Abstract
Background
Obesity increases the risk of fatty liver disease and liver cancer. There are several models of obesity-associated hepatocellular carcinoma, but tumor development in these models is slow.
Materials and methods
We investigated Zucker fatty rats, a model of obesity and insulin resistance, to discover if diethylnitrosamine (DEN), a potent liver carcinogen, might enhance liver carcinogenesis. We also investigated the effect of branched chain amino acids (BCAA) against the development of liver cancer.
Results
Incidence and number of hepatocellular carcinomas and adenomas were significantly greater in DEN-treated Zucker fatty rats than in DEN-treated lean rats. All treated Zucker fatty rats developed hepatocellular carcinoma within 16 weeks. Long-term BCAA supplementation significantly reduced expression of CyclinD1, PCNA, thymidine kinase, Bcl-2, and GST-p and increased expression of p21 in the liver. Furthermore, BCAA treatment significantly reduced the area of GST-p positive foci.
Conclusion
Long-term BCAA treatment may induces cell cycle arrest and apoptotic induction, thus suppressing pre-neoplastic lesions.
Keywords: BCAA, hepatocellular carcinoma, obesity
Abbreviations: DEN, diethylnitrosamine; BCAA, branched chain amino acids; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; DTT, dithiothreitol; TK, thymidine kinase; PCNA, proliferation cell nuclear antigen; GST-p, glutathione S-transferase P-form; FLS, fatty liver shionogi
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of global cancer-related deaths.1 Its prognosis is very poor, because conventional chemotherapy is usually inefficient2 and relapse occurs with high frequency. Therefore, chemoprevention could play an important role in the therapeutic strategy for this disease.
Major risk factors for HCC include infection with Hepatitis B virus (HBV) or Hepatitis C virus (HCV), alcoholic liver disease, and nonalcoholic steatohepatitis.3 Previous studies have suggested that host factors, such as sex, age, alcohol consumption, smoking, steatosis, diabetes mellitus,4 and obesity5 are important risk factors for HCC. The prevalence of obesity is increasing worldwide, and these trends are likely to lead to higher HCC disease burden in the future. There are several animal models of obesity-dependent HCC,6,7 but tumor development is slow.
Diethylnitrosamine (DEN) is a potent hepatocarcinogenic dialkyl nitrosamine used in animal models of HCC. Among several chemically induced, genetically modified mice, DEN-induced HCC was most similar to the expression patterns of the poor survival group of human HCCs.8 Therefore, we treated Zucker fatty rats with DEN; and then, using this model, we estimated the effect of long-term BCAA administration, which significantly reduced the risk of HCC in cirrhotic patients with a BMI of ≥25.9 The mechanism by which BCAA reduces the risk of HCC in cirrhotic patients is not clear; however, Muto et al suggested the effect of BCAA on glucose metabolism may reduce the incidence of liver cancer in cirrhotic patients with a BMI score of 25 or higher, since they may have a particularly higher incidence of hyperinsulinemia and peripheral insulin resistance. This rat model does not develop cirrhosis, but exhibits insulin resistance and hyperinsulinemia; therefore, we selected this model to evaluate tumor suppression by long-term BCAA administration.
Materials and methods
Animals
Male 8 week-old Zucker fatty rats and male 8 week-old Zucker lean rats, all purchased from Charles River Laboratories (Charles River Laboratories, Japan), were used in this study after 2 weeks acclimatization. The animal facilities and protocol were reviewed and approved by the Institutional Animal Care and Use Committee of Ajinomoto Co., Inc. All animals were maintained at 23 ± 2 °C on a 12 h light–dark cycle. They were provided a commercial diet (CRF-1, Oriental Yeast Co., Ltd., Tokyo, Japan) and water ad libitum.
Carcinogen-induced Hepatocellular Carcinoma
Diethylnitrosamine (0.04%, DEN; Sigma Chemical Co, St. Louis, MO, USA) was continuously administered via drinking water for 4 weeks, beginning at age 10 weeks. Rats were sacrificed at age 30 weeks to investigate liver tissues and collect plasma samples. The weight of each rat was measured before sacrifice and the liver weight, number, and diameter of all macroscopically visible liver tumors were recorded. Liver tissues were fixed in 10% buffered formalin for hematoxylin-eosin and immunohistochemical staining. A portion of each tissue was snap-frozen in liquid nitrogen and stored at −80 °C for mRNA analysis.
Branched Chain Amino Acids and Casein Supplementation
Sixteen Zuker fatty rats were divided into 2 groups matched for body weight. Supplementation with 3% BCAA (w/w, Ajinomoto Co., LTD, Tokyo, Japan) (n = 8) or 3% casein (w/w, Oriental Yeast Co., LTD, Tokyo, Japan) (n = 8) was provided with the commercial diet, CRF-1 (Charles River Laboratories Japan, INC., Kanagawa, Japan) for 20 weeks beginning at age 10 weeks. The BCAA composition (Leucine:Isoleucine:Valine = 2:1:1.2) was the same as the clinical dosage used for the treatment of decompensated liver cirrhosis in Japan.10
Measurement of Plasma Biochemical Makers and Liver Triglycerides
Plasma ALT, AST, γ-GTP, and triglycerides (TG) were determined using the multilayer analytical slide method in a Fuji Dri-Chem 5500 analyzer (Fuji Photo Film, Tokyo, Japan).
Liver TG were extracted with chloroform/methanol by the modified Folch method11 and measured using the TG E-test WAKO (Wako Pure Chemical Industries, Ltd. Osaka, Japan).
Real-time Reverse-transcription Polymerase Chain Reaction for mRNA in Liver Tissue
RNA was isolated from pieces of liver tissue (ca. 100 mg) with ISOGEN reagents (Nippon Gene, Japan) according to the manufacturer's instructions. One microgram of RNA was used to synthesize cDNA using first-strand buffer, dithiothreitol (DTT), oligo (dT) primer, recombinant ribonuclease inhibitor, and RNase H–reverse transcriptase from Gibco BRL (Life Technologies GmbH, Karlsruhe, Germany), and deoxynucleoside triphosphates from Invitrogen (Groningen, The Netherlands). For real-time reverse-transcription polymerase chain reaction (RT-PCR), we used the Light Cycler Fast Start DNA Master SYBR Green I method (Roche Diagnostics GmbH, Mannheim, Germany), and cDNA was amplified using an OPTICON (MJ Research). Primers were selected for rat GAPDH, CyclinD1, PCNA, Bcl-2, TK, p21, and GST-p.
GAPDH: Forward 5′-GATCTCGCTCCTGGAAGATG-3′ Reverse 5′-ATGACTCTACCCACGGCAAG-3′ CyclinD1: Forward 5′-GGAGATGTGGGTCTCCTTGA-3′ Reverse 5′-GCAAGAATGTGCCAGACTCA-3′ PCNA: Forward 5′-TTATTTGGCTCCCAAGATCG-3′ Reverse 5′-CATCTCAGAAGCGATCGTCA-3′ Bcl-2: Forward 5′-AGTCTTTCCGACCAAGAGCA-3′ Reverse 5′-GCCGAACCACAAAGAGAAAG-3′ TK: Forward 5′-TGCCAGGAGAGTCAGGAGAT-3′ Reverse 5′-TCTGAGCCGTTTCCTCAACT-3′ p21: Forward 5′-GACATCTCAGGGCCGAAAAC-3′ Reverse 5′-CGGCGCTTGGAGTGATAGAA-3′ GST-p: Forward 5′-GCTCCCCAAGTTTGAAGATG-3′ Reverse 5′-AGCCTCCTTCTGGTCTTTCC-3′.
Forty cycles of real-time RT-PCR were performed as follows: denaturation, 95 °C, 15 s; primer annealing and polymerization 60 °C, 60 s. The relative mRNA expression level of the these gene were estimated using GAPDH as the reference gene. We used delta–delta Ct method. Ct value of each gene was divided by average of Ct value of GAPDH.
Immunohistochemistry
Immunohistochemical staining with antibody against GST-p was performed at Biopathology Institute Co., Ltd. (Ooita, Japan). The working titer of GST-p antibody (Medical & Biological laboratories Co., LTD., Nagoya, Japan) was 1:1000. The area of GST-p positive pre-neoplastic foci was measured using WinROOF image processing software (Mitani Corp., Tokyo, Japan), and the ratio of the immunostained area to the corresponding area was calculated and expressed as a percentage of the ratio in the control group.
Statistical Analysis
All results are presented as the mean ± S.E.M. Statistical analysis of differences between mean values was assessed by Student's t-test. Differences were defined as significant at P < 0.05, 0.01, 0.001.
Results
Diethylnitrosamine-induced Hepatocarcinogenesis in Zucker Fatty and Lean Rats
We sacrificed Zucker fatty and lean rats 20 weeks after DEN administration. Tumors >2 cm in diameter (Figure 1A) and an incidence of 45.5% was observed in 11 Zucker fatty rats; in contrast tumor development was observed in only 1 of 8 Zucker lean rats. Although there were 5 Zucker fatty rats with pulmonary metastases, no lean rats exhibited metastases. The number of hepatic tumors in the liver of DEN-treated Zucker fatty rats was significantly greater than in Zucker lean rats (Figure 1D). Surprisingly, the incidence of hepatocellular carcinoma and adenoma in Zucker fatty rats was 100% (Figure 1E).
Figure 1.
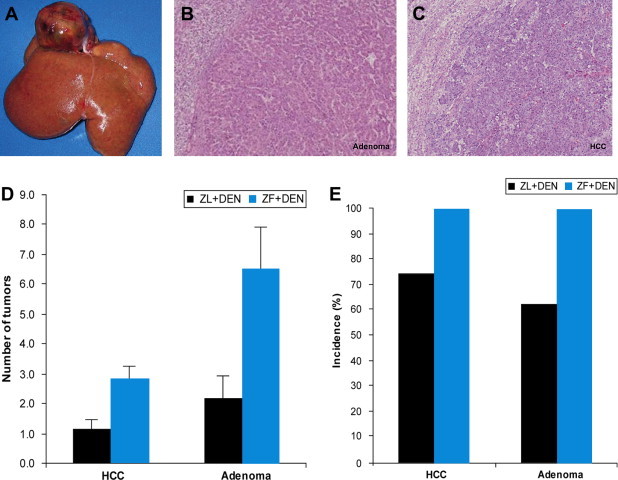
Development of hepatic tumors within 4 weeks DEN treatment (A) Macroscopic findings of the liver of Zucker fatty rat after 16 weeks DEN treatment. (B), (C) Microscopic findings of the liver of Zucker fatty rat after 16 weeks DEN treatment. (D), (E) Incidence and multiplicity of carcinoma and adenoma in the liver.
Next, we examined how DEN affected the liver, particularly in the context of liver toxicity (ALT, AST, γ-GTP, liver weight) and lipids (liver TG and plasma TG). Body weight, liver weight, plasma TG, plasma T-CHO, and liver TG of DEN-treated Zucker fatty rats were significantly increased and plasma albumin was significantly reduced in comparison to DEN-treated Zucker lean rats (Table 1).
Table 1.
Body weight, liver weight, liver TG, and serum marker of liver function in ZL + DEN and ZF + DEN.
| ZL + DEN | ZF + DEN | |
|---|---|---|
| Body Weight (g) | 498.2 ± 9.0 | 765.6 ± 27.4∗∗∗ |
| Liver weight (g) | 15.6 ± 0.6 | 45.7 ± 5.1∗∗∗ |
| ALT (U/L) | 65.5 ± 4.6 | 385.0 ± 269.7 |
| AST (U’L) | 114.0 ± 13.2 | 629.5 ± 391.3 |
| ALB (g/dl) | 4.1 ± 0.1 | 3.3 ± 0.1∗∗∗ |
| Plasma TG (mg/dl) | 120.2 ± 7.9 | 1650.0 ± 624.9∗∗ |
| Plasma T-CHO (mg/dl) | 153.9 ± 7.9 | 516.3 ± 105.7∗∗∗ |
| Liver TG (mg/g tissue) | 22.4 ± 4.0 | 61.0 ± 13.3∗∗ |
Data are expressed as mean ± SE.
**P < 0.01, ***P < 0.001 vs. ZL + DEN.
Effect of Branched Chain Amino Acids in Diethylnitrosamine induced Hepatocellular Carcinoma model
Next, we estimated the chemopreventive effect of BCAA in this model. The mean tumor size in BCAA-treated rats was reduced in comparison to casein-treated rats (211.2 ± 88.7 vs. 95.5 ± 18.5 mm3, mean ± S.E. P = 0.13), but tumor incidence did not differ between groups (data not shown). After 20 weeks treatment, body weight, liver weight, plasma ALT, plasma AST, plasma TG, plasma T-CHO, and liver TG did not differ between groups, but plasma γ-GTP was significantly reduced in BCAA-treated rats (Table 2). Next, we investigated gene expression in the liver (Figure 2). Expression of cyclin D, which mediates cell cycle progression, was reduced in the liver of BCAA-treated vs. casein-treated rats. Thymidine kinase (TK), which has a key function in the synthesis of DNA, was also reduced in the liver of BCAA-treated rats. Proliferation cell nuclear antigen (PCNA), a marker of the DNA synthesis phase of the cell cycle, was reduced in the liver of BCAA-treated rats. p21, a regulator of cell cycle progression that prevents cell proliferation, was enhanced in the liver of BCAA-treated vs. casein-treated rats. Bcl-2, an apoptosis regulator, was reduced in the liver of BCAA-treated rats. Glutathione S-transferase P-form (GST-p), a marker of pre-neoplastic foci, was reduced in the liver of BCAA-treated rats (Figure 3).
Table 2.
Effect of BCAA treatment on body weight, liver weight, and serum markers of liver function in Zucker fatty rats.
| ZF + DEN/casein | ZF + DEN/BCAA | |
|---|---|---|
| Body weight (g) | 671.2 ± 16.2 | 718.3 ± 18.5 |
| Liver weight (g) | 41.1 ± 2.8 | 38.5 ± 2.0 |
| ALT (U/L) | 202.0 ± 23.6 | 174.9 ± 26.1 |
| AST (U/L) | 257.5 ± 19.0 | 237.1 ± 39.9 |
| γ-GTP (U/L) | 148.6 ± 14.3 | 93.9 ± 19.1∗ |
| Plasma TG (mg/dl) | 537.7 ± 180.6 | 268.4 ± 67.5 |
| Plasma T-CHO (mg/dl) | 354.5 ± 36.0 | 322.3 ± 17.9 |
| Liver TG (mg/g tissue) | 89.8 ± 4.4 | 96.7 ± 8.8 |
Data are expressed as mean ± SE.
∗P < 0.05 vs. ZL + DEN.
Figure 2.
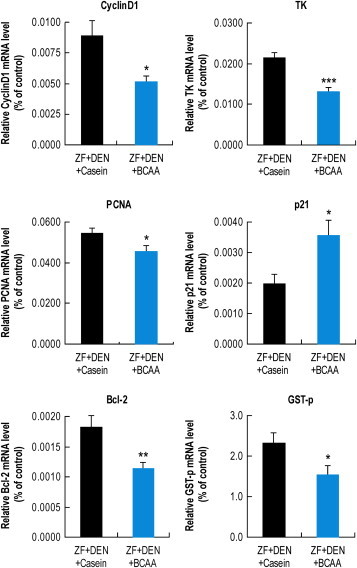
Effect of BCAA on gene expression in the liver Liver gene expression with casein or BCAA supplementation. GAPDH served as internal control. Ct value of each gene was divided by average of Ct value of GAPDH. Results are the mean ± SE and are expressed relative to the control. *P < 0.05, **P < 0.01, ***P < 0.001 vs. ZF + DEN/casein.
Figure 3.
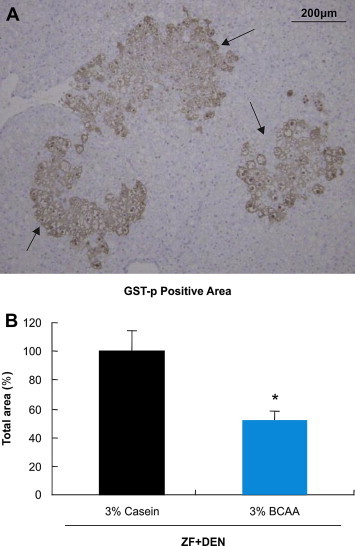
Immunohistochemical staining of GST-p positive foci (A) GST-p stained Liver sections of Zucker Fatty rat after 16 weeks DEN treatment (B) Estimated cell area of pre-neoplastic foci. The ratio of the immunostained area to the corresponding total area was calculated and expressed as a percentage of the ratio in the control group. *P < 0.05 vs. ZF + DEN/casein.
Discussion
In vivo Evaluation of Diethylnitrosamine-induced Liver Tumors in Zucker Fatty Rats
A lack of leptin response is characteristic of obese Zucker fatty rats,12 which bear a mutation (fa) in the leptin receptor gene.13 Regardless of whether Zucker fatty rats develop insulin resistance and liver steatosis, there is a low incidence of liver tumor development at <6 months of age.
DEN is a potent hepatocarcinogenic nitrosamine; therefore, DEN-induced HCC animal models are one of the most accepted and widely used experimental models for hepatocarcinogenesis. The natural history of cancer development involves several stages including initiation and promotion, and initiation is linearly dependent on the applied dose of DEN.14 In this study, 0.04% DEN was provided in drinking water for only 4 weeks without promotion treatment (no phenobarbital treatment); however, 100% of the rats developed HCC within only 20 weeks (Figure 1E). Previous DEN-induced hepatocarcinogenesis obese animal models require more time for tumor development and the incidence of HCC is lower. For example, less than 27% of DEN-treated db/db mice developed HCC by 34 weeks,15 and 33% of DEN-treated Fatty liver Shionogi (FLS) mice developed HCC by 26 weeks.16
Leptin receptor-mediated signaling is important to hepatic fibrosis. Several studies showed that hepatic fibrosis induced by chemical compounds17 or by feeding a choline-deficient, l-amino acid-defined diet18 was almost completely prevented in Zucker fatty rats. In these studies, hepatocarcinogenesis of Zucker fatty rats was also impaired in comparison to Zucker lean rats. These results are different from our observations, but in the liver cancer models, DEN induces hepatocellular carcinoma without liver fibrosis. Therefore, leptin receptor-mediated signaling may not mediate inhibition of hepatocarcinogenesis induced by DEN in Zucker fatty rats.
The reasons why DEN-treated Zucker fatty rats developed tumors more easily than other models are not clear, but the difference may correlate with the higher concentration of plasma insulin in Zucker Fatty rats than in FLS19 or db/db mice.20 Ours could be a good model of obesity-induced hepatocellular carcinoma, because the high frequency of carcinogenesis reduces the number of experimental animals required for experimentation. Furthermore, results can be obtained more quickly, as carcinogenesis occurs in less than 20 weeks.
Effect of Branched Chain Amino Acids on Diethylnitrosamine-Induced Liver Tumor Zucker Fatty Rats
In Japan, BCAA granules are indicated for decompensated cirrhosis patients with hypoalbuminemia despite adequate dietary intake, and oral administration of BCAA is widely used to recover hypoalbuminemia. Long-term BCAA treatment in patients with liver cirrhosis improved event-free survival time and quality of life in several randomized trials.21,22 Furthermore, long-term oral supplementation with BCAA granules inhibits liver carcinogenesis in overweight and obese patients with liver cirrhosis.9 Long-term administration of BCAA improves insulin resistance15 and inhibits VEGF production,23 which may therefore inhibit carcinogenesis.
Immediately after DEN administration, hepatic damage (the degree of plasma ALT and γ-GTP elevation) did not differ between the BCAA and casein groups (data not shown), so BCAA did not affect the hepatic metabolism and toxicity of DEN.
Pre-neoplastic lesions in the liver induced by chemical carcinogens, which are usually detected by glutathione S-transferase placental form (GST-P),24 develop into HCC in rodents.25 When BCAA was administered for 20 weeks in DEN-treated Zucker fatty rats, GST-p transcripts and GST-p protein-positive areas in the liver were reduced. Therefore, BCAA may affect tumor development.
Hepatic steatosis is a risk factor for HCC in patients with chronic HCV infection.26 Long-term supplementation of Zucker fatty rats with BCAA improved hepatic steatosis and reduced hepatic triglycerides.27 Diabetes is also associated with increased levels of insulin and insulin-like growth factors, which are potential cancer-promoting factors.
In this model, long-term BCAA administration did not improve insulin resistance (data not shown). Hagiwara and group reported that BCAA inhibited insulin-dependent hepatoma cell growth in vitro.28 Zucker fatty rats present with hyperinsulinemia, so BCAA may inhibit insulin-dependent tumor cell growth. Future studies will explore the mechanism of tumor growth inhibition by BCAA.
Conflicts of interest
The authors have no conflict of interest to declare.
References
- 1.Herszenyi L., Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci. 2010;14:249–258. [PubMed] [Google Scholar]
- 2.Cao H., Phan H., Yang L.X. Improved chemotherapy for hepatocellular carcinoma. Anticancer Res. 2012;32:1379–1386. [PubMed] [Google Scholar]
- 3.El-Serag H.B. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. doi: 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag H.B., Tran T., Everhart J.E. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460–468. doi: 10.1053/j.gastro.2003.10.065. [DOI] [PubMed] [Google Scholar]
- 5.Saunders D., Seidel D., Allison M., Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma—epidemiological evidence. Aliment Pharmacol Ther. 2010;31:1051–1063. doi: 10.1111/j.1365-2036.2010.04271.x. [DOI] [PubMed] [Google Scholar]
- 6.Hill-Baskin A.E., Markiewski M.M., Buchner D.A. Diet-induced hepatocellular carcinoma in genetically predisposed mice. Hum Mol Genet. 2009;18:2975–2988. doi: 10.1093/hmg/ddp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park E.J., Lee J.H., Yu G.Y. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J.S., Chu I.S., Mikaelyan A. Application of comparative functional genomics to identify best-fit mouse models to study human cancer. Nat Genet. 2004;36:1306–1311. doi: 10.1038/ng1481. [DOI] [PubMed] [Google Scholar]
- 9.Muto Y., Sato S., Watanabe A. Long-Term Survival Study (LOTUS) Group: overweight and obesity increase the risk for liver cancer in patients with liver cirrhosis and long-term oral supplementation with branched-chain amino acid granules inhibits liver carcinogenesis in heavier patients with liver cirrhosis. Hepatol Res. 2006;35:204–214. doi: 10.1016/j.hepres.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Sato S., Watanabe A., Muto Y. Clinical comparison of branched-chain amino acid (l-Leucine, l-Isoleucine, l-Valine) granules and oral nutrition for hepatic insufficiency in patients with decompensated liver cirrhosis (LIV-EN study) Hepatol Res. 2005;31:232–240. doi: 10.1016/j.hepres.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Folch J., Lees M., Sloane Stanley G.H. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 12.Cusin I., Rohner-Jeanrenaud F., Stricker-Krongrad A., Jeanrenaud B. The weight-reducing effect of an intracerebroventricular bolus injection of leptin in genetically obese fa/fa rats: reduced sensitivity compared with lean animals. Diabetes. 1996;45:1446–1450. doi: 10.2337/diab.45.10.1446. [DOI] [PubMed] [Google Scholar]
- 13.Phillips M.S., Liu Q., Hammond H.A. Leptin receptor missense mutation in the fatty Zucker rat. Nat Genet. 1996;13:18–19. doi: 10.1038/ng0596-18. [DOI] [PubMed] [Google Scholar]
- 14.Travis C.C., McClain T.W., Birkner P.D. Diethylnitrosamine-induced hepatocarcinogenesis in rats: a theoretical study. Toxicol Appl Pharmacol. 1991;109:289–304. doi: 10.1016/0041-008x(91)90176-f. [DOI] [PubMed] [Google Scholar]
- 15.Iwasa J., Shimizu M., Shiraki M. Dietary supplementation with branched-chain amino acids suppresses diethylnitrosamine-induced liver tumorigenesis in obese and diabetic C57BL/KsJ-db/db mice. Cancer Sci. 2010;101:460–467. doi: 10.1111/j.1349-7006.2009.01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iwai S., Murai T., Makino S. High sensitivity of fatty liver Shionogi (FLS) mice to diethylnitrosamine hepatocarcinogenesis: comparison to C3H and C57 mice. Cancer Lett. 2007;246:115–121. doi: 10.1016/j.canlet.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Ikejima K., Takei Y., Honda H. Leptin receptor-mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122:1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- 18.Kitade M., Yoshiji H., Kojima H. Leptin-mediated neovascularization is a prerequisite for progression of nonalcoholic steatohepatitis in rats. Hepatology. 2006;44:983–991. doi: 10.1002/hep.21338. [DOI] [PubMed] [Google Scholar]
- 19.Soga M., Hashimoto S., Kishimoto Y., Hirasawa T., Makino S., Inagaki S. Insulin resistance, steatohepatitis, and hepatocellular carcinoma in a new congenic strain of Fatty Liver Shionogi (FLS) mice with the Lep(ob) gene. Exp Anim. 2010;59:407–419. doi: 10.1538/expanim.59.407. [DOI] [PubMed] [Google Scholar]
- 20.Djiogue S., Nwabo Kamdje A.H., Vecchio L. Insulin resistance and cancer: the role of insulin and IGFs. Endocr Relat Cancer. 2013;20:R1–R17. doi: 10.1530/ERC-12-0324. [DOI] [PubMed] [Google Scholar]
- 21.Muto Y., Sato S., Watanabe A. Long-Term Survival Study (LOTUS) Group: effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 22.Marchesini G., Bianchi G., Merli M. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 23.Yoshiji H., Noguchi R., Kitade M. Branched-chain amino acids suppress insulin-resistance-based hepatocarcinogenesis in obese diabetic rats. J Gastroenterol. 2009;44:483–491. doi: 10.1007/s00535-009-0031-0. [DOI] [PubMed] [Google Scholar]
- 24.Sato K. Glutathione S-transferases and hepatocarcinogenesis. Jpn J Cancer Res. 1988;79:556–572. doi: 10.1111/j.1349-7006.1988.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito N., Imaida K., Tamano S., Hagiwara A., Shirai T. Medium-term bioassays as alternative carcinogenicityi test. J Toxicol Sci. 1998;23:103–106. doi: 10.2131/jts.23.supplementii_103. [DOI] [PubMed] [Google Scholar]
- 26.Ohata K., Hamasaki K., Toriyama K. Hepatic steatosis is a risk factor for hepatocellular carcinoma in patients with chronic hepatitis C virus infection. Cancer. 2003;97:3036–3043. doi: 10.1002/cncr.11427. [DOI] [PubMed] [Google Scholar]
- 27.Ishizaki S. BCAA granules and hepatic metabolism. Bio Clinica. 2009;24:70–74. [Google Scholar]
- 28.Hagiwara A., Nishiyama M., Ishizaki S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J Cell Physiol. 2012;227:2097–2105. doi: 10.1002/jcp.22941. [DOI] [PubMed] [Google Scholar]


