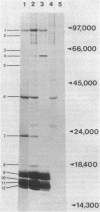
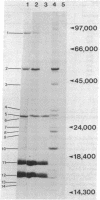
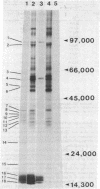
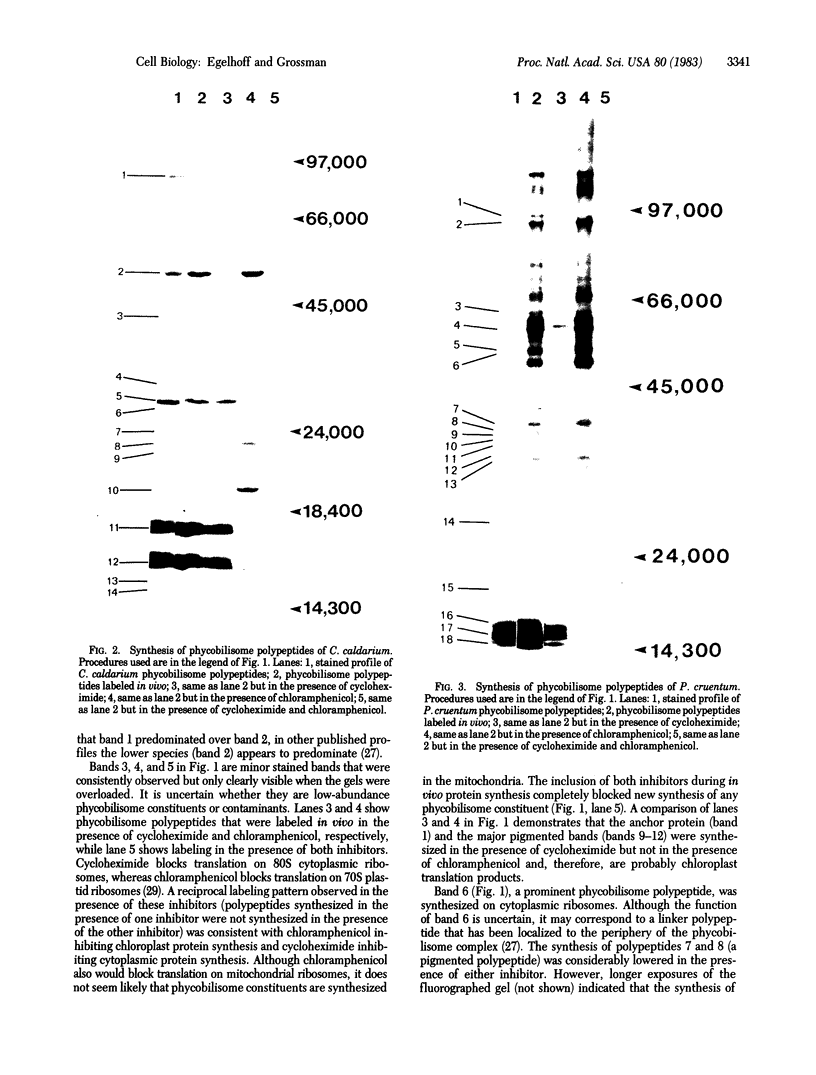
Abstract
In vivo labeling of eukaryotic phycobilisomes in the presence of inhibitors of translation on 70S and 80S ribosomes demonstrates that some of the polypeptides of this light-harvesting complex are synthesized in the cytoplasm while others are synthesized in the chloroplast. The major pigmented polypeptides, the α and β subunits of the biliproteins (molecular weights between 15,000 and 20,000) and the anchor protein (molecular weight about 90,000) are translated on 70S ribosomes. This suggests that these polypeptides are made within the algal chloroplast. Because the α and β subunits comprise a group of closely related polypeptides, the genes encoding these polypeptides may reside in the plastid genome as a multigene family. Other prominent phycobilisome polypeptides, including a nonpigmented polypeptide that may be involved in maintaining the structural integrity of the complex, are synthesized on cytoplasmic ribosomes. Because the synthesis of phycobilisomes appears to require the expression of genes in two subcellular compartments, this system may be an excellent model for: (i) examining interaction between nuclear and plastid genomes: (ii) elucidating the molecular processes involved in the evolution of plastid genes: (iii) clarifying the events in the synthesis and assembly of macromolecular complexes in the chloroplast.
Keywords: translation inhibitors, phycobiliproteins, anchor protein, linker protein
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. B. Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol. 1959;32(3):270–277. doi: 10.1007/BF00409348. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Gantt E., Lipschultz C. A., Zilinskas B. Further evidence for a phycobilisome model from selective dissociation, fluorescence emission, immunoprecipitation, and electron microscopy. Biochim Biophys Acta. 1976 May 14;430(2):375–388. doi: 10.1016/0005-2728(76)90093-1. [DOI] [PubMed] [Google Scholar]
- Gingrich J. C., Blaha L. K., Glazer A. N. Rod substructure in cyanobacterial phycobilisomes: analysis of Synechocystis 6701 mutants low in phycoerythrin. J Cell Biol. 1982 Feb;92(2):261–268. doi: 10.1083/jcb.92.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N., Apell G. S., Hixson C. S., Bryant D. A., Rimon S., Brown D. M. Biliproteins of cyanobacteria and Rhodophyta: Homologous family of photosynthetic accessory pigments. Proc Natl Acad Sci U S A. 1976 Feb;73(2):428–431. doi: 10.1073/pnas.73.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer A. N. Structure and molecular organization of the photosynthetic accessory pigments of cyanobacteria and red algae. Mol Cell Biochem. 1977 Dec 29;18(2-3):125–140. doi: 10.1007/BF00280278. [DOI] [PubMed] [Google Scholar]
- HUGHES E. O., GORHAM P. R., ZEHNDER A. Toxicity of a unialgal culture of Microcystis aeruginosa. Can J Microbiol. 1958 Jun;4(3):225–236. doi: 10.1139/m58-024. [DOI] [PubMed] [Google Scholar]
- Harington A., Thornley A. L. Biochemical and genetic consequences of gene transfer from endosymbiont to host genome. J Mol Evol. 1982;18(5):287–292. doi: 10.1007/BF01733893. [DOI] [PubMed] [Google Scholar]
- Harris J. U., Berns D. S. Letter: Sequences of the N-terminus portions of biliproteins. J Mol Evol. 1975 Jul 11;5(2):153–163. doi: 10.1007/BF01732519. [DOI] [PubMed] [Google Scholar]
- Koller K. P., Wehrmeyer W., Mörschel E. Biliprotein assemble in the disc-shaped phycobilisomes of Rhodella violacea. On the molecular composition of energy-transfering complexes (tripartite units) forming the periphery of the phycobilisome. Eur J Biochem. 1978 Nov 2;91(1):57–63. doi: 10.1111/j.1432-1033.1978.tb20936.x. [DOI] [PubMed] [Google Scholar]
- Lipschultz C. A., Gantt E. Association of phycoerythrin and phycocyanin: in vitro formation of a functional energy transferring phycobilisome complex of Porphyridium sordidum. Biochemistry. 1981 Jun 9;20(12):3371–3376. doi: 10.1021/bi00515a010. [DOI] [PubMed] [Google Scholar]
- Porter G., Tredwell C. J., Searle G. F., Barber J. Picosecond time-resolved energy transfer in Porphyridium cruentum. Part I. In the intact alga. Biochim Biophys Acta. 1978 Feb 9;501(2):232–245. doi: 10.1016/0005-2728(78)90029-4. [DOI] [PubMed] [Google Scholar]
- Redlinger T., Gantt E. A M(r) 95,000 polypeptide in Porphyridium cruentum phycobilisomes and thylakoids: Possible function in linkage of phycobilisomes to thylakoids and in energy transfer. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5542–5546. doi: 10.1073/pnas.79.18.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redlinger T., Gantt E. Phycobilisome Structure of Porphyridium cruentum: POLYPEPTIDE COMPOSITION. Plant Physiol. 1981 Dec;68(6):1375–1379. doi: 10.1104/pp.68.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle G. F., Barber J., Porter G., Tredwell C. J. Picosecond time-resolved energy transfer in Porphyridium cruentum. Part II. In the isolated light harvesting complex (phycobilisomes). Biochim Biophys Acta. 1978 Feb 9;501(2):246–256. doi: 10.1016/0005-2728(78)90030-0. [DOI] [PubMed] [Google Scholar]
- Taylor F. J. Symbionticism revisited: a discussion of the evolutionary impact of intracellular symbioses. Proc R Soc Lond B Biol Sci. 1979 Apr 11;204(1155):267–286. doi: 10.1098/rspb.1979.0027. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Foster J. A., Brown A. S., Franzblau C. The alpha and beta subunits of Cyanidium caldarium phycocyanin: properties and amino acid sequences at the amino terminus. Biochemistry. 1975 Jan 28;14(2):268–274. doi: 10.1021/bi00673a012. [DOI] [PubMed] [Google Scholar]
- Troxler R. F., Greenwald L. S., Zilinskas B. A. Allophycocyanin from Nostoc sp. phycobilisomes. Properties and amino acid sequence at the NH2 terminus of the alpha and beta subunits of allophycocyanins I, II, and III. J Biol Chem. 1980 Oct 10;255(19):9380–9387. [PubMed] [Google Scholar]
- Williams R. C., Gingrich J. C., Glazer A. N. Cyanobacterial phycobilisomes. Particles from Synechocystis 6701 and two pigment mutants. J Cell Biol. 1980 Jun;85(3):558–566. doi: 10.1083/jcb.85.3.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams V. P., Freidenreich P., Glazer A. N. Homology of amino-terminal regions of C-phycocyanins from a prokaryote and a eukaryote. Biochem Biophys Res Commun. 1974 Jul 24;59(2):462–466. doi: 10.1016/s0006-291x(74)80002-1. [DOI] [PubMed] [Google Scholar]
- Yamanaka G., Glazer A. N., Williams R. C. Cyanobacterial phycobilisomes. Characterization of the phycobilisomes of Synechococcus sp. 6301. J Biol Chem. 1978 Nov 25;253(22):8303–8310. [PubMed] [Google Scholar]
- de Marsac N. T., Cohen-bazire G. Molecular composition of cyanobacterial phycobilisomes. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1635–1639. doi: 10.1073/pnas.74.4.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]