: Global Vaccine Introduction. October 2012:10. [Google Scholar]
- 72.World Health Organization. WHO vaccine-preventable diseases: monitoring system. 2013 global summary. 2013 Available at: http://apps.who.int/immunization_monitoring/globalsummary/schedules. Accessed June 12, 2013. [Google Scholar]


