Abstract
Background
Heart failure is common and highly morbid in older adults. Performance measurement systems for this condition may work best when they account for the reasons why physicians do not provide guideline-recommended interventions.
Objective
To develop a conceptual framework for understanding the proximate, patient-centered reasons why physicians do not prescribe ACE inhibitors and beta blockers to patients with heart failure.
Design
Focus group study using a two-stage design. First, we asked participants to describe reasons for not prescribing ACE inhibitors and beta blockers to patients with heart failure and impaired ejection fraction. Second, we asked groups to develop concept maps that organized these reasons into categories and described the relationships between these categories.
Participants
Seven focus groups comprising 31 academically-affiliated clinicians of different specialties and levels of training. Participants were recruited via invitations sent to clinicians within each target group.
Approach
We synthesized each group’s concept maps to develop a consensus scheme for categorizing reasons for non-prescribing.
Results
We identified two broad themes. First, clinicians hinted at their own attitudinal barriers to prescribing. However, they framed their comments largely around patient-centered reasons for non-prescribing that arose in individual patient encounters. Second, decision-making about heart failure drug therapy often involved a complex and overlapping series of considerations. Five categories of reasons for not prescribing ACE inhibitors and beta blockers emerged: 1) adverse effects of drug therapy, 2) non-adherence to therapeutic and monitoring plan, 3) patient preferences and beliefs, 4) co-management and transitions of care, and 5) prioritization and patient benefit.
Conclusions
Physician reasons for not prescribing guideline-recommended drugs for heart failure are complex but can be organized into a useful taxonomy. This taxonomy may be helpful for performance measurement and quality improvement programs that seek to understand and account for reasons for physician non-adherence to guidelines.
Keywords: Decision making, Guideline adherence, Heart failure, Aged, Physician’s Practice Patterns, Angiotensin-Converting Enzyme Inhibitors, Adrenergic beta-Antagonists
INTRODUCTION
Performance measurement programs have been criticized for translating clinical practice guidelines into performance measures in an overly simplistic manner that does not account for the varying circumstances of individual patients.1–2 In response, a growing literature has worked to improve performance measurement by exploring the actual content of clinical encounters, with the aim of understanding reasons why clinicians may not meet performance measurement-based goals. For example, Kerr and colleagues found that many patients who did not meet laboratory-based targets for lipid and diabetes management were in the midst of treatment intensification for these conditions, while “failures” to control blood pressure to recommended levels were often attributable to uncertainty over the patient’s true baseline blood pressure.3–4 Such issues may be particularly common in older adults, given their higher prevalence of several reasons for avoiding guideline-recommended drugs, including justifiable reasons (e.g., adverse drug reactions, goals of care) and problematic ones (e.g., unexplained undertreatment).
Efforts to study and improve assessments of care quality would benefit from a systematic approach to understanding physician reasons for not providing guideline-recommended interventions. However, existing frameworks from the literature on guideline adherence are not well-suited to this task. Much of the conceptual work in this area has focused on underlying physician and system barriers to guideline adherence, for example, physician knowledge of guidelines, physician attitudes toward guideline recommendations, and systems barriers to implementing these recommendations.5–10 In contrast, less developmental work has focused on the proximate reasons that explain prescribing decisions at individual patient encounters. Such models are needed to better understand prescribing decisions for individual patients, and in doing so help to improve both the measurement of care quality and efforts to improve it.
We encountered this gap in conceptual understanding as we prepared for a research study on physician reasons for not prescribing guideline-recommended medications to patients with heart failure. In response, we conducted a focus group study to develop a conceptual framework for understanding and categorizing these reasons. In doing so, our goal was to create a model that could be used to study guideline adherence and to evaluate and improve performance measurement programs based on guideline recommendations.
METHODS
Focus group recruitment and composition
We conducted 7 focus groups with practicing physicians and residents between May and October, 2008. We recruited participants from a range of specialties and levels of training from four sites: a university medical center, VA medical center, safety net hospital, and community-based clinic, all affiliated with a school of medicine. For each target group, we sent email invitations to each potentially eligible physician. Among those who agreed to participate, we identified a meeting time that would allow as many as possible to participate. To be included, participants needed to have a current practice in an ambulatory setting in which they encountered patients with heart failure.
In order to reduce the influence of physician seniority and specialty training on the discussion, we stratified the groups so that each group was homogenous by site, specialty, and level of training. For example, one group comprised staff geriatricians from the VA medical center, another group comprised family practice residents from the safety-net hospital, and so forth.
Focus group methods
Before each group began, participants completed a brief demographic questionnaire. Two of the authors moderated the groups (MS, SK) and two took notes and made audio recordings (SP, PK). Focus group meetings lasted for 1 to 1.5 hours and were divided into two sections. In the first section, we used semi-structured methods to elicit reasons for not prescribing ACE inhibitors (and/or angiotensin receptor blockers) and beta blockers to patients with heart failure and impaired systolic function, as recommended by heart failure guidelines.11 (Of note, we did not otherwise review guideline recommendations with the groups, nor did we ask participants about their familiarity with guidelines). We encouraged participants to focus on the physician’s decision to not prescribe a drug, rather than on reasons why a patient may not take a drug which they were prescribed. Where necessary, we used question probes to elicit discussion on key topic areas that did not emerge spontaneously from conversation. We recorded reasons generated by the group on slips of paper and taped them to a whiteboard.
Following the idea-generation phase, we engaged each group in a concept-mapping exercise. In this exercise, we asked the group to arrange the slips of paper, each representing a single reason for not prescribing guideline-recommended medications, into clusters on the whiteboard. For example, participants in several groups organized reasons for not prescribing related to adverse drug reactions (e.g., hyperkalemia, cough, bradycardia) into a single cluster, and reasons related to problems accessing health care (e.g., inability to pay for care, lack of a regular source of care) into another cluster. Decisions on how to categorize reasons were made by group consensus. During this process, we asked each group to identify closely related concepts and to indicate whether these would be better represented as a single concept. Finally, we asked groups to identify linkages between clusters, including which clusters were related more closely or more distantly to one another. We used a different approach in our first focus group (asking the group to comment on a draft taxonomy based on previous literature). Because the first group generated new categories and indicated relationships among categories that had not been included in the original taxonomy, we transitioned to the concept mapping technique in subsequent groups in order to more fully capture and understand the perceived relationships among new categories.
Analyses
Our analytic plan proceeded in several steps. First, 4 authors (MS, SP, PK, SK) independently reviewed the concept maps created by each group, and through a process of iterative discussion we developed a single, consensus concept map that synthesized the maps of each group, further refined by our judgment. Based on this map, we developed an approach to coding the group discussions. Two authors (MS, SK) read through transcripts and developed operational definitions for the coding guide. Then, 3 authors (MS, SP, PK) independently coded transcripts from the first three groups, comparing coding results and refining the coding scheme after each group until we had reached a consistent and inclusive set of coding guidelines. Using this scheme, these 3 authors independently coded results for all 7 groups.
Using our coding system, we identified blocks of discussion that reflected a single category or sub-category of reasons for not prescribing guideline-recommended drugs. A discussion was defined as a conversation focusing on a specific theme up to the point that a new topic was introduced. For example, a discussion in which participants serially noted different clinical contraindications to prescribing would be counted as a single discussion under the category “adverse effects of drug therapy.” In certain cases where conversation contained closely interwoven comments related to two categories, we counted this as a single discussion but separately coded each category. Disagreements between the 3 primary reviewers were resolved by consensus; where consensus could not be reached, the final coding decision was made by the senior author (SK). After finishing the coding, we tabulated the number of times each category and sub-category was discussed. We then re-reviewed discussions in the categories where these counts suggested a potential deficit in conceptual clarity, and based on this review made a final change to our consensus concept map.
RESULTS
I. Characteristics of focus groups and participants
Thirty-one physicians participated in 7 focus groups, including clinicians in general internal medicine (3 groups), family practice (2 groups), geriatrics (1 group), and cardiology (1 group). Four groups were composed of staff physicians, and 3 of residents; the distribution of practice sites included a university medical center (1 group), VA hospital (4 groups), safety-net public hospital (1 group), and community-based clinic (1 group). Characteristics of focus group participants are shown in Table 1. The median age of participants was 31 years, and 54% were women. Participants spent a median of 2 half-days per week in ambulatory care. This relatively low number reflects the composition of the groups, including residents (whose schedule predominantly involves inpatient care) and academically-affiliated physicians (who engage in teaching, administration, and research in addition to direct patient care).
Table 1.
Characteristics of focus groups and participants
| Participant characteristics | n (%); or median (interquartile range) (N=31 participants) |
|---|---|
|
| |
| Age | |
| <30 years | 11 (35) |
| 30–44 years | 15 (48) |
| >=45 years | 5 (16) |
|
| |
| Female | 17 (54) |
|
| |
| Professional status | |
| Practicing physician | 13 (42) |
| Resident | 18 (58) |
|
| |
| Number of half-days of clinic per week, median (IQR) | 2 (1–3) |
|
| |
| Estimated percent of clinic patients with heart failure, median (IQR) | 10 (4–20) |
|
| |
| Estimated percent of clinic patients with low income, median (IQR) | 50 (30–85) |
II. Emergent themes
Each group identified 9 to 20 distinct types of reasons for not prescribing an ACE inhibitor or beta blocker to patients with heart failure and impaired ejection fraction. Several over-arching themes emerged. One key finding was that clinician attitudes, beliefs, and practice styles were an important undercurrent to the discussion of reasons for non-prescribing. For example, some clinicians suggested that they were more or less fearful than their colleagues about causing side effects. One primary care physician stated “I’m just nervous about starting a beta-blocker and it needs to be done so carefully.” In contrast, a cardiologist noted ” By the time a patient makes it to us, I don’t know if it’s that we’re more comfortable with slower heart rates … and higher potassiums than the average primary care doctor, but I think that if anything we in the Cardiology Clinic are perhaps a little more likely to try someone on a drug.”
However, such physician-centered perspectives were not commonly articulated. Rather, participants focused mainly on concrete patient and system factors that arose at individual patient encounters as reasons for not prescribing guideline-recommended medications. To expand the example above, participants spent much more time discussing specific clinical contraindications to prescribing (e.g., bradycardia, hyperkalemia) than their own attitudes or fears about prescribing in the face of these contraindications. This focus on the proximate reasons for not prescribing was particularly evident early in the process of analyzing the focus group data, where we roughly divided reasons into “patient-centered reasons” (e.g., proximate reasons related to specific patient clinical or psychosocial attributes) and “physician-centered reasons” (broader issues of physician attitudes, time pressures, co-management, and the like). While it was clear that many physician-centered reasons were present as subtext to the conversations, the great majority of explicit and readily codeable reasons for non-prescribing were described in patient-centered terms.
Another key theme that emerged was complexity, whereby reasons for non-prescribing involved a complex interplay of factors. For example, some participants noted a reluctance to start an ACE inhibitor at hospital discharge if the patient did not have well-established outpatient care, out of concern that the patient might not obtain laboratory-based safety monitoring. Discussions of this topic often included a tightly interwoven commentary on both systems factors (e.g., the difficulty of establishing outpatient care in a timely manner) and patient factors (e.g., the patient’s ability to successfully navigate the health care system to obtain appropriate follow-up).
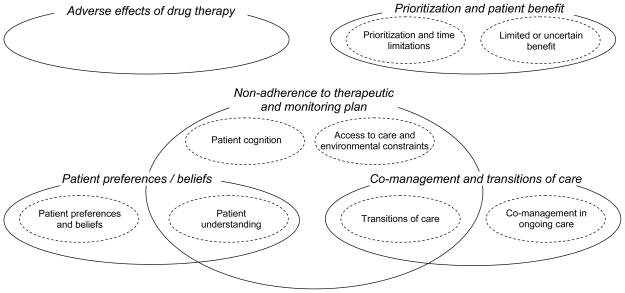
Complexity was also apparent in the finding that many reasons could not be distinctly isolated within a single conceptual category, but instead were shared between two categories. This is represented by areas of overlap in Figure 1. For example, in the sub-category of “patient understanding,” communication issues might underlie decisions to withhold a recommended drug due to difficulty communicating with the patient about how to use and monitor the drug properly (a reason subsumed under the category “non-adherence to therapeutic and monitoring plan”). At other times, communication barriers can interfere with discussions of patients’ beliefs and preferences toward drug use, negatively impacting their willingness to accept a prescription (a reason subsumed under the category “patient preferences and beliefs”).
Figure 1. Concept map.
The figure shows the relationship between the categories and subcategories of reasons for not prescribing guideline-recommended medications in patients with heart failure
Similarly, the complexity of decision-making processes was often manifested in reasons which crossed two or more sub-categories within a larger category. For example, discussions about prescribing to patients with cognitive impairment involved consideration of both the patient’s own capacity to regularly and safely administer medications (patient cognition) and the presence or absence of a caregiver who could facilitate proper medication use (environmental constraints). As noted by one participant, “I have a floridly manic patient who has dementia as well and [was] just unable to take his medications appropriately…so we stopped ACE inhibitors until we could get home care in there…. Now that he has a more structured setting we were able to put several things back on.”
III. Taxonomy of reasons for not prescribing guideline-recommended medications
We identified 5 categories of reasons for not prescribing guideline-recommended medications. These are described below and in Figure 1 and Table 2. The number of conversations involving each category and sub-category are shown in Table 3.
Table 2.
Taxonomy of reasons for non-prescribing – definitions and illustrations
| Category / Subcategory | Definition | Illustration |
|---|---|---|
| 1. Adverse effects of drug therapy | Adverse clinical effects of drug therapy, including adverse drug reactions and drug contraindications | Hyperkalemia on ACE inhibitors |
|
| ||
| 2. Non-adherence to therapeutic and monitoring plan | Improper use of medications, including non-adherence, improper dose and dosing frequency (e.g., with risk of overdose), and problems with followup monitoring. Often discussed as a safety issue | |
| Cognitive capacity | Patient confusion about proper use or monitoring of medications | Cognitive impairment |
| Access to care and environmental constraints | Environmental and systems factors impacting proper use or monitoring of medications | Lack of health insurance impacting ability to pay for medications |
| Patient understanding * | Challenges to communication between patient and their physician or the health system | Poor health systems literacy impacting patient ability to obtain follow-up care |
| Transitions of care * | Challenges to physician-physician communication as patient is transferred from one clinician or care setting to another | Discharge from inpatient to outpatient care with fear that patient will not receive follow-up labs after being started on ACE inhibitor |
| Cross-cutting and non-specific barriers | Reason involving more than one of the above sub-categories, or sufficiently general to prevent classification into a specific sub-category | Cognitive impairment in absence of caregiver to assist with medication administration |
|
| ||
| 3. Patient preferences | Patient reluctance to take medications due to preferences, health beliefs, and goals of care. | |
| Patient preferences and beliefs | Same as general category definition | Patient refusal to add more medications to current regimen |
| Patient understanding * | As above (see “non-adherence to therapeutic and monitoring plan” category) | Language barriers that impede clinician attempts to understand and overcome patient reluctance to use medications |
| Cross-cutting and non-specific barriers | Reason involving more than one of the above sub-categories, or sufficiently general to prevent classification into a specific sub-category | Non-English-speaking patients with non-scientific approach to medicine |
|
| ||
| 4. Co-management and transitions of care | Patient receiving care from more than one physician or health system | |
| Co-management in ongoing care | Delegation of responsibility in ongoing care of a patient, or other failure to coordinate ongoing care. | Primary care physician defers responsibility for heart failure care to patient’s cardiologist |
| Transitions of care * | As above (see “non-adherence to therapeutic and monitoring plan” category) | As above |
| Cross-cutting or non-specific barriers | Reason involving more than one of the above sub-categories, or sufficiently general to prevent classification into a specific sub-category | Receiving physician not understanding reasons why patient is not on a recommended drug |
|
| ||
| 5. Prioritization and patient benefit | Prioritization among competing demands for time, and perceived benefit of the guideline-recommended intervention | |
| Prioritization and time limitations | No long term reason not to prescribe, but cannot practically address recommended medications until other issues have stabilized | Acute medical or psychiatric illness |
| Limited or uncertain benefit | Uncertainty that patient will derive net benefit from drug | Patient with limited life expectancy; patient with borderline or changing ejection fraction |
These sub-categories are shared between 2 categories.
Table 3.
Frequency of discussions of reasons for non-prescribing
| Category / subcategory | # of discussions | |
|---|---|---|
| 1. Adverse effects of drug therapy | 20 | |
|
| ||
| 2. Non-adherence to therapeutic and monitoring plan | 33 | |
| Patient cognition | 15 † | |
| Access to care and environmental constraints | 7 † | |
| Patient understanding* | 2 | |
| Transitions of care | 1 | |
| Cross-cutting and non-specific barriers | 11 | |
|
| ||
| 3. Patient preferences | 17 | |
| Patient beliefs and preferences | 15 | |
| Patient understanding* | 0 | |
| Cross-cutting and non-specific barriers | 2 | |
|
| ||
| 4. Co-management with other providers and health systems | 12 | |
| Co-management in ongoing care | 9 | |
| Transitions of care * | 3 | |
| Cross-cutting and non-specific barriers | 0 | |
|
| ||
| 5. Prioritization / benefit | 20 | |
| Prioritization and time limitations | 13 | |
| Limited or uncertain benefit | 7 | |
These subcategories are shared across more than one category. Counts are allocated to only one category, based on what was considered the “primary” category for that reason.
Three discussions included a combination of patient cognition and access / environmental issues; these discussions are counted in each of these subcategories.
Adverse effects of drug therapy
When asked to list reasons for not prescribing guideline-recommended drugs, each group began by discussing clinical contraindications to drug use. This included adverse reactions to drugs as well as comorbidities that precluded a drug from ever being initiated. Correspondingly, these reasons were among the most commonly mentioned concepts across the 7 focus groups (see Table 2).
Non-adherence to therapeutic and monitoring plan
Issues related to improper use and monitoring of drugs were the most common reasons for non-prescribing cited by focus group participants. This category includes problems with underuse of drugs (e.g., non-adherence), misuse (including inappropriate dosing schedules and the risk of overdose), and lack of follow-up for safety monitoring (e.g., potassium and renal function testing for ACE inhibitors). Issues of complexity were particularly evident in this category, as almost one-half (14 of 33) of reasons for non-prescribing involved more than one sub-category or were sufficiently general to prevent sub-classification.
In these discussions, environmental constraints were commonly cited alongside patient factors. As noted by one physician, “The standard recommendation [for ACE inhibitors] to check the creatinine or potassium in one week is not a benign recommendation in a lot of patients because really for some patients coming here is a major journey.”
Patient preferences and beliefs
A number of focus group participants noted that patient preferences or beliefs sometimes led them to avoid prescribing guideline-recommended medications. The most common of these patient preferences included a general disinclination to take medications or patients’ concern that they were already on too many medications. As one physician reflected, “There are some patients who just philosophically do not like medicines; they want to restrict medicines as much as possible.”
In addition, in two circumstances clinicians noted patients’ reluctance to take medications due to perceived side effects that in the clinician’s judgment were not in fact attributable to the drug.
Co-management with other providers and health systems
Groups discussed issues of co-management and transitions of care in a manner consistent with the health system and care settings in which each group practiced. For example, focus groups of residents (who spend much of their time in inpatient settings) raised questions about safe prescribing as patients were discharged from hospital to home. One resident stated: “The wait list [for a new clinic appointment] could be months…and you start an ACE and suddenly their creatinine hasn’t been checked in four months, and it’s 3.7…It’s in the discharge summary, it’s the plan but knowing that there’s going to be this gap… [Let’s] not put them on something that could potentially kill them in the interim.”
In contrast, VA-based clinicians cited reluctance to prescribe new medications to patients followed by clinicians in the community whose main reason for coming to VA clinics was to obtain drugs at low cost from the VA pharmacy. Similarly, primary care clinicians noted sometimes deferring decision-making to their patients’ cardiologists. As noted by one primary care physician, “I definitely have patients who are followed by Cardiology as well, and if they’re not on a medicine that is a cardiology medicine, sometimes I won’t start it because I feel like Cardiology needs to make that decision.”
Prioritization and benefit
In a number of cases, clinicians noted that they could not realistically prescribe drugs for heart failure until more pressing clinical issues were addressed. In other patients, clinicians perceived little benefit to using ACE-inhibitors or beta blockers, for example patients with limited life expectancy or patients who despite technically meeting criteria for guideline eligibility presented with atypical clinical scenarios. As noted by one clinician, “The treatment of heart failure particularly to prolong, to maximize survival time and not improve function becomes just one of 23 things, not the most important thing that you are trying to manage.”
DISCUSSION
In this focus group study of reasons for not prescribing guideline-recommended medications for patients with heart failure, two broad themes emerged. First, while there was a clear undercurrent of clinician attitudes, beliefs, and practice styles, clinicians focused mainly on the proximate, patient-centered reasons for non-prescribing that arose in individual patient encounters. Second, in many cases the decision to not prescribe involved a complex and overlapping series of reasons. Within these broad themes, we identified 5 categories of reasons why physicians do not prescribe ACE inhibitors and beta blockers to patients with heart failure and impaired systolic function: 1) adverse effects of drug therapy, 2) non-adherence to therapeutic and monitoring plan, 3) patient preferences and beliefs, 4) co-management and transitions of care, and 5) prioritization and patient benefit.
The taxonomy of reasons for not prescribing guideline-recommended drugs that we identified is consistent with prior research. A series of studies using physician interviews, focus groups and surveys have variably identified barriers to guideline adherence that correspond to each of the five categories of reasons for non-prescribing that we identified.5–6, 12–20 Of note, in much of this work patient-centered reasons for non-prescribing were discussed largely in the context of how they intersected with physician-centered attitudes and behavioral styles. For example, the role of adverse drug effects was often discussed in the context of physician fears of causing side effects rather than the specific side effects themselves.6, 15, 19–20 Other work has focused on the intrinsic characteristics of clinicians and their environment, for example finding that older physicians and clinicians in certain practice settings are less likely to provide guideline-recommended treatments.14, 21–22
This body of research provides an important foundation for our work by providing a basis for understanding the wide range of barriers that contribute to physicians not providing guideline-recommended care. However, such approaches are largely based on physician knowledge, attitudes, and health system interactions, and are not well-suited to optimizing performance measurement systems. Rather, performance measurement systems are best served by a framework for assessing the care of individual patients and the specific reasons why a patient may or may not be an appropriate candidate for an intervention.
Unfortunately, many performance measurement systems have lacked this type of underlying framework, relying instead on an approach that measures care patterns without consideration for individual patient circumstances. This approach has been critiqued insofar as it fails to consider that certain physicians and institutions care for more patients in whom the intervention or outcome is more difficult to achieve or not clinically warranted.2, 23 As a result, such performance measures encounter biases in comparing quality across providers or health care systems, and often lack credibility.24
To confront these problems, other measures have been developed that provide an opportunity to exclude from consideration patients who have a contraindication to the recommended intervention or outcome. However, the decision rules that underlie such measures often fail to capture many important reasons for non-prescribing. Although some of this is due to limitations in available data, in other settings it results from inadequate design. For example, in the VA healthcare system, clinicians receive clinical reminders when computer algorithms detect potential problems, such as a patient with ischemic heart disease who is not prescribed a beta blocker. To clear these reminders, clinicians must click one of several checkboxes to indicate how they plan to address the concern or their reason for not doing so. The options presented in this system, which predominantly focus on clinical issues such as drug intolerance or presence of a contraindicating drug, often do not correspond to the actual reason for not providing the intervention.
Our taxonomy could be used to improve the measurement and delivery of quality care by informing the type of data to be collected in performance measurement and clinical reminder systems. This approach would have several potential benefits. These data could be used in decision rules to better differentiate between patients with appropriate vs. inappropriate reasons for not achieving a recommended intervention or outcome, thus improving the quality of measurement and improving clinician buy-in to performance measurement systems.25 In addition, such data would help health system leaders better understand why physicians are not providing recommended services.26 This information could be employed to develop quality improvement programs and systems interventions targeted toward these reasons. For example, if concerns about patient adherence or misuse of drugs commonly arose as reasons for not prescribing, an appropriate solution might be strategically integrating pharmacy services (including medication teaching and adherence and safety aids) into clinics.
Moreover, the optimal use of our type of taxonomy would be to directly link reasons for guideline non-adherence to the supports that would help to overcome these barriers (where appropriate). In this way, the categories developed for this work could be mapped to potential solutions. For example, if a clinician clicked the checkbox indicating that concerns about drug misuse were their reason for not prescribing a given drug, the activated checkbox could prompt the physician with options for addressing the problem, such a clickable link that would order a pharmacist consult. In creating such systems, it will be important to avoid implying that all reasons for non-prescribing are justified, but to nonetheless capture these reasons to help drive improvement for the individual patient and the health system overall.
Research by Keefe et al. provides an early signal for how information on reasons for not performing guideline-recommended interventions might help performance measurement and quality improvement systems.27 As part of a computer-based decision support tool for heart failure, investigators recorded and classified free-text responses written by physicians in response to computer-generated care recommendations. The most common reason for ignoring a guideline-based recommendation was that the patient would not tolerate the intervention. Patient refusal and deference to another provider occurred much less commonly. Similar results have been found in chart-review studies, in which the majority of non-prescribing of guideline-recommended drugs in heart failure was attributed to drug contraindications or intolerance.28–29 Such results, although lacking great precision, can provide insights and stimulate in-depth review to guide decisions about where and how quality improvement efforts should be focused.
In considering such quality improvement programs, insights gained through our taxonomy should complement rather than replace the large body of work that focuses on the underlying attitudinal, knowledge, and behavior-based barriers to guideline adherence. These issues intersect broadly with the proximate, largely patient-centered reasons that are the focus of our approach. For example, underlying physician fears of causing patient harm can provide an important subtext for several of the categories in our taxonomy, including fears of causing an adverse drug reaction, fears of patients not obtaining follow-up monitoring, a different calculation of the benefit-to-risk ratio of a drug, and so forth.
Although our study focused on prescribing for heart failure, our findings likely apply to decision-making for a number of other chronic conditions. That said, each disease and type of intervention has unique features that need to be accounted for. For example, core recommendations for heart failure treatment are widely known, whereas there may be substantial knowledge gaps for other guidelines. In addition, there is an important difference between “prescriptive” guidelines, which recommend expanding treatment (e.g., drug therapy for heart failure), and “proscriptive” guidelines, which focus on reducing use of overused services (e.g., limiting the use of advanced imaging for patients with uncomplicated low back pain).7 In the former case, dominant issues typically involve adapting guidelines to individual patient circumstances and practical constraints in implementing recommended services.7 In the latter case, key issues include the physician’s role as a gate-keeper and maintaining the doctor-patient relationship in the face of concerns about rationing. Thus, non-adherence to such guidelines may best be understood by a taxonomy that is different than ours.
Of note, our research approach focused on generating a conceptual model that was closely linked to our data and that had some basis in existing published data on physician non-prescribing, rather than beginning with a preexisting conceptual framework. This approach is useful in two ways. First, it allows greater fidelity to the data. Second, this approach can generate novel insights that directly apply to interventions that measure and (where appropriate) help to address reasons for not prescribing guideline-recommended medications. However, existing theoretical models can also provide a useful framework in which to understand our findings. In particular, Gollwitzer’s Action Phase model of setting goals, planning and enacting their execution, and followup evaluation can be broadly mapped to our taxonomy and provides a well-developed theoretical framework for considering decision-making and actions around prescribing.30
There are several limitations to our research. Although we recruited physicians from a variety of clinical settings and levels of training, all participants were affiliated with a single medical school. Because of their academic affiliation, group members were paid a fixed salary (rather than on a fee-for-service model) and on average had fewer outpatient sessions per week than many practicing physicians (and none were engaged full-time in direct-patient-contact ambulatory practice). To the extent that these practice features may impact reasons for not prescribing, replication of our work in a non-academic setting will be important to validate our results.31–32
CONCLUSIONS
Understanding reasons for non-adherence to guidelines is essential for finding ways to improve the measurement and delivery of high-quality care. Attempts to improve performance measurement systems will particularly benefit from considering proximate, largely patient-centered reasons for guideline non-adherence. An optimal system remains an elusive goal, as no conceivable measurement framework will be able to precisely capture every reason for guideline non-adherence and make informed judgments about their appropriateness. Nonetheless, applying a conceptual framework to performance measurement can help design systems that provide better measures of quality and directly inform and link to quality improvement efforts.
Acknowledgments
Funding:
This work was supported by the Department of Veterans Affairs Health Services Research and Development Service (IAF 06-080 and CDTA 01-013) and by the National Institute on Aging and the American Federation for Aging Research (K23-AG030999)
The authors thank Mary Jo Pugh, RN, PhD for her careful review of the manuscript.
Footnotes
Presentation:
This work was presented at the annual meetings of the Society of General Internal Medicine (Miami, May 2009) and the American Geriatrics Society (Chicago, May 2009)
Conflict of Interest:
The authors have no conflicts of interest with topics discussed in this manuscript.
Author Contributions:
Developing research question and methods: Steinman, Knight
Conducting focus groups: Steinman, Patil, Kamat, Knight
Coding and analysis of data: Steinman, Patil, Kamat, Knight, Peterson
Initial authorship of manuscript: Steinman
Critical review of manuscript: Steinman, Patil, Kamat, Peterson, Knight
References
- 1.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. Jama. 2001 Jun 6;285(21):2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 2.Hayward RA. Performance measurement in search of a path. N Engl J Med. 2007 Mar 1;356(9):951–953. doi: 10.1056/NEJMe068285. [DOI] [PubMed] [Google Scholar]
- 3.Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008 May 20;148(10):717–727. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
- 4.Kerr EA, Smith DM, Hogan MM, et al. Building a better quality measure: are some patients with ‘poor quality’ actually getting good care? Med Care. 2003 Oct;41(10):1173–1182. doi: 10.1097/01.MLR.0000088453.57269.29. [DOI] [PubMed] [Google Scholar]
- 5.Cabana MD, Rand CS, Powe NR, et al. Why don’t physicians follow clinical practice guidelines? A framework for improvement. Jama. 1999;282(15):1458–1465. doi: 10.1001/jama.282.15.1458. [DOI] [PubMed] [Google Scholar]
- 6.Francke AL, Smit MC, de Veer AJ, Mistiaen P. Factors influencing the implementation of clinical guidelines for health care professionals: a systematic meta-review. BMC Med Inform Decis Mak. 2008;8:38. doi: 10.1186/1472-6947-8-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsen B, Glenton C, Pope C. Thou shalt versus thou shalt not: a meta-synthesis of GPs’ attitudes to clinical practice guidelines. Br J Gen Pract. 2007 Dec;57(545):971–978. doi: 10.3399/096016407782604820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foley KA, Denke MA, Kamal-Bahl S, et al. The impact of physician attitudes and beliefs on treatment decisions: lipid therapy in high-risk patients. Med Care. 2006 May;44(5):421–428. doi: 10.1097/01.mlr.0000208017.18278.1a. [DOI] [PubMed] [Google Scholar]
- 9.Freeman AC, Sweeney K. Why general practitioners do not implement evidence: qualitative study. BMJ. 2001 Nov 10;323(7321):1100–1102. doi: 10.1136/bmj.323.7321.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim C, Hofer TP, Kerr EA. Review of evidence and explanations for suboptimal screening and treatment of dyslipidemia in women. A conceptual model. J Gen Intern Med. 2003 Oct;18(10):854–863. doi: 10.1046/j.1525-1497.2003.20910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2005 Sep 20;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 12.Elisabeth AB, Denig P, van Vliet T, Dekker JH. Reasons of general practitioners for not prescribing lipid-lowering medication to patients with diabetes: a qualitative study. BMC Fam Pract. 2009;10:24. doi: 10.1186/1471-2296-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavazos JM, Naik AD, Woofter A, Abraham NS. Barriers to physician adherence to NSAID guidelines: A qualitative study. Aliment Pharmacol Ther. 2008 Jul 4; doi: 10.1111/j.1365-2036.2008.03791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haagen EC, Nelen WL, Hermens RP, Braat DD, Grol RP, Kremer JA. Barriers to physician adherence to a subfertility guideline. Hum Reprod. 2005 Dec;20(12):3301–3306. doi: 10.1093/humrep/dei220. [DOI] [PubMed] [Google Scholar]
- 15.Powell-Cope GM, Luther S, Neugaard B, Vara J, Nelson A. Provider-perceived barriers and facilitators for ischaemic heart disease (IHD) guideline adherence. J Eval Clin Pract. 2004 May;10(2):227–239. doi: 10.1111/j.1365-2753.2003.00450.x. [DOI] [PubMed] [Google Scholar]
- 16.Kasje WN, Denig P, de Graeff PA, Haaijer-Ruskamp FM. Perceived barriers for treatment of chronic heart failure in general practice; are they affecting performance? BMC Fam Pract. 2005 May 3;6(1):19. doi: 10.1186/1471-2296-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Short D, Frischer M, Bashford J, Ashcroft D. Why are eligible patients not prescribed aspirin in primary care? A qualitative study indicating measures for improvement. BMC Fam Pract. 2003 Jul 18;4:9. doi: 10.1186/1471-2296-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips SM, Marton RL, Tofler GH. Barriers to diagnosing and managing heart failure in primary care. Med J Aust. 2004 Jul 19;181(2):78–81. doi: 10.5694/j.1326-5377.2004.tb06178.x. [DOI] [PubMed] [Google Scholar]
- 19.Hickling JA, Nazareth I, Rogers S. The barriers to effective management of heart failure in general practice. Br J Gen Pract. 2001 Aug;51(469):615–618. [PMC free article] [PubMed] [Google Scholar]
- 20.Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. Bmj. 2003 Jan 25;326(7382):196. doi: 10.1136/bmj.326.7382.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKinlay JB, Link CL, Freund KM, Marceau LD, O’Donnell AB, Lutfey KL. Sources of variation in physician adherence with clinical guidelines: results from a factorial experiment. J Gen Intern Med. 2007 Mar;22(3):289–296. doi: 10.1007/s11606-006-0075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.James PA, Cowan TM, Graham RP. Patient-centered clinical decisions and their impact on physician adherence to clinical guidelines. J Fam Pract. 1998 Apr;46(4):311–318. [PubMed] [Google Scholar]
- 23.Mehta RH, Liang L, Karve AM, et al. Association of patient case-mix adjustment, hospital process performance rankings, and eligibility for financial incentives. JAMA. 2008 Oct 22;300(16):1897–1903. doi: 10.1001/jama.300.16.1897. [DOI] [PubMed] [Google Scholar]
- 24.Greenfield S, Kaplan SH, Kahn R, Ninomiya J, Griffith JL. Profiling care provided by different groups of physicians: effects of patient case-mix (bias) and physician-level clustering on quality assessment results. Ann Intern Med. 2002 Jan 15;136(2):111–121. doi: 10.7326/0003-4819-136-2-200201150-00008. [DOI] [PubMed] [Google Scholar]
- 25.Persell SD, Wright JM, Thompson JA, Kmetik KS, Baker DW. Assessing the validity of national quality measures for coronary artery disease using an electronic health record. Arch Intern Med. 2006 Nov 13;166(20):2272–2277. doi: 10.1001/archinte.166.20.2272. [DOI] [PubMed] [Google Scholar]
- 26.Halm EA, Atlas SJ, Borowsky LH, et al. Understanding physician adherence with a pneumonia practice guideline: effects of patient, system, and physician factors. Arch Intern Med. 2000 Jan 10;160(1):98–104. doi: 10.1001/archinte.160.1.98. [DOI] [PubMed] [Google Scholar]
- 27.Keeffe B, Subramanian U, Tierney WM, et al. Provider response to computer-based care suggestions for chronic heart failure. Med Care. 2005 May;43(5):461–465. doi: 10.1097/01.mlr.0000160378.53326.f3. [DOI] [PubMed] [Google Scholar]
- 28.Bart BA, Gattis WA, Diem SJ, O’Connor CM. Reasons for underuse of angiotensin-converting enzyme inhibitors in patients with heart failure and left ventricular dysfunction. Am J Cardiol. 1997 Apr 15;79(8):1118–1120. doi: 10.1016/s0002-9149(97)00060-x. [DOI] [PubMed] [Google Scholar]
- 29.Baker DW, Persell SD, Thompson JA, et al. Automated review of electronic health records to assess quality of care for outpatients with heart failure. Ann Intern Med. 2007 Feb 20;146(4):270–277. doi: 10.7326/0003-4819-146-4-200702200-00006. [DOI] [PubMed] [Google Scholar]
- 30.Gollwitzer PM. Action Phases and Mind-Sets. In: Higgins ET, Sorrentino RM, editors. Handbook of motivation and cognition: Foundations of social behavior. Vol. 2. New York: The Guilford Press; 1990. pp. 53–92. [Google Scholar]
- 31.Havranek EP, Wolfe P, Masoudi FA, Rathore SS, Krumholz HM, Ordin DL. Provider and hospital characteristics associated with geographic variation in the evaluation and management of elderly patients with heart failure. Arch Intern Med. 2004 Jun 14;164(11):1186–1191. doi: 10.1001/archinte.164.11.1186. [DOI] [PubMed] [Google Scholar]
- 32.Subramanian U, Sutherland J, McCoy KD, Welke KF, Vaughn TE, Doebbeling BN. Facility-level factors influencing chronic heart failure care process performance in a national integrated health delivery system. Med Care. 2007 Jan;45(1):28–45. doi: 10.1097/01.mlr.0000244531.69528.ee. [DOI] [PubMed] [Google Scholar]