Abstract
Since its discovery in 1988 as an endothelial cell-derived peptide that exerts the most potent vasoconstriction of any known endogenous compound, endothelin (ET) has emerged as an important regulator of renal physiology and pathophysiology. This review focuses on how the ET system impacts renal function in health; it is apparent that ET regulates multiple aspects of kidney function. These include modulation of glomerular filtration rate and renal blood flow, control of renin release, and regulation of transport of sodium, water, protons and bicarbonate. These effects are exerted through ET interactions with almost every cell type in the kidney, including mesangial cells, podocytes, endothelium, vascular smooth muscle, every section of the nephron, and renal nerves. In addition, while not the subject of the current review, ET can also indirectly affect renal function through modulation of extrarenal systems, including the vasculature, nervous system, adrenal gland, circulating hormones and the heart. As will become apparent, these pleiotropic effects of ET are of fundamental physiologic importance in the control of renal function in health. In addition, to help put these effects into perspective, we will also discuss, albeit to a relatively limited extent, how alterations in the ET system can contribute to hypertension and kidney disease.
Keywords: blood pressure, sodium homeostasis, water homeostasis, acid base, microcirculation
The exciting discovery of ET, and in particular ET-1, stimulated a great deal of studies by the renal research community. Initial work demonstrated that the kidney produced ET-1 in relatively high amounts. For example, Kitamura et al found that the inner medulla of the kidney contained a much higher concentration of ET-1 as compared to multiple other tissues in the body (using the pig as a model) (209). Subsequently, it was determined that almost every cell type within the kidney synthesized ET-1. In addition, the kidney was noted to contain abundant ET receptors, particularly in the vasculature and the medulla. Indeed, as best as could be determined, every cell type within the kidney expresses ET receptors. The kidney is also extremely sensitive to ET-1, having up to 10-fold greater sensitivity to the vascular effects of the peptide as compared to other organ beds (272, 325). Given the almost ubiquitous expression of ET-1 and its cognate receptors within the kidney, it is perhaps not surprising that our current understanding of renal ET biology emphasizes that renal cell-derived ET acts primarily in an autocrine or paracrine manner. Thus, as the various effects of ET are discussed in this chapter, it is important to bear in mind that the renal ET system must be viewed within the context of the local microenvironment. In addition, given such high ET production and receptor expression, it was perhaps predictable that ET regulates multiple renal functional parameters. We now know that the ET system can affect total and regional renal blood flow (RBF), glomerular filtration rate (GFR), Na and water excretion, and acid/base handling. In addition, ET-1 can exert a variety of pathophysiologic effects such as regulation of cell proliferation, extracellular matrix accumulation, and inflammation. These latter effects of ET-1 have been shown to be of substantial importance in the development and/or maintenance of a number of forms of acute and chronic renal injury. This chapter will, however, primarily focus on the physiologic role of ET-1 in the kidney.
Although not the subject of this chapter, it is important to recognize that ET-1 may affect renal function through direct modulation of non-renal systems. ET-1 is produced and bound by, and exerts physiologic effects upon, the adrenal gland (both aldosterone and catecholamine synthesis), the heart (regulating inotropy, chronotropy and atrial natriuretic peptide production), the vasculature throughout the body, and the central nervous system (modifying vasopressin release and central mechanisms affecting blood pressure). Discussion of how ET affects these systems can be found elsewhere (37, 79, 363, 365, 366, 370).
I. Biology of ET peptides and receptors
A. ET synthesis and degradation
1. ET genes, mRNA and prepropeptide
There are three mammalian ET isoforms: ET-1, ET-2 and ET-3. Each of these peptides contain 21 amino acids, two intrachain disulfide bonds, vary by not more than 6 amino acids, and are highly conserved between species (Figure 1). The three isoforms are encoded by separate genes that do not undergo alternate splicing. Most studies examining ET regulation of renal function have focused on ET-1; hence this discussion will focus on ET-1. The human ET-1 gene consists of 5 exons distributed over 6838 base pairs (184), is located on chromosome 6 (17), and encodes a 2026 base pair mRNA (184). ET-1 production and secretion is largely controlled at the gene transcription level. Cooperation between a large host of tissue-specific transcription factors permits tissue-selective ET-1 gene transcription and helps ensure that ET-1 is appropriately activated (510). Such cooperation is facilitated by multiple regulatory elements in the ET-1 promoter, including activator protein 1 (AP-1), nuclear factor of activated T-cells (NFAT)-binding domains, GATA binding protein 2 (GATA-2), CAAT-binding nuclear factor-1 (NF-1) and many others (411).
Figure 1.
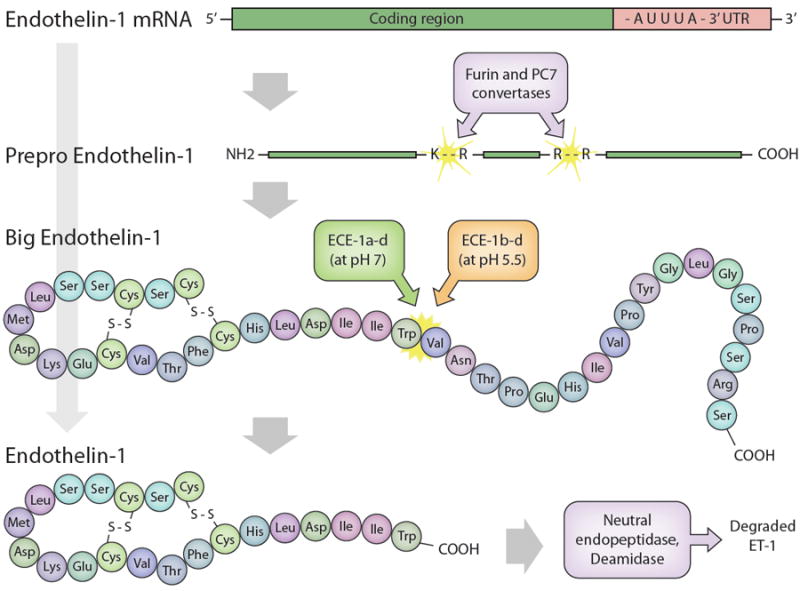
Biosynthetic and degradation pathways for ET-1. ET-1 mRNA encodes preproET-1. The short signal peptide is cleaved to yield proET-1 which, in turn, is cleaved by furin or PC7 convertases at dibasic amino acids to yield Big ET-1. Big ET-1 is cleaved by different ET converting enzymes (ECE) to mature ET-1. ET-1 is degraded by neutral endopeptidase and deamidase.
ET-1 mRNA has a short half-life (~15 minutes) which is partly due to the presence of three destabilizing AUUUA motifs in the 3’-untranslated region (184). Alterations in ET-1 synthesis can be effected by modification of ET-1 mRNA stability (87, 283, 361). Human ET-1 mRNA encodes a 212 amino acid prepropeptide that undergoes removal of a short signal sequence by a signal peptidase to yield proET-1. ProET-1 is cleaved by dibasic-pair-specific endopeptidases, including furin and PC7, to yield the 38 amino acid Big ET-1 (279, 512).
2. Conversion of Big ET-1 to ET-1
Big ET-1 is present in the circulation, however it has at least two orders of magnitude less vasoconstrictor potency than ET-1 (71). Conversion of Big ET-1 to ET-1 occurs primarily by ET converting enzymes (ECE) (Figure 1). ECE are integral membrane zinc peptidases; three isoforms (ECE-1, -2 and -3) have been identified (71, 456). ECE-1 is present mainly in endothelial cells, has the greatest affinity for Big ET-1, and has a pH optimum of 6.8 (506). There are four ECE-1 isoforms (ECE-1a, -1b, -1c and -1d) that are derived from alternative splicing of a single gene (392, 397, 461) and differ only at the amino terminus. ECE-1a is located in intracellular vesicles and on the cell surface; ECE-1b is located in the endosomal compartment near the trans-Golgi network, while ECE-1c and -1d are found primarily on the extracellular face of the plasma membrane (71, 278). The different ECE-1 locations indicate that ET-1 can be formed from Big ET-1 both intracellularly and extracellularly. Notably, Big ET-1 is sometimes present in the plasma in concentrations greater than ET-1, suggesting that extracellular conversion is of physiological importance. Since ECE is also localized to vesicles, the potential exists for ET-1 to be stored and secreted upon demand. Such a possibility has been confirmed in endothelial cells, wherein ET-1 can be found in Weibel-Palade bodies and released from vesicles upon stimulation (148, 371, 372).
ECE-2 has about 60% homology with ECE-1, hydrolyzes ET-1, and also consists of four isoforms with varying amino termini that may confer different intracellular cellular localization (99, 179). It’s pH optimum is 5.5, indicating that ECE-2 is particularly involved with the trans-Golgi network (99).
Mice with combined knockout of ECE-1 and ECE-2 have measurable ET-1 levels, indicating that other proteases may catalyze ET-1 formation (511). ET-1 may also be generated by other enzymes, although their physiologic relevance is uncertain (71). There is no convincing evidence that ECE are rate limiting in mature ET-1 synthesis. Finally, Big ET-1 can be converted to ET-1 [1-31] by chymase; ET-1 [1-31] can potently vasoconstrict (amongst other properties) (71). This suggests that alternative processing of Big ET-1 may be of biologic relevance.
3. ET catabolism
ET-1 is degraded by at least two enzymes. These include neutral endopeptidase (71, 463) and deamidase (189, 191) (Figure 1). The enzymes have different optimum pH profiles (deamidase at acid pH and neutral endopeptidase at neutral pH), suggesting different biologic roles. Renal catabolism of ET-1 may be of particular importance in that ET-1 causes prolonged BP elevation in bilaterally nephrectomized rats (224). In addition, circulating ET-1 does not appear in the urine (2).
B. ET receptors
1. Molecular biology of ET receptors
Mammalian ET receptors derive from two separate genes. The human ETA receptor (ETA) contains 427 amino acids, its gene is located on chromosome 4, and the receptor binds ET-1 ≥ ET-2 ≫ ET-3 (168). Splice variants have been described, however their relevance to kidney function has not been examined (150). The human ETB receptor (ETB) contains 442 amino acids, its gene is located on chromosome 13, and the receptor binds all ETs with equal affinity (15, 376). Several ETB splice variants have been described, including one with minimal signaling activity that may function as a clearance receptor (398). As for the ETA variant, the physiologic significance of these ETB splice variants in the kidney remains unknown. Finally, variable ET glycosylation has been described, but no functional significance has been identified (33).
2. ET receptor localization and signaling
ETA and ETB are widely expressed in the kidney; a given cell can express one or both receptor isoforms. In general, ETA predominate in vascular smooth muscle, while ETB predominate in endothelial cells and renal tubules. ET receptors activate multiple signaling systems that vary depending upon the cell type. ET receptors couple to members of the Gi, Gq, Gs and Ga12/13 G-protein families (88, 181, 208) with resultant regulation of a variety of signaling cascades, including adenylyl cyclases, cyclooxygenases (COX), cytochrome P-450, nitric oxide synthase (NOS), the nuclear helix-loop-helix protein p8 (138), serine/threonine kinases, tyrosine kinases and others (411). ETA and ETB often have opposing actions, i.e., in the vasculature, ETA activation causes vasoconstriction, while ETB activation, at least initially, causes vasodilation. However, many exceptions exist in which ETA and ETB elicit similar biologic responses. Given this complexity and cell-specific responses, detailed discussion of ET receptor signaling will be done in the context of each renal cell type.
A number of pharmacologic agents have been used to characterize ET receptor isoform function. However, substantial uncertainty exists about ET receptor isoform function. Part of this problem may relate to ET receptor dimerization (30). In vitro studies indicate that ETA and ETB can form homodimers (140) and heterodimers (139). Interestingly, ETA and ETB heterodimerized though a PDZ finger; mutation of the PDZ domain caused delayed ET receptor internalization and a prolonged increase in intracellular Ca2+ concentration ([Ca2+]I) in response to ET-1, suggesting that ETA/ETB heterodimerization affects receptor function (105). ETB may also heterodimerize with receptors other than ETA, including dopamine D3 and angiotensin II (AII) AT1 receptors (522, 523). However, to date there is no evidence that ET receptor heterodimers exist in vivo or subserve a biologic function.
ET-1 binding to its receptors causes prolonged biologic effects that are due, at least in part, to the almost irreversible binding of the peptide. In fact, ET-1 can remain bound to its receptor for up to 2 hours after endocytosis, suggesting that the peptide activates signal transduction long after internalization (63). ET receptors can also be present in the cell nucleus where they could conceivably exert different biologic effects than ET receptors on the plasma membrane (193).
C. Fundamental concepts about ET biology
A fundamental concept is that ET biology is best understood in the context of the local microenvironment. Plasma ET levels are generally too low to activate its cognate receptors. In contrast, local tissue ET concentrations are very likely much greater than that found in the circulation and can exert physiologic effects. Polarized cells, such as endothelial and renal tubular cells, secrete ET-1 predominantly towards the abluminal side. ET receptors are also mainly located on the abluminal side of polarized cells, thereby facilitating autocrine and/or paracrine regulation. Thus, ET appears to function primarily as an autocrine and paracrine regulator. Such considerations help explain how ET exerts such a broad range of effects, some of which can even oppose one another.
Another important concept is that ET-1 production is primarily regulated at the level of gene transcription. While alterations in mRNA stability, preproET-1 processing, ECE activity, or even vesicular trafficking have been described for ET-1, they have been infrequently identified as playing a significant role in the control of ET-1 synthesis and release. This control of ET-1 gene transcription involves numerous factors activating a panoply of intracellular signaling pathways. In general, vasoconstrictors tend to increase, while vasodilators decrease, ET-1 release. Further, since ET-1 release is largely dependent upon gene transcription, its regulation can lead to not only amplification of the magnitude of a given factor’s physiologic effect, but also substantial prolongation of the given factor’s duration of action. This latter concept may be particularly important in that ET-1 exerts long-lasting effects. Hence, the combination of stimulated gene transcription leading to sustained ET-1 production together with prolonged ET-1 binding to its receptors provides a powerful mechanism to lengthen the effect of a given stimulus (e.g. cell contraction) exerted by a given physiologic agent. This scenario may be relevant to all renal cell types; once the ET system is activated, it can exert potent and long-acting effects.
II. ET and the renal vasculature
A. Renal vascular ET receptor expression
ET-1 influences renal microvascular function by activation of ETA and/or ETB (Figures 2 and 3). The relative proportion and distribution of ETA and ETB expression varies between animal models and humans, possibly reflecting regional differences within the kidney. For example, in the canine renal cortex, the proportion of ETA/ETB expressed is approximately 22/78 based on binding studies using cortical membranes (36). The ratios in medullary and papillary tissues average 40/60 and 50/50, respectively (36). In contrast, the ETA/ETB distribution approaches 30/70 in the rat renal cortex and medulla alike (301). Descending vasa recta in the outer medulla express both ETA and ETB. The relative ETA/ETB proportion in porcine kidneys averages approximately 60/40 in glomerular membranes, but only ETB binding could be identified in papillary membranes (19). Approximately 30% of the receptors expressed in the human renal cortex and medulla are ETA (300).
Figure 2.
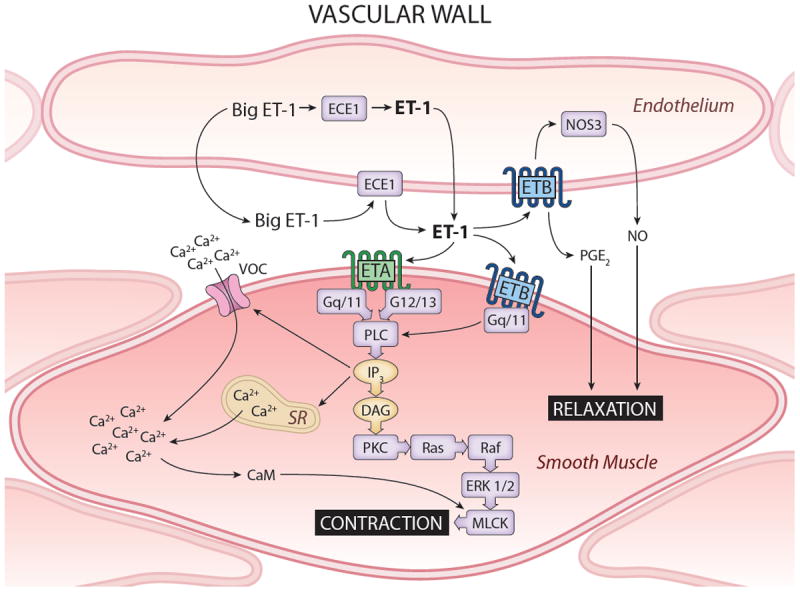
Schema of ET in vasculature. Endothelial cells express ETB exclusively and are the predominant vascular source of ET-1. ET-1 and nitric oxide synthase 3 (NOS3) can increase ETB activity or amount, respectively, leading to NO and PGE2 production with resulting vasolaxation. Activation of vascular smooth muscle ETA or ETB leads to a signaling cascade involving G-proteins, phospholipase C (PLC) and inositol triphosphate (IP3) that activate voltage-operated Ca2+ channel (VOC) and sarcoplasmic reticulum (SR)-mediated increases in [Ca2+]i and calmodulin (CaM) activation. CaM, together with activation of protein kinase C (PKC) by diacylglycerol (DAG) and Ras/Raf/ERK1/2 activation, causes myosin light chain kinase (MLCK) activation and cell contraction.
Figure 3.

Schema of functional ET receptors in the glomerulus and renal arterioles (Panel A), nephron (Panel B) and vasa recta (Panel C). A = contractile ETA; B = contractile ETB; white B with shadow: relaxant or natriuretic ETB that stimulates NO production; white A with shadow: natriuretic ETA. The amount of ET receptor shown in a given area is representative of the level of ET receptor activity in that region. Afferent arteriolar smooth muscle has more vasoconstrictive ET receptors than do efferent arterioles, while efferent arteriole endothelium has more vasodilatory ETB than does afferent arteriole (Panel A). Podocytes and mesangial cells contain primarily contractile ETA (Panel A). The inner medullary collecting duct (IMCD) has the greatest density of natriuretic ET receptors, although natriuretic ET receptors exist in the cortical collecting duct (CCD), thick ascending limb (TAL) and proximal tubule (PT) (Panel B). Vasa recta express contractile ETA on pericytes and vasodilatory ETB on endothelial cells (Panel C).
While the renal microvasculature clearly expresses ET receptors, the fractional expression that vascular receptors represent of the total renal ET receptor expression is not well understood. Physiological regulation of renal microvascular ET receptor expression has not been thoroughly investigated. Functional evidence established that both receptor subtypes are expressed by preglomerular vessels and efferent arterioles (92, 101, 186, 249, 268), however there are only limited data pertaining to the relative distribution of receptors in specific renal microvascular segments. Edwards and Trizna (91) showed that preglomerular microvessels of rats and rabbits express ETA/ETB in approximately a 40/60 proportion and 125I-ET-1 bound in a manner consistent with a single binding site. Dissociation constants were nearly identical across species and averaged approximately 20-21 pmoles/mg protein suggesting that preglomerular microvascular membranes from rats and rabbits exhibit nearly identical affinities for ET-1. ETA expression in rats (determined by 125I-ET-1 binding) was lower in glomeruli and inner medullary collecting duct than in preglomerular microvessels, whereas the ETA/ETB proportion was similar for the microvasculature and inner medullary collecting duct of rabbits, but glomeruli exhibited primarily (80%) ETB binding. Davenport and colleagues used an ETA selective radioligand to demonstrate that ETA was expressed along human arcuate, interlobular arteries and veins and along arterioles and glomeruli (75). More recently, Wendel et al used immunofluorescence to survey rat renal ET receptor expression and found that ETA were detected on vascular smooth muscle cells of the large interlobar, arcuate and interlobular arteries and in veins (481). ETA immunoreactivity also appeared on smooth muscle cells of afferent and efferent arterioles, but not on endothelial cells of these vascular elements. Weak immunostaining for ETB was noted on endothelial cells of interlobular arteries, but ETB immunoreactivity was much stronger on the endothelial cells of peritubular capillaries. Importantly, endothelial cells of afferent or efferent arterioles did not exhibit detectable ETB immunostaining.
Vascular ET receptor expression can be influenced by changes in physiological status. For example, chronic increases in ET-1 suppress ETA expression while having little effect on ETB expression (240, 438). Surface flow and NO enhance vascular smooth muscle ETA expression and affinity (351, 352). Recently, chronic salt feeding was shown to increase renal vascular ETB, but not ETA, expression (382). The possibility that physiological challenges alter renal microvascular ET receptor expression is exciting because it implies a new level of regulation/compensation, which may reveal novel roles for ET in kidney function.
B. Renal microvascular ET production
There is little information defining which renal vascular cells actually produce ET peptides and under what conditions that production can be modulated. Immunocytochemistry studies confirmed that endothelial cells of human interlobular and arcuate arteries and adjacent veins produce mature ET-1 and Big ET-1 (198). Positive staining was also detected in glomerular capillary endothelial cells, but not endothelia from other intrarenal capillaries. Interestingly, no immunostaining was evident in the vascular smooth muscle cells of these endothelium-positive arterial segments.
ECE-1 was detected in human kidney on the endothelial surface of arcuate and interlobular arteries and on vascular smooth muscle and endothelial cells of glomerular arterioles (342). In the medulla, ECE-1 was detected in vasa recta bundles and tubular elements. Endothelial staining was confirmed by immunohistochemical detection with von Willebrand factor, which paralleled ECE-1 mRNA distribution (342).
ET-1 release occurs soon after it is formed by ECE-1 from pre-pro ET-1. Accordingly, secreting cells do not contain significant stores of biologically active ET-1. Endothelial cells produce biologically active ET-1 from pre-pro ET-1, mainly through the catalytic actions of ECE-1; however, other mechanisms are implicated under physiological and pathophysiologic conditions, and in tissue specific manners (71, 227). ET-1 production can come from many sources and conditions. Renal production is facilitated by conditions such as shear stress, inflammation, oxidative stress and the renin/AII system (30, 96, 130, 303). Most ET-1 is probably generated by tubular epithelial cells. How much renal ET-1 is derived directly from renal microvascular endothelial and smooth muscle cells, and how this ET-1 might influence renal microvascular or tubular function, is unknown.
C. Effect of ET on the renal microcirculation
The renal vascular effects of ET are preceded by activation of ETA and ETB. ET produces a powerful and prolonged renal vasoconstriction following either lumenal or adventitial peptide delivery (29, 80, 101, 134, 186, 249, 268, 332, 334, 335, 402). This vasoconstriction involves activation of either ETA or ETB and occurs in a segment specific manner (101, 186, 249, 268). ET also influences mesangial cell signaling, migration and proliferation, but does not appear to exert a direct influence on tubuloglomerular feedback (202, 411, 425). Therefore, the relationship between ET and regulation of renal hemodynamics or renal microvascular function are complex and highly varied. This section will highlight how ET influences renal microvascular function and how these actions translate into modulation of renal hemodynamics.
Probably the best information on the segment specific actions of ET on the renal microcirculation has come from in vitro studies using isolated arteries and arterioles from rat and rabbit and in vivo or in vitro hydronephrotic kidney models. The earliest work used isolated arterioles to assess microvascular reactivity to ET-1, ET-2 and ET-3, and revealed that ET-1 produced a long-lasting, concentration-dependent vasoconstriction of afferent and efferent arterioles (92). The ED50 averaged approximately 1.4 and 0.9 nM for afferent and efferent arterioles, respectively. ET-2 mediated vasoconstriction of these arterioles was similar to ET-1, but ET-3 was significantly less potent. In similar work, using isolated rat microvessels, efferent arterioles were approximately 10-fold more sensitive to ET-1 compared to afferent arterioles (249, 321). This implicates ET as a paracrine regulator of glomerular hemodynamics and glomerular filtration pressure.
1. Studies in the hydronephrotic kidney
Much of our knowledge of the renal microcirculation has benefitted from using the in vitro and in vivo hydronephrotic kidney. This is a kidney model that is devoid of renal tubules while most of the vascular architecture is retained and visible for study (306). Hydronephrotic kidney studies provided the first in situ resolution of ET’s actions on intrarenal microvascular elements; demonstrating that ET potently vasoconstricts afferent arterioles in vitro (268, 432, 433) and in vivo (119, 142, 413), whereas ET-1 exerts more modest, and more variable effects on the efferent arterioles. Variability in the efferent response may arise from data collected in vitro or in vivo. For example, ET-1 caused modest efferent arteriole vasoconstriction in vitro (268, 433), but evoked a much greater efferent response in the in vivo hydronephrotic kidney (101, 119). It is possible that ET-1-mediated afferent vasoconstriction in the in vivo hydronephrotic kidney reflects activation of both ETA and ETB (47, 101), whereas efferent vasoconstrictor responses may be through ETB (101). ETA blockade reduces afferent vasoconstriction to ET-1 without affecting efferent arteriolar responses. Conversely, ETB blockade, or ETB agonists influenced vessel diameter of both afferent and efferent arterioles. Using a different in vivo approach, Gulbins et al infused antibodies directed at ET-1 and ET-3 to scavenge endogenous ET peptides, and then monitored changes renal microvascular diameter (142). Anti ET-1/ET-3 antibody infusion evoked vasorelaxation from arcuate and interlobular arteries and the proximal portion of afferent arterioles. The diameter of efferent and distal afferent arterioles did not change. Thus ET-1 may produce a more prolonged vasoconstriction of distal afferent arterioles and efferent arterioles than more upstream preglomerular segments
2. Studies in the blood perfused juxtamedullary nephron preparation
The blood perfused juxtamedullary nephron preparation was developed in the mid-1980’s by Daniel Casellas to evaluate inner cortical nephron function and microvascular reactivity (45, 46). The major advantage of this approach is that the kidney is perfused with blood and the vascular-tubular associations remain intact. Investigation of ET’s effects on the renal microvasculature using this technique has clearly revealed that ET-1, ET-2 and ET-3 vasoconstrict both afferent and efferent arterioles (183, 186, 382). ET-1 and ET-3 constricted afferent arterioles more than efferent arterioles whereas the magnitude of afferent and efferent responses to ET-2 were similar (186). ET-1 is significantly more potent than ET-2 or ET-3 (183, 186). Accordingly, much of the vasoconstriction induced by lower concentrations of ET-1 is ETA-dependent, and is consistent with earlier in vivo studies showing that ETA blockade could completely block the ET-1-mediated decline in RBF and GFR (334, 335).
Afferent arteriole vasoconstriction involves activation of both ETA and ETB. ET-1-mediated vasoconstriction of afferent arterioles is blunted by ETA blockade and abolished by combined ETA/ETB blockade (186). Efferent vasoconstriction also involves both ETA and ETB, but a more complex interaction may exist. Acute ETA blockade converts prominent efferent vasoconstriction to a modest vasodilation at lower ET-1 concentrations (10-100 pM) before a stronger vasoconstriction appears when the ET-1 concentration reaches 1 and 10 nM. Interestingly, blockade of ETB shifts the ET-1 concentration response curve slightly to the left suggesting increased ET-1 potency. The ETB agonist, S6c, also vasodilates efferent arterioles and reverts to a modest vasoconstriction during ETB blockade (186). Thus, these studies suggest that vasodilatory ETB present on vascular endothelium may exert a dominant role on the efferent arteriole and ETB-dependent constriction is only observed at higher agonist concentrations. This also suggests that vascular smooth muscle ETB might have lower affinity for ET-1 than endothelial ETB.
Data from the juxtamedullary nephron model suggest that ETB provide a vasodilatory influence on normal efferent arteriolar vascular tone whereas it is mainly a vasoconstrictor of afferent arterioles. These data may explain the variability noted in efferent arteriole responses in the hydronephrotic kidney models because efferent arterioles can respond to ET with either vasoconstriction or vasodilation, depending on ambient conditions. Notably a high salt diet attenuates afferent arteriolar vasoconstrictor responses to ET-1, presumably by enhanced preglomerular microvascular expression of vasodilatory ETB (382).
3. Renal blood flow, renal hemodynamics, in vivo and in isolated perfused Kidneys
Whole kidney responses to systemic or intrarenal infusion of ET uniformly demonstrate profound vasoconstriction (20, 41, 104, 333, 375, 474, 480). Early in vitro and in vivo work demonstrate that intrarenal ET-1 infusion reduced RBF, increased renal vascular resistance and reduced GFR, all variables consistent with significant vasoconstriction of the preglomerular vasculature. However, whole kidney studies have limited utility for establishing which intrarenal microvascular elements respond to ET-1 and how they respond. Using intra-vital video-microscopy, Saito et al examined surface glomeruli and arterioles in isolated perfused kidneys from Munich-Wistar rats (375). They found that ET-1 potently vasoconstricted surface afferent arterioles at concentrations approaching 30 fM. Efferent diameter decreased by just 3% with 30 pM ET-1 (375). These findings in intact kidneys agree with in vitro studies suggesting that ET-1 is a more effective vasoconstrictor of pre-glomerular versus post-glomerular vessels.
Micropuncture studies uniformly support ET-1 as a preglomerular vasoconstrictor, but efferent arteriolar responses are equivocal with studies suggesting more, less, or no difference in the efferent vasoconstrictor response to ET-1. ET-1 reduces rat RBF and increases afferent and efferent arteriolar resistance (20, 205). ET-1 also decreases Kf and single nephron GFR declined significantly (20, 205). In other studies, Kf remained unchanged during ET-1 infusion but proportionally greater increases in afferent arteriolar resistance were observed compared to efferent changes (227, 228). Antagonist studies indicate that the renal microcirculation is under the influence of endogenous ET-1. Infusion of the combined ETA/ETB antagonist, bosentan, reduced arterial blood pressure slightly and significantly decreased glomerular capillary pressure (344). Under the same conditions, selective ETA blockade had no effect on mean arterial pressure or glomerular hemodynamics. These data suggest that endogenous ET exerts a tonic vasodilatory influence on the renal microcirculation that is of ETB origin. Endogenous NO contributes to ET-modulation of renal vascular resistance (57, 77, 343, 344).
In the canine kidney, intrarenal infusion of ET-1 or ET-3 reduced RBF and GFR (57, 153). This effect was enhanced by NOS inhibition, suggesting that NO buffers the renal vasoconstrictor actions of ET (57). Inhibition of COX augmented ET-1 mediated renal vasoconstriction but abolished the ET-3-mediated renal vasoconstriction (57). The renal microvascular response to ET appears to involve direct effects of ET-1 on microvascular resistance and indirect actions of ET-1 to stimulate production of other vasoactive mediators, such as thromboxane, adrenergic influences or AII.
D. Effect of ET on medullary blood flow
Thus far, discussion has focused on the effects of ET on afferent and efferent arteriolar resistance. These resistance changes can profoundly impact whole kidney and cortical blood flow, but how does ET influence medullary blood flow? The observation that ET-1 potently vasoconstricts both afferent and efferent arterioles of the juxtamedullary nephrons provide some clues to address this question (186). The juxtamedullary microvasculature provide blood flow to the vasa recta that perfuse the renal medulla (322), thus ET should reduce medullary blood flow in concert with reducing cortical blood flow. However, ET’s influence on medullary blood flow seems more complex (107). Indeed, ET-1, ET-2 and ET-3 potently vasoconstrict isolated rat descending vasa recta with threshold effects visible at concentrations of approximately 10-16 M, 10-14 M, and 10-9 M, for the three peptides, respectively (322). The constrictor effects of ET-1 and ET-2, but not ET-3, are attenuated during ETA blockade. In contrast, ET-3-mediated constriction is inhibited by ETB, but not ETA, blockade. Interestingly, PGE2 could relax descending vasa recta preconstricted with ET-1 (1pM) or ET-3 (10nM) (399). ET also reportedly increases medullary blood flow in rabbit kidneys in vivo, while at the same time reducing whole kidney and renal cortical blood flow (106). ETA blockade decreased arterial blood pressure and increased RBF by increasing both cortical and medullary blood flow. ETB blockade increased arterial pressure and reduced renal and cortical blood flow while having no significant effect on medullary blood flow. ETB blockade also potentiated ET-1-mediated reductions in renal and cortical blood flow and abolished ET-1-mediated increases in medullary blood flow. Qualitatively similar results were observed in rats and mice (35, 298, 422, 462). These data argue that ETB are important regulators of the medullary blood flow response to ET-1, while ETA play a more important role in regulating ET-1’s influence on renal cortical blood flow (Figure 3).
The role of prostanoid metabolites in modulating ET-1’s influence on renal perfusion is unclear. Prostaglandins or NO appear to modulate ET-1’s effects on RBF (57, 142, 155, 156, 347), but how they influence intrarenal regional blood flow is not well defined. While thromboxane A2 is implicated in the dog kidney, it may not influence ET’s actions on renal perfusion in the rat (57). Blockade of thromboxane receptors had no effect on ET-1-mediated reduction of cortical or medullary blood flow (496).
The impact of ET-1 on medullary perfusion is influenced by gender and environmental conditions. Infusion of ET-1 directly into the renal medulla had no detectable effect on medullary blood flow in normal or ETB-deficient female rats, but produced a marked reduction in medullary blood flow in male rats of both strains (298). Ovariectomy eliminated this gender difference. Big ET-1 decreases cortical and medullary blood flow in rats fed a normal salt diet, but the medullary vasoconstriction is abrogated on a high salt diet (462). Notably, ECE-1 expression is significantly enhanced in the renal medulla of rats fed high salt. In addition, the sensitivity of juxtamedullary afferent arterioles to ET-1 was shifted to the right in rats fed high salt. This shift is accompanied by increases in ETB expression by the preglomerular microvasculature (382). These data support involvement of medullary ECE-1 and ETB activation in the medullary perfusion response to ET precursors and peptides.
E. ET signaling pathways in the renal circulation
This section focuses on the intracellular signaling mechanisms responsible for ET-mediated renal microvascular vasoconstriction. Studies have clearly shown that renal microvascular vasoconstriction is strongly linked to elevation of [Ca2+]I and that changes in [Ca2+]i involve both Ca2+ influx and Ca2+ mobilization signaling cascades (16, 303) (Figure 2). Interestingly, afferent and efferent arterioles often use different Ca2+ signaling mechanisms to produce vasoconstriction evoked by the same agonist (43, 44, 152, 266, 267, 269, 303).
At the vascular smooth muscle cell level, ET increases [Ca2+]i rapidly. ET-1, ET-2 and ET-3 stimulate peak increases in cytosolic Ca2+ concentration in preglomerular smooth muscle cells with a rank order potency of ET-1>ET-2≫ET-3 (389). This pharmacological potency profile suggests that ET-mediated elevations in [Ca2+]i arise primarily through activation of ETA, with perhaps a smaller component arising through ETB stimulation. The peak response to ET-1 and ET-2 was followed by a smaller but sustained plateau elevation of [Ca2+]i. Peak Ca2+ responses were unaffected by depletion of extracellular Ca2+, but the sustained increases in Ca2+ were abolished. The peak responses to ET-1 are primarily generated by mobilization of Ca2+ from intracellular stores, whereas the sustained increases in Ca2+ reflects influx of extracellular Ca2+. ETA- and ETB-stimulated Ca2+ responses were corroborated by Fellner and Arendshorst who noted that ET-1, and the ETB agonist, IRL-1620 both stimulated increases in [Ca2+]i in preglomerular smooth muscle cells and isolated rat afferent arterioles (112). The relative ETA- and ETB-dependent responses were attenuated by their respective receptor antagonists, and combined ETA/ETB blockade virtually eliminated the response, demonstrating select receptor-dependency.
Early studies performed in hydronephrotic kidneys provide the first data using intact, pressurized arterioles. ET-1-mediated vasoconstriction of afferent arterioles was inhibited by blockade of L-type voltage-gated Ca2+ channels while having a more modest effect on efferent arterioles (268). Activation of sarcolemma chloride channels may initiate the depolarization necessary to activate voltage-dependent Ca2+ channels. Indeed, Takenaka showed that inhibition of voltage-gated Ca2+ channels or chloride channels reversed ET-1-mediated vasoconstriction of afferent arterioles in hydronephrotic kidneys, in vitro(432, 433). In vivo however, Fretschner and co-workers found no effect of Ca2+ channel blockade on the afferent or efferent response to ET-1 (119), whereas Pollock et al. showed that nifedipine significantly attenuated the renal vasoconstriction evoked by ET-1, or the ETB agonist, S6c (332). Ca2+ channel blockade attenuated Ca2+ signaling events in preglomerular smooth muscle cells and blunted afferent arteriolar vasoconstriction to lower (1.0 and 10 pM), but not higher (100 pM), ET-1 concentrations.
Collectively, ET peptides activate ETA and ETB to increase [Ca2+]i in preglomerular smooth muscle cells by stimulating Ca2+ influx and Ca2+ release mechanisms. Voltage-dependent Ca2+ influx may occur through ET receptor-mediated opening of chloride channels leading to membrane depolarization and subsequent activation of L-type, voltage-dependent Ca2+ channels. The explanation for discrepant results pertaining to the efficacy of Ca2+ channel blockers to blunt or eliminate ET-1 mediated signaling in vivo and in vitro is unclear, but may reflect differences in physiological or hemodynamic status among preparations.
Ca2+ mobilization from intracellular stores involves activation of several receptor-specific Ca2+ signaling cascades (303). ET-mediated Ca2+ release in the renal microcirculation appears to involve activation of Ca2+ -induced Ca2+ release/cADP-ribose mechanisms, reactive oxygen species, Rho-kinase, PKC, chloride channels and cytochrome P450 metabolites (16, 48, 111, 183, 196, 207, 303, 433, 443). Preglomerular smooth muscle Ca2+ signaling experiments suggest that release of Ca2+ from intracellular stores is an important signaling mechanism employed by ET. Activation of renal microvascular ETA or ETB presumably stimulates ADP-ribosyl cyclase to produce cyclic ADP-ribose (cADP-ribose) and nicotinic acid adenine dinucleotide phosphate (NAADP) leading to Ca2+ release (111, 443, 444). cADP ribose activates ryanodine receptor-dependent Ca2+ release and NAADP may stimulate Ca2+ release through a lysosome dependent pathway. ET-1 or S6c-stimulated Ca2+ signaling events and reductions in RBF were attenuated during inhibition of ADP-ribosyl-cyclase activity and during ryanodine receptor blockade (111, 444). Mice with defective ADP-ribosyl cyclase exhibit markedly reduced renal hemodynamic sensitivity to ET-1 infusion compared to wild type controls.
More recent work implicates a novel lysosomal Ca2+ signaling mechanism (443). Interventions such as inhibition of NAADP actions, or disruption of lysosomal pH and Ca2+ regulation, simultaneously blunt ET-1 and norepinephrine-mediated increases in [Ca2+]i in rat afferent arteriolar smooth muscle. Thus, ET-1 employs novel mechanisms to stimulate Ca2+ signaling events in renal microvascular smooth muscle.
Reactive oxygen species are important modulators of renal microvascular function and appear to influence preglomerular responsiveness to ET (111, 196). ET-1 increases [Ca2+]i and superoxide accumulation in preglomerular smooth muscle cells, and this effect was markedly diminished in the presence of the free radical scavenger, Tempol (111). ETB activation with S6c also increases [Ca2+]i, but only slightly influences superoxide accumulation. At the whole kidney level, the NADPH oxidase inhibitor, apocynin, attenuated ET-1 or S6c’s ability to reduce RBF (196). Thus, oxidative status in the renal microvasculature can significantly influence renal microcirculatory responses to ET.
ET-1 binding to ETA and ETB also stimulates phospholipase A2 activation, arachidonic acid release, and production of COX and cytochrome P450 metabolites from renal tubular and vascular cells (86, 182, 183, 319, 380, 505). Cytochrome P450 and COX metabolites contribute to ET’s renal vasoconstrictor actions, but comparatively little is known about the vascular sites of action and cellular signaling mechanisms involved. In pioneering studies, Imig et al. determined that COX and cytochrome P450 metabolites modulate Ca2+ signaling events in preglomerular smooth muscle cells and directly influence afferent arteriolar vasoconstrictor responses to ET-1 (182, 183). Inhibition of COX or cytochrome P450 hydroxylase activity attenuated ET-1 mediated afferent arteriolar vasoconstriction. Conversely, inhibition of cytochrome P450 epoxygenase activity enhanced ET-1 mediated afferent arteriolar vasoconstriction. Inhibition of COX or cytochrome P450 hydroxylase activity attenuated ET-1- mediated increases in [Ca2+]i in preglomerular smooth muscle cells, but cytochrome P450 epoxygenase inhibition had no effect. These data establish that ET-1 stimulates production of COX and CYP450 hydroxylase metabolites by renal microvascular smooth muscle cells, and these metabolites contribute to the ET-1–mediated vasoconstriction. In contrast, inhibition of the CYP450 epoxygenase pathway enhanced the vasoconstrictor response to ET-1. Thus ET-1 mediated production of vasodilatory epoxyeicosatrienoic acids (EETs) may counteract the vasoconstrictor actions of ET-1.
While ET-1-mediated increases in [Ca2+]i are important signaling mechanisms by which ET influences renal microvascular resistance, changes in Ca2+ sensitivity can also regulate vascular function (409). Presently, two major mechanisms appear to alter Ca2+ sensitivity; these include PKC and receptor-mediated activation of the RhoA/Rho-kinase pathway, which inhibits myosin light chain phosphatase (8, 270, 326). In two separate studies, different PKC inhibitors did not alter ETA (ET-1) or ETB (IRL-1620) mediated vasoconstriction of afferent arterioles (48, 433). PKC does not appear to play a major role in mediating renal microvascular vasoconstriction by ET. In contrast, the data on Rho-kinase is more interesting and deserves more study. Rat afferent arterioles express elements of the Rho-kinase signaling pathway and arteriolar reactivity is influenced by inhibitors of Rho-kinase activity (48, 185). In addition, ETB activation reportedly activates RhoA of the Rho-kinase signaling pathway (208). Acute exposure to Rho-kinase inhibitors rapidly reduces microvascular resistance in normal and hydronephrotic kidneys. Furthermore, pretreatment with Rho-kinase inhibitors virtually eliminates the preglomerular vasoconstriction induced by the ETB agonist, IRL-1620, and attenuates IRL-1620-mediated vasoconstriction of the efferent arteriole. Unfortunately, ETA-dependent effects have not been examined, so the contributions of the Rho-kinase system on ETA-mediated renal microvascular responses remain to be determined. Nevertheless, the limited data available thus far support a role for Rho-kinase activity in ET-1-mediated renal microvascular reactivity.
Integration of the data presented on the microvascular effects of ET-1 argues that ET-1 is a potent vasoconstrictor of the renal microcirculation and may act in an important, segment-specific manner. Renal microvascular vasoconstriction involves both ETA and ETB subtypes, and may be altered under differing environmental conditions, such as high salt diets or hypertension. The physiological implications of regional influences along the vascular tree remain to be determined. There is general agreement that ET regulates microvascular tone by modulating [Ca2+]i, and may also modulate Ca2+ sensitivity, thus providing a remarkably sensitive mechanism for ET-1-dependent regulation of renal cortical and medullary resistance.
III. ET and glomerular function
ET can modify glomerular cell function, although most of the peptide’s actions have been described in the context of pathophysiologic conditions associated with glomerular sclerosis, cell proliferation and/or proteinuria (25). There is limited data that suggests the ET system can alter GFR through control of glomerular cell function; this section focuses on this topic. Please note that endothelial cell ET biology is discussed in the context of the renal vasculature in the previous section.
A. Glomerular epithelial cells
1. Glomerular epithelial cell ET production and receptor expression
Glomerular podocytes from rat, human and mouse can synthesize ET-1 (67, 70, 117, 200, 292). Rat glomerular epithelial ET-1 production is increased by activation of PKC and inhibited by blockade or depletion of PKC (70). An interesting study using mouse podocytes reported that reorganization of the actin cytoskeleton via Rho kinase-dependent focal adhesion kinase (FAK) activation of nuclear factor- KB (NF-KB) and activator protein-1 (AP-1) led to increased ET-1 synthesis (292). Thus, podocytes release ET-1; such production may be regulated by factors associated with changes in podocyte conformation.
Glomerular epithelial cells from human and rat express ET receptors (121, 350) (Figure 3). There is limited information on the ET receptor isoforms in these cells, however ETB have been localized to podocytes in rat glomeruli (507), while ET-1 induction of thrombin receptor internalization in human glomerular epithelial cells occurred by ETA activation (55).
2. ET actions in glomerular epithelial cell
There is no direct evidence that ET modulation of glomerular epithelial cell function leads to alterations in GFR or RBF. However, if one can speculate that contraction of these cells might alter glomerular surface area or glomerular sieving, then the possibility does exist that ET-1 could affect glomerular hemodynamics. Indeed, ET-1 has been demonstrated to modulate alterations in the cytoskeleton in glomerular epithelial cells. For example, ET-1 induces nephrin shedding from glomerular epithelial cells, in part due to cytoskeleton redistribution. In addition, ET-1 caused cytoskeletal rearrangement leading to increased protein permeability in mouse podocytes (293); this effect was mediated via ETA activation, and phosphatidylinositol-3 kinase (PI3K) and Rho-kinase pathways. This group also noted that shigatoxin-stimulated podocyte cytoskeletal changes were mediated by ET-1, raising the possibility of an autocrine ET-1 function in these cells. ET-1 increases [Ca2+]I in podocytes (350) and parietal cells (277); this latter effect was associated with contraction of the parietal sheet. Taken together, the above studies suggest that ET-1 can contract glomerular epithelial cells, most likely via ETA activation; whether this impacts glomerular function remains to be determined.
B. Mesangial cells
1. Mesangial cell ET production and receptor expression
Mesangial cells have been well described to produce ET-1 (Figure 4). A wide variety of agents modulate mesangial cell ET-1 gene synthesis, including vasoactive substances, growth factors, cytokines, reactive oxygen species, and others. Factors that enhance ET-1 synthesis include ET-1 itself (via ETB) (190), AII (178, 222), AVP (178, 377, 424), thromboxane A2 (529), thrombin, tumor necrosis factor (TNF) and interleukin-1 (IL-1). These stimulatory effects are mediated by a number of signaling systems. Typically, preproET-1 expression is increased by activation of p38 mitogen-activated protein kinase (MAPK) and PKC, while extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinases/stress-activated protein kinase (JNK/SAPK), or intracellular Ca2+ release are typically not involved (115, 158). Subsequent to p38 MAPK activation in mesangial cells, TAK1 kinase, TAK1 binding protein-1 (TAB1), TNF receptor associated factor 2 (TRAF2) and several mitogen activated protein kinase/extracellular signal regulated kinase kinases (MEKKs) play a role in ET-1 gene transcription. Finally, atrial and brain natriuretic peptides decrease mesangial cell ET-1 production (225).
Figure 4.
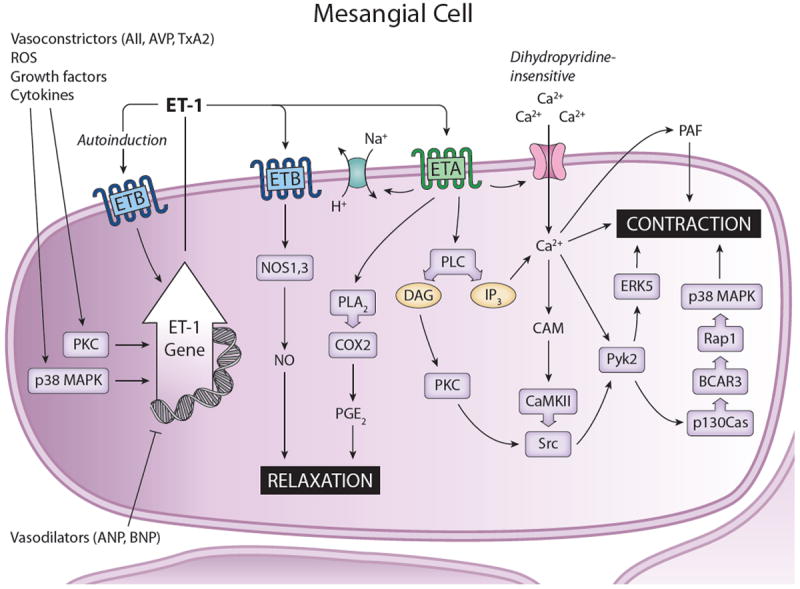
Synthesis and actions of ET-1 in mesangial cells. A large variety of substances stimulate ET-1 production, including ET-1 itself. Vasodilators tend to inhibit mesangial cell ET-1 synthesis. ET-1 likely acts in an autocrine manner. In general, ETA activation leads to cell contraction, while ETB activation causes relaxation. Please see text for definitions of abbreviations.
Mesangial cells express both ETA and ETB (Figures 3 and 4). Initial studies, conducted before anti-ET receptor antibodies and a large variety of receptor-specific agonists and antagonists were available, demonstrated that mesangial cells expressed ET receptor mRNA, bound ET, and that ET elicited Ca2+ signaling and cGMP production, however the specific isoforms involved were uncertain (6, 23, 66, 318, 405, 420, 423, 429, 516). Subsequently, strong evidence for both ETA- and ETB-mediated actions in mesangial cells has been obtained. In addition, ETA and ETB are expressed by cultured human mesangial cells (158, 315), while prominent mesangial cell ETA immunostaining is present human kidney sections (481). A notable aspect of mesangial cell ET receptors is that their binding and signaling are downregulated by pre-incubation with ET-1 (receptor desensitization). ET-1 markedly reduced ET-1 binding capacity without affecting its affinity in cultured rat mesangial cells (23). Similar desensitization, in terms of evoked [Ca2+]I responses, was observed in these cells (403).
2. ET actions in mesangial cells
ET-1 exerts multiple effects on mesangial cells, including cell contraction, hypertrophy, proliferation and extracellular matrix accumulation (411). The latter responses may be important in the pathophysiologic effects of ET-1, however this discussion will focus primarily on cell contraction as this may be involved in modulating GFR and/or RBF. ET-1 causes mesangial cell contraction primarily through ETA activation (20, 401, 428) (Figure 4). ET-1 activates phospholipase C (PLC) (194, 406, 407) and PKC (403), leading to augmented inositol triphosphate levels, increased Na/H exchange, cell alkalinization and elevated [Ca2+]I (20, 403, 405, 406). The increased [Ca2+]I derives from intracellular stores and influx through dihydropyridine-insensitive pathways (403, 516). Low ET-1 concentrations (0.1-10 pM) cause slow sustained increases in [Ca2+]I that are dependent upon Ca2+ influx through a voltage channel-independent mechanism, whereas higher ET-1 concentrations (≥ 100 pM) elicit a rapid and transient increase that is dependent upon Ca2+ release from intracellular stores through activation of PLC and PKC (406). The increase in [Ca2+]I is primarily due to ETA (516); ETB activation can increase [Ca2+]I, albeit much less than that seen with ET-1 (516).
Several pathways involved in cell contraction downstream of ET-1-induced increases in [Ca2+]I in mesangial cells have been identified. Proline-rich tyrosine kinase-2 (Pyk2), the only cytoplasmic tyrosine kinase activated by mobilization of [Ca2+]I (255), undergoes ET-1-stimulated autophosphorylation in a Ca2+-dependent manner in mesangial cells (412). Pyk2 causes p38 MAPK activation in mesangial cells, another kinase known to mediate cell contraction (412). Pyk2 can also phosphorylate p130Cas, causing increased interaction with BCAR3, a protein with a GDP exchange-factor-like domain; this may lead to BCAR3-mediated GTP-loading of Rap1 with resultant alterations in the actin cytoskeleton and cell contraction (367). ET-1 can also increase CrkII adapter protein association with BCAR3 in mesangial cells, another potential mechanisms for regulating cell contraction (368). In addition to the Pyk2 pathway, ERK5 may be involved since ET-1 activates ERK5 in human mesangial cells, while ERK5 knockdown inhibits mesangial cell contraction (85). ET-1 may also modulate mesangial cell contraction through activation of Src tyrosine kinases; this may involve ß-arrestin-1-mediated recruitment of Src to complex with the ET receptor (181), adhesion-dependent activation of Src by interaction with focal adhesion kinase (49), and Ca2+/CaM-dependent protein kinase II (CaMK II) (473). In addition, ET-1 actions on mesangial cells may relate, at least in part, to its transactivation of the EGF receptor, an effect that has been shown to activate the adaptor protein p66Shc, Gαi3, ß1Pix and ERK1/2 (51, 171). Finally, ET-1 induces platelet activating factor (PAF) release by mesangial cells, while inhibition of PAF release decreases ET-1-mediated mesangial cell contraction (265, 289).
ET-1-induced mesangial cell contraction could be partially mitigated by the vasorelaxants prostaglandin E2 (PGE2) and NO. ET-1, via ETA, increases phospholipase A2 and COX2 (but not COX1) activity, leading to elevated PGE2, and to a lesser extent, thromboxane A2 (120, 174, 404). ET-1 stimulated COX2 activity in mesangial cells depends upon intracellular Ca2+ release, CAMK, non-receptor linked protein tyrosine kinases, and nuclear factor of activated T cell 2 (NFAT2) (68, 419). ET-1 may also stimulate NO production by mesangial cells. ET-3 rapidly increases NO-dependent cGMP production by isolated rat glomeruli and cultured rat mesangial cells though activation of ETB, release of Ca2+ from intracellular stores, and CaM (89, 318, 423). However, the system is clearly more complex in that prolonged exposure (many hours) to ET-1 inhibits cytokine-induced NOS2 activity in rat mesangial cells (via ETA) (27, 162). The explanation of these diverse effects of ET on NO production is unknown, however it seems likely that, in contrast to the likely inhibition of NOS2 gene transcription, the rapid induction of NO occurs through activation of NOS1 or NOS3 and does not involve regulation of gene transcription.
All of the above studies have been done using in vitro preparations, hence the physiologic relevance of ET regulation of mesangial cell function is an open question. Given this limitation, it is notable altered mesangial cell ET-1 production has been observed in hypertension. In particular, Ikeda et al. reported that phorbol ester, AII, and vasopressin (AVP) increased ET-1 secretion to a greater extent in mesangial cells from SHR hypertensive as compared to WKY normotensive rats (178). These findings suggest that heightened ET-1-mediated mesangial cell contraction, resulting in reduced GFR, might pay a role in impaired renal Na excretion in some hypertensive states.
IV. ET and sodium transport
A. Overview of ET regulation of Na transport
As has been alluded to previously, ET in the kidney exerts complex regional actions, involving paracrine and autocrine pathways. This has generally confounded interpretation of studies that either: 1) examined the effects of systemically or intrarenally administered ET agonists or antagonists; or 2) analyzed changes in renal ET-1 levels or urinary ET-1 excretion in response to stimuli. Nonetheless, an abbreviated review of these types of studies can provide some insight into the role of renal ET in the regulation of Na transport, as well as the problems encountered with using these types of approaches.
Exogenously administered ET-1, if given at a sufficient dose, typically reduces RBF and GFR in experimental animals and humans (5, 166, 201, 334, 345, 410). Such renal hemodynamic effects are usually associated with reduced urinary Na and water excretion, particularly when RBF is reduced by at least 25% (64, 201). However, when ET receptor agonists were given at doses with only modest or no effects on RBF or GFR, urinary Na excretion either increased (81, 147, 163, 387) or did not change (118, 137, 197, 383). One group found that renal decapsulation or maintaining renal perfusion pressure at baseline values with an aortic clamp prevented ET-1-induced natriuresis, suggesting that the natriuretic effect of exogenous ET-1 was due to increased BP (460). In addition, as will be discussed in the section on Na transport, inconsistent findings about ET agonist-induced natriuresis may relate to differential activation of ET receptor isoforms. The bottom line from these studies, however, is that the role of the ET system in controlling renal Na excretion cannot be clearly defined by maneuvers that exert generalized renal effects.
Changes in renal ET levels or urinary ET-1 excretion have been measured in response to alterations in fluid volume status. While a few studies have seen either decreased (in humans) (287, 349, 394) or unchanged (in humans) (13, 114, 165) urinary ET-1 excretion following Na loading, the majority of studies have found increases in urinary ET-1 excretion (in humans and experimental animals) (4, 69, 170, 176, 192, 220, 274, 374, 381). Thus, taken together, the bulk of evidence indicates that volume expansion is associated with increased renal ET-1 production. This conclusion suggests that endogenous renal ET-1 exerts a net natriuretic effect; as will be seen from the discussion below, this does indeed appear to be the case.
B. ET regulation of proximal tubule Na transport
1. ET production by the proximal tubule
Although early studies did not routinely detect ET-1 mRNA in the proximal tubule (PT) (458, 459), the majority of studies have demonstrated that this nephron segment synthesizes ET-1 (Figure 5). Proximal tubule ET-1 protein release and/or mRNA content have been confirmed using LLC-PK1 cells (a porcine PT cell line) (396), cultured rabbit PT cells (214), isolated or in situ rat PT (54, 291, 495), and cultured human PT cells (313, 514). In addition, ECE-1 protein was detected in human PTs (342). Despite this evidence for PT ET synthesis, the available data suggests that PT produce ET peptides in relatively small amounts compared to most other nephron segments. In cultured rabbit tubular cells, PT released much less ET-1 and ET-3 as compared to thick ascending limb and collecting duct (214).
Figure 5.
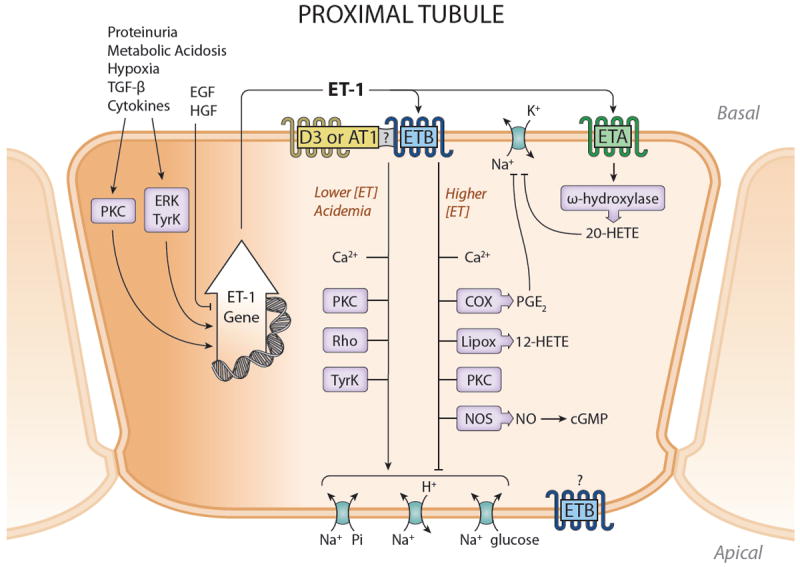
Synthesis and actions of ET-1 in the proximal tubule. ET-1 production is enhanced during inflammation, hypoxia, glomerular injury and acidemia. Most studies implicate ETB in mediating ET effects on the proximal tubule, although ETA activation may result in inhibition of Na reabsorption. ETB effects appear to depend upon the concentration of ET-1, with lower concentrations stimulating Na transport processes and higher concentrations having the opposite effect. It is likely that ET-1 exerts primary a natriuretic effect on the proximal tubule under physiologic conditions. Please see text for definitions of abbreviations.
ET-1 production by the PT can undergo regulation, however the identified factors may be more involved in disease than physiologic control. The only agents shown to inhibit PT ET-1 release are epidermal growth factor (EGF) and hepatocyte growth factor (HGF), factors typically associated with renal injury (151). In contrast, a variety of substances and conditions associated with renal injury or inflammation enhance PT ET-1 production, including hypoxia (305), thrombin, transforming growth factor-ß (TGF-ß), TNF-α and IL-1ß (308). Additionally, high density lipoproteins stimulated ET-1 release by human proximal tubular cells (313), while plasma proteins such as albumin and immunoglobulin G increased ET-1 release by a rabbit PT cell line (528); these findings suggest that nephrotic syndrome may lead to enhanced PT ET-1 production. Finally, activation of PKC increases ET-1 secretion by LLC-PK1 cells (308). Taken together, the above studies indicate that PT ET-1 production is subject to regulation, however whether or not this is involved in control of Na transport is unknown. That such alterations might occur is suggested by one study in which an increase in ET-1 mRNA and protein was observed in PTs of uninephrectomized SHR rats as compared to WKY controls (250). Clearly, studies on the effect of blood pressure, extracellular fluid volume (ECFV), interstitial pressure and vasoactive factors on PT ET-1 production are needed.
2. ET receptor expression by the proximal tubule
Peptide binding, immunostaining, RT-PCR and functional studies convincingly demonstrate that the PT expresses ET receptors, albeit in smaller amounts than more distal nephron segments (226, 304, 431) (Figure 3). Despite some disparate results, the bulk of evidence indicates that PT (rat and human) likely express both ETA and ETB. ETB mRNA, protein and/or binding patterns in PT have been generally, albeit not unequivocally detected (314, 320, 439, 481, 509). In addition, as described below, ET-1 modulation of PT function via ETB activation has been repeatedly demonstrated. Proximal tubules in rat and human also likely express ETA (314, 508), although this has not been universally observed (481). In summary, although expression is low, the PT appears to express both ETA and ETB.
ET receptor expression by the PT may be regulated, both in terms of degree of expression and interaction with other plasma membrane receptors. Dopamine D3 receptors may interact with ETB; D3 and ETB in rat PT co-immunoprecipitated, while WKY PT cell ETB expression was increased by D3 activation (this was dependent upon extracellular Ca2+ or dihydropyridine-sensitive Ca2+-channels) (517). Notably, SHR PT ETB levels were reduced as compared to WKY, and were also decreased by D3 activation, raising the possibility that D3 regulation of ETB is aberrant in some forms of hypertension (517). AT1 receptors may also interact with ETB; WKY PT AT1 and ETB co-localize and co-immunoprecipitate (523). Further, AT1 activation increased ETB cell surface expression in WKY, but not SHR, PT cells, while ETB activation, in turn, reduced AT1 receptor expression in WKY, but not SHR, PT cells (523, 524). Thus, ETB may physically associate with D3 and AT1 receptors. Further, alterations in ETB regulation, leading to diminished ETB activity, may occur in some forms of hypertension.
3. ET actions in the proximal tubule
ET-1 modulation of PT Na transport is complex (Figure 5); conflicting results have been obtained by several laboratories. Some groups have found that ET-1 reduces PT Na and fluid transport. Whole animal studies, using lithium clearance, found that intravenous ET-1 decreased rat PT fluid reabsorption (147, 324). In support of this, ET-1 (1 nM) reduced fluid and bicarbonate absorption, in part by inhibition of Na/K ATPase activity, in microperfused isolated rat proximal straight tubule (127). ET-1 also decreased rat PT brush border membrane Na-glucose co-transporter activity (273), while both ET-1 and ET-3 inhibited Na/K ATPase activity in isolated rat PT (312). However, other groups have found no effect of ET-1 on PT Na reabsorption using lithium clearance in humans (410) or isolated PT Na/K ATPase activity (520). Finally, yet other investigators have reported that ET-1 enhances PT Na reabsorption. ET-1 (given apically) increased rat PT fluid reabsorption in micropuncture studies, albeit SNGFR was also greatly augmented (362). ET-1 also increased Na/H antiporter and Na/HCO3 co-transporter activity in rabbit renal cortex plasma membrane vesicles (93). In addition, ET-1 stimulated Na/H exchange and Na/Pi co-transport by rat renal cortical slices (via activation of protein kinase A (PKA) and PKC (144) and increased Na/H exchange activity in PT-like OKP cells (via PKC) (469).
The reasons for the divergent results on ET-1 regulation of PT Na transport are not fully understand, but may relate, at least in part, to the concentration of the peptide. In a key study, Garcia and Garvin found that low concentrations of ET-1 (0.1 pM) enhanced PT fluid absorption (via PKC), while high concentrations of ET-1 (1 nM) reduced fluid transport (via PKC-, COX-, and lipoxygenase-dependent mechanisms) (123). In agreement, blockade of 5-lipoxygenase or leukotriene C4/D4 abrogated ET-1-induced natriuresis in rats (as assessed by lithium clearance) (324). Similarly, inhibition of ω-hydroxylase prevented ET-1 reduction of rat PT Na/K ATPase activity, while ET-1 stimulated PT 20-hydroxyeicosatetraenoic acid (20-HETE) production (103). Thus, it may be that low concentrations of ET-1, sufficient to only activate PKC, lead to augmented PT Na reabsorption; high ET-1 levels may reduce PT Na transport through modulation of arachidonate-dependent pathways. However, the net effect of ET-1 on PT Na reabsorption under physiologic conditions remains an open question.
The mechanisms by which ET-1 exerts biphasic effects of ET-1 on PT Na reabsorption are speculative. One possibility is differential activation of ET receptor isoforms, however this has not been clearly demonstrated. Little data is available with regard to ETA in the PT, however ET-1 stimulation of 20-HETE (which inhibits Na/K ATPase activity) in rat PT has been reported to be mediated by ETA (103). In contrast, ET-1 and ET-3 were equipotent in decreasing Na/K ATPase activity in rat PT (312), suggesting that ETB activation may also be involved. Similarly, ETB mediated reduced Na/K ATPase activity in PT from WKY rats (517). ETB may also decrease PT Na transport via induction of NO. ETB activation increases cGMP levels in LLC-PK1 cells (most likely through a Ca2+-dependent pathway) (187, 320); cGMP, in turn, can inhibit PT Na transport (360, 471). Taken together, these data suggest that both ETA and ETB mediate inhibition of PT Na transport, albeit the signaling pathways may differ.
ETB also appear capable of augmenting PT Na transport; this effect occurs largely through activation of Na/H exchange. Initial studies demonstrated that ET-1 increases Na/H exchange in OKP cells transfected with ETB, but not ETA (60). The ETB effect was due to enhanced Na/H exchanger 3 (NHE3) activity, was mediated by the COOH-terminal tail and the second intracellular loop of ETB (248), and was involved tyrosine kinase pathways (61). ETB stimulation of Na/H exchange activity is Ca2+- and Rho kinase-dependent, requires phosphorylation and exocytosis of NHE3 into the apical membrane (339), and may involve Gi-mediated inhibition of cAMP accumulation (144, 320).
It is apparent from the above discussion that the different effects of ET-1 on PT Na transport pathways are not readily explained by differences in receptor isoform-specific responses. While identification of the responsible mechanisms remain elusive, it may be germane to note that ET-1 modulation of NHE3 and Na/K ATPase may serve different purposes. In particular, and as will be discussed in the section on acid/base, ET-1-induced NHE3 activation may be primarily involved in augmenting H+ secretion in response to metabolic acidosis (339). In contrast, Na/K ATPase regulation may be more involved in responses to alterations in blood pressure or ECFV. Clearly, further elucidation of the factors regulating ET-1 production and ET-1 responsiveness is needed.
C. ET and the thin limb of Henle’s loop
The biology of ET in the thin limb of Henle’s loop has been minimally investigated. In general, the data on thin limb ET production is conflicting. Some investigators have reported ET-1 protein or mRNA in this nephron segment in the rat (291) and human (314), whereas others have failed to observe ET-1 protein or mRNA in rat (38, 495) or human (341). Even if the thin limb does makes ET-1, it is most likely in very small amounts.
Only one study has examined ET receptors and signaling in thin limbs; in acutely isolated rat thin descending limbs contained ETA and ETB mRNA, while only ETA activation increased [Ca2+]i (21). The ET-1 stimulated Ca2+ signal was reduced in descending limbs from hypertensive rat strains (SHR and Lyon hypertensive) as compared to normotensive WKY and Lyon strains, however the biologic relevance of this is unknown.
D. ET regulation of thick ascending limb NaCl transport
1. ET production and receptor expression by the thick ascending limb
There is not universal agreement on whether or not thick ascending limb (TAL) synthesizes ET-1. Several groups failed to detect ET-1 protein and/or mRNA in rat or human TAL (38, 341, 458, 459, 495). In contrast, ET-1 production was observed in TAL from rabbit and rat (54, 159, 214, 215), while ECE-1 mRNA was observed in rat TAL (84). TAL ET-1 release was greater than PT, but less than collecting duct (214, 215). Taken together, these data suggest that TAL likely do produce ET-1, albeit in relatively low amounts that can make its detection difficult (Figure 6).
Figure 6.
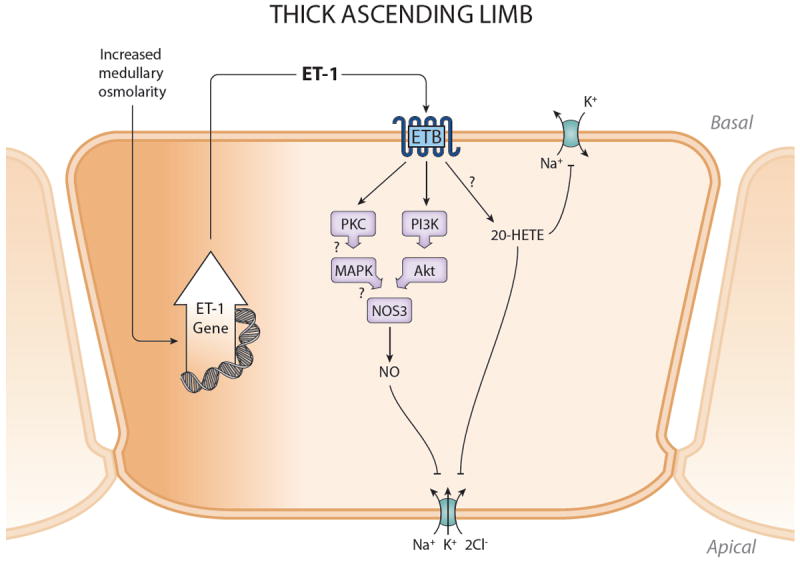
Synthesis and actions of ET-1 in the thick ascending limb. ET-1 production is stimulated by increased medullary osmolality which occurs during high Na intake. ET-1 can then act in an autocrine manner, via ETB, to stimulate NOS3 activity and inhibit NKCC2. ETB may also increased 20-HETE with possible inhibition of Na/K ATPase activity, although this is unproven. Please see text for definitions of abbreviations.
Factors regulating TAL ET-1 production are not well known. One study found that increasing osmolarity stimulated cultured rat TAL ET-1 production; since high salt intake raises outer medullary tonicity, this suggests that TAL ET-1 release may be coupled to Na load (159). As will be described below, ET-1 can inhibit TAL Na reabsorption, hence Na intake-induced alterations in TAL ET-1 activity may provide one mechanism for regulating ECFV.
TAL respond to ET-1 via ETB activation (159). However, the available data is contradictory on ET receptor expression. Rat TAL were found to express ETB, but not ETA, protein and mRNA (116, 440). In contrast, no or extremely faint ET receptor expression was detected in rat TAL (78, 431, 481). Thus, TAL appear to contain ETB, although at very low levels.
2. ET actions in the thick ascending limb
ET-1 can inhibit Cl transport in the TAL through NO-, and possibly PKC-, dependent decreases in NKCC2 activity (Figure 6). ET-1 reduced Cl transport in rat cortical TAL through activation of ETB and ensuing increases in [Ca2+]i. and stimulation of NO (the ET-1 effect was abolished by L-NAME) (330). In a similar study, ET-1 inhibited rat medullary and cortical Cl transport vis ETB and PKC, but was independent of prostaglandins, cAMP or alterations increases in [Ca2+]I (76). ET-1 increases NOS3 expression and NO production by rat MTAL, an effect that is mediated by ETB and PI3K (160). In addition, ET-1 stimulated of MTAL NO production is absent in MTAL from NOS3 deficient mice (161); this effect was Akt-dependent and involved phosphorylation of NOS3 at Ser1177. Since NO can inhibit Na-K-2Cl cotransport in TAL (reviewed in (316)), the above studies paint a convincing picture that ET-1, via ETB activation, stimulates NO and potentially other signaling pathways, to reduced TAL NKCC2 activity. There is evidence that this system is physiologically relevant. As mentioned earlier, high salt intake raises medullary osmolality (159); this was associated with increased TAL NO3 expression which could be prevented by non-selective ET receptor blockade. Further, increased osmolarity stimulated cultured rat TAL NOS3 expression and this could be prevented by ETB blockade (159). Thus, high salt intake increases medullary tonicity, releases ET-1 which, via ETB stimulation of NO, inhibits TAL NaCl reabsorption. The ultimate role of autocrine ET system in controlling BP and ECFV remains uncertain; insights may be gained from studies using mice deficient in TAL ETB and/or ET-1.
In addition to NO, ET may activate other signaling mechanisms that could impact TAL NaCl transport. ET-1 activates the MAPK cascade in rat MTAL, potentially involving non-NO pathways (442). Another interesting possibility is 20-HETE. While not studied in the TAL, ET-1 increases PT 20-HETE, a potent inhibitor of the Na/K ATPase (103). The TAL also produces 20-HETE where it has been shown to inhibit NKCC2 activity, in part by blocking Na/K ATPase as well as a 70 pS apical potassium channel (reviewed in (378, 526)). Thus, studies on ET-1 regulation of TAL 20-HETE, and how this is involved in mediating responses to Na intake, are clearly indicated.
E. ET regulation of collecting duct Na transport
1. Baseline ET production by the collecting duct
The CD is the predominant source of ET-1 in the kidney (212). Within the CD, numerous studies have repeatedly shown that the inner medullary CD (IMCD) produces the most ET-1, at least in the rat and rabbit (54, 214, 458, 459). In addition, porcine and human IMCD produce ET-1 mRNA and protein (19, 213, 341). The outer medullary CD (OMCD) produces the second most amount of ET-1 in the CD (54, 216, 341, 458, 459); both OMCD and IMCD have high ECE-1 content (342). The cortical CD (CCD) synthesizes less ET-1 than elsewhere in the CD, but more than any other nephron segment (214, 291). Similarly, the entire CD expresses relatively high ET-3, albeit much less than ET-1 (214, 441). While specific CD cell types have not been examined, given that the IMCD produces the most ET-1, it is likely that the principal cell is the major site of ET-1 synthesis in the CD.
Cultured rat IMCD, mouse CCD and MDCK cells secrete 2-10 fold greater ET-1 onto the basolateral as compared to the apical side (218, 386, 447, 457). Since most CD ET receptors are located basolaterally, this suggests that ET-1 potentially functions as an autocrine factor in the CD.
2. Regulation of ET production by the collecting duct (Figure 7)
Figure 7.
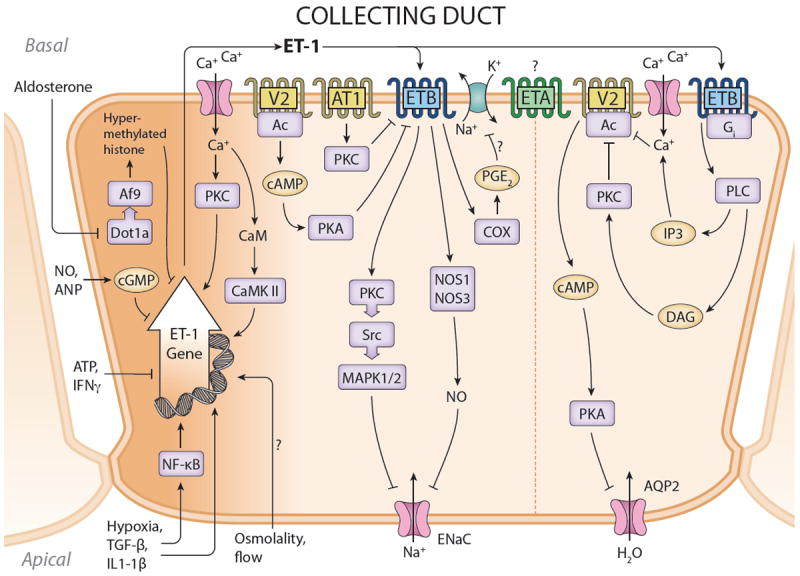
Synthesis and actions of ET-1 in the collecting duct. ET-1 gene transcription is under complex control, involving transactivators binding to cis elements in the ET-1 promoter, as well as histone methylation. The latter effect mediates aldosterone stimulation of collecting duct ET-1 production; this may serve as a negative feedback regulator of aldosterone-stimulated Na transport in this nephron segment. ETB mediates ET-1 inhibition of water transport, primarily through inhibition of AVP-stimulated adenylyl cyclase (AC) activity. ETB also mediates ET-1 inhibition of ENaC activity; this involves both NO and MAPK. V2 and AT1 receptors have been reported to inhibit ETB expression in this nephron segment. The role of ETA in regulating collecting duct Na and water transport is uncertain. Please see text for definitions of abbreviations.
CD ET synthesis is controlled by ECFV status. Indirect evidence for this comes from studies in which Na loading increases rat medullary ET-1 mRNA, ECE-1 mRNA and protein, and urinary ET-1 excretion (4, 110). More direct evidence comes from studies in which the increase in urinary ET-1 excretion observed following Na loading is substantially blunted in mice with CD-specific knockout of the ET-1 gene (4).
How ECFV expansion stimulated CD ET-1 production is unclear, however it does not appear to relate to circulating hormones. AVP, bradykinin and norepinephrine have no effect on ET-1 release of cultured mouse, rat and/or porcine IMCD cells (OMCD or CCD have not been studied, primarily due to inability to obtain sufficient tissue) (216, 219, 284, 446, 447). Aldosterone increases renal ET-1 mRNA in adrenalectomized rats (499), while DOCA/high Na increases medullary and IMCD ET-1 content (170). Aldosterone enhances cultured mouse IMCD ET-1 gene expression (143); this effect has been ascribed to stimulation of Sgk1 through derepression of ET-1 gene expression by inhibition of Dot1a-Af9-induced histone hypomethylation (525). As will be discussed below, ET-1 has a pronounced natriuretic effect on the CD, hence aldosterone induction of CD ET-1 mitigate the anti-natriuretic effects of aldosterone, but does not explain how ECFV expansion increased CD ET-1 production.
The effect of local factors that respond to ECFV on CD ET-1 production has been examined, however there is as yet no clear consensus. High Na intake increases medullary (at least outer medullary) osmolality (159) and medullary ECE-1 content (110), while renal medullary administration of hypertonic, but not isotonic, NaCl or mannitol into rats increased urinary ET-1 excretion (31). Media made hyperosmolar with betaine or urea enhanced ET-1 production (via p38 MAPK and ERK activation) by MDCK cells, a canine distal nephron cell line (236). In addition, media made hyperosmolar with NaCl or urea stimulated ET-1 synthesis by porcine IMCD cells (284), while hyperosmolar NaCl increased ET-1 protein and/or mRNA content in rat and rabbit IMCD (513). While this makes a nice story, wherein Na loading increases medullary tonicity, leading to increased ET-1 and an ensuing natriuresis, unfortunately there is much conflicting data. For example, hypertonic urea or mannitol have not been consistently found to increase IMCD ET-1 production (513). To make matters even more puzzling, several groups have reported that hyperosmolar media, particularly when due to relatively impermeable solutes like NaCl, raffinose or mannitol, inhibited ET-1 secretion by MDCK or rat IMCD cells (220, 386). The reasons for these disparate results are not readily apparent. The possible of a biphasic ET-1 response to media hyperosmolality was raised by the findings that 6 hr exposure to hypertonic NaCl increased ET-1 release by mouse CD cell lines, while 24 hr exposure reduced ET-1 secretion (447). However, this pattern has not been observed by others. This latter group also noted that AVP was required in order to see CD cell responsiveness to hyperosmolarity, but AVP was not needed for the response in other studies. Thus, there are not obvious differences in study conditions or cell types that explain the varying results. In addition, all of these studies have been conducted on cells in vitro. In essence, it appears that the effect of environmental osmolarity, including the effect of specific solutes, will not be resolved until studies can be conducted using in vivo models. For example, studies examining the effect of intramedullary infusion of hypertonic saline, or correlation of CD ET-1 mRNA with medullary tonicity, may begin to shed some light on this issue. Finally, from a strictly teological standpoint, while interstitial tonicity can change in the medulla in response to alterations in Na intake, such effects are not likely to occur in the renal cortex; thus, CCD ET-1 production is probably regulated by other factors.
An interesting possibility for local control of CD ET-1 production relates to tubule fluid flow. It is notable that conditions that increase tubule flow, including high intake or administration of salt, salt plus water, or water alone virtually always increase urinary ET-1 excretion in humans and experimental animals (62, 69, 176, 274, 281, 282, 286, 287, 374, 434, 503, 521). Since, as mentioned above, a significant component of urinary ET-1 excretion derives from the CD (2, 4), then tubule fluid flow, which is common to salt and/or water loading, could play a role in increasing CD ET-1 synthesis. This intriguing possibility has not been examined in CD, however there is evidence from other cell types to support this notion. Increasing flow, with resultant increased shear stress, enhances ET- release by endothelial or mesothelial cells (73, 74, 290, 468). In addition, CD cells are capable of sensing and responding to changes in flow. Such regulation includes alterations in K secretion by mouse CCD in response to varying flow (435, 504), while increased tubule fluid flow augments ENaC open channel probability (Po) in rabbit CCD (294). Furthermore, a potential mechanism of flow detection has been indentified in CD whereby flow-induced bending of primary cilia alters [Ca2+]I (169, 264, 337, 338, 408). Of course, even if flow does regulate CD ET-1 production, in of itself, it would not discriminate between Na and water loading, so other mechanisms must also be involved.
A number of factors have been shown to affect CD (primarily using cultured rat IMCD) ET-1 synthesis, however these are not clearly associated with ECFV. These include inhibitors of cultured rat IMCD ET-1 release (reduced pH (417), interferon-γ (219), and ATP (175)), while stimulatory factors include hypoxia (285), IL-1ß and TGFß (167, 284, 384).
Relatively little is known about the signaling pathways that control ET-1 synthesis in the CD. However, Ca2+ appears to play a pivotal role. Increasing [Ca2+]I with a Ca2+ ionophore augmented ET-1 production by IMCD cells (284), while chelation of intracellular Ca2+ markedly decreased IMCD ET-1 synthesis (415). Alterations in [Ca2+]I likely act through calmodulin (CaM) or CaM kinase since inhibition of these pathways also greatly reduced IMCD ET-1 secretion and mRNA content (415). These effects of the Ca/CaM pathway are mediated, at least in part, through changes in ET gene transcription. Inhibition of CaM did not alter ET-1 mRNA stability, however it did greatly reduced activity of a transfected ET-1 promoter-reporter construct in IMCD cells (415). An interesting aspect of these latter studies was that the transfected ET-1 promoter-reporter constructs revealed maximal activity within 1.72 kb 5’ to the transcription start site with only one-third of this activity in the region 0.36 kb 5’ to the transcription start site; this region of the ET-1 promoter has not been typically described as being involved in mediating the known mechanisms for control of ET-1 gene transcription. In addition, CaM inhibition markedly decreased activity of the 1.72 kb, but not 0.36 kb, promoter regions, while it did not affect activity of transfected ET-1 promoter-reporter constructs in aortic endothelial cells. Thus, CD ET-1 synthesis may be under unique regulation involving Ca2+/CaM-dependent pathways. Further exploration of these intracellular signaling pathways controlling ET-1 production by CD should lead to important insights into how not only CD ET-1 synthesis is regulated, but may suggest how ECFV is coupled to this system.
Finally, it is pertinent to note that hypertension has been associated with possible changes in CD ET-1 production. For example, IMCD from hypertensive SHR rats secreted less ET-1 than did IMCD from normotensive WKY rats, while SHR had reduced ET-1 in the outer and/or inner medulla as compared to WKY rats (136, 173, 210). Renal papillary ET-1 content and mRNA are also reduced in Prague hypertensive and Dahl S rats (465). While studies have been quite limited in humans, involving only a small number of patients, individuals with essential hypertension (especially of the salt-sensitive type), have reduced urinary ET-1 excretion (164, 176, 527). Thus, CD ET-1 production may be reduced in the hypertension. As will be discussed below, such an alteration in CD ET-1 could potentially be involved in the pathogenesis and/or maintenance of the hypertensive state.
3. ET receptor expression by the collecting duct
Numerous studies, using binding or autoradiography, have found that the medulla in general, and the CD in particular, is the predominant site of renal ET binding in several species, including man (215, 428, 492, 515) (Figures 3 and 7). Binding, autoradiographic, immunofluorescent, and mRNA studies reveal that the renal medulla and the CD primarily express ETB, with the greatest expression being in the IMCD (19, 58, 199, 217, 239, 431, 440, 481). CD also express ETA, albeit in much smaller amounts than ETB. While some studies have failed to detect ETA message or protein in rat or human CD (58, 198, 431, 440), several other groups, using binding, immunostaining, autoradiography or electron microscopy have found ETA in CD from dog, rat and rabbit IMCD and/or CCD (50, 91, 217, 311, 481, 518). A recent study demonstrated ETA in the basal infoldings of the CD, and particularly in the IMCD (508). Furthermore, as described below, evidence exists for functional ETA in the CD. Finally, as alluded to earlier, the bulk of ET receptors are expressed on the basolateral side of the CD (albeit only IMCD was studied) (218). Thus, given the preferential CD basolateral ET-1 secretion (218, 386, 446, 457), the stage is set for an autocrine ET effect in the CD.
CD ET receptor expression may be subject to regulation. AVP has been shown to downregulate CD ET receptors. AVP, forskolin or dibutyryl cAMP decreased ET-1 binding affinity to rat CCD (430). Interestingly, this effect was prevented by PKA inhibition; ETB, but not ETA, contain a consensus amino acid sequence for PKA-mediated phosphorylation. Similarly, AVP, via cAMP, reduced the Bmax for ET-1 binding to rat IMCD associated with a decrease in ETB mRNA (498). AII may also downregulate CD ET receptors; AII has been shown to decrease ETA and ETB binding and mRNA content in rat IMCD (via a PKC) (497). It is notable that CD ETB expression is reduced in congestive heart failure models (116, 500), while AII receptor blockade can mitigate this downregulation (500). These findings raise the interesting possibility that AVP and AII, which can stimulate tubule Na reabsorption, reduce ET receptor expression in order to mitigate the natriuretic effects of ET-1 in the CD.
4. Overview of ET regulation of Na transport in the collecting duct
ET-1 can directly reduce Na reabsorption in the CD (Figure 7); the peptide decreases Na transport by rat and rabbit CCD (243, 448). This effect of ET-1 is likely due to inhibition of the major Na reabsorptive pathways in the CCD, namely Na/K ATPase and the epithelial Na channel (ENaC). ET-1 reduces ouabain-sensitive oxygen consumption (which presumably reflects Na/K ATPase activity) in rabbit IMCD cells (520). Beyond this, however, ET effects on Na/K ATPase in the CD have not been investigated. In contrast, much more is known about ET-1 modulation of ENaC in the CD. The peptide decreases amiloride-sensitive Na uptake by amphibian distal nephron A6 cells (via an increase in mean Na channel closed time) (122) and by 3T3 cells stably expressing ENaC (135). Furthermore, ET-1 reduced ENaC open probability in isolated split-open rat CCD (40). The physiologic relevance of CD-derived ET in controlling urinary Na excretion was demonstrated using mice with CD-specific knockout of the ET-1 gene (CD ET-1 KO) (4). These mice lack ET-1 specifically in principal cells of the CD; they are hypertensive by about 15 mmHg systolic BP during normal salt intake, and develop marked hypertension (by about 35 mmHg systolic BP) on a high Na diet. The hypertension in CD ET-1 KO mice is associated with excessive weight gain and reduced Na excretion when the animals are subjected to Na loading. In addition, increases in renal perfusion pressure were associated with reduced Na excretion in CD ET-1 KO mice (381). Increased ENaC-mediated Na reabsorption may be involved since amiloride largely prevented the elevated BP and Na retention in the CD ET-1 KO mice (4). Thus, CD-derived ET-1 is an important physiologic regulator of Na reabsorption and arterial BP, most likely through, at least in part, inhibition of CD ENaC activity.
5. ET receptor isoforms mediating collecting duct Na transport
ETB appears to be the primary mediator of ET-1 inhibition of CD Na transport (Figure 7). ETB agonism inhibits Na channel activity in A6 cells (122), while ETB, but not ETA, antagonists prevent ET-1 inhibition of ENaC open channel probability in rat CCD (40). Infusion of an ETB antagonist directly into the rat renal medulla infusion decreases volume expansion-induced natriuresis (145), while intramedullary infusion of an ETB agonist increases Na excretion in wild type, but not ETB-deficient, rats (299). These ETB-deficient rats are generated using animals with a null mutation in ETB, while containing a transgene that confers relatively gut-specific ETB expression (the latter prevents intestinal aganglionosis) (126). The rescued ETB deficient rate develop exaggerated hypertension in response to DOCA and high salt intake that is ameliorated by amiloride (125, 126). CD ETB has been most directly implicated in regulation of Na excretion in studies using mice with CD-specific knockout of the ETB gene (CD ETB KO) (129). CD ETB KO mice had about an 8 mmHg elevation in systolic BP during normal salt intake, while systolic BP was increased by about 20 mmHg during high salt intake; the hypertension is associated with impaired ability to excrete a Na load. Note that the magnitude of the hypertension in these CD ETB KO mice is about 50-60% of that seen in CD ET-1 KO animals, suggesting that CD ETB do not account for all of the antihypertensive and natriuretic effects of CD-derived ET-1.
There is conflicting data on the ability of ETA to modulate Na transport in the CD. In A6 cells, ET-1, In the presence of ETB antagonism, enhances Na channel activity (122). In contrast, intramedullary administration of ET-1 causes a natriuresis in ETB-deficient rats that is prevented by ETA blockade (298). Curiously, this latter study found apparent ETA-mediated natriuresis only in female ETB-deficient rats. Only male ETB-deficient animals had ET-1- induced reductions in medullary blood flow, suggesting that ET-1 (presumably via ETA) natriuresis in males is prevented by a concurrent fall in medullary perfusion, thereby mitigating any direct tubular effect of the peptide. Perhaps of greatest direct relevance is the finding that mice with CD-specific knockout of ETA (CD ETA KO) have no detectable alterations in arterial BP or urinary Na excretion on a normal or high salt diet (132). Thus, taken together, the above studies do not provide convincing evidence that ETA plays a role in the physiologic regulation of CD Na reabsorption. However, a subsequent study indicates that the simple paradigm of CD ETA and ETB operating as individual receptors with well-defined agonists and signaling pathways may not be valid. In this study, mice with CD-specific knockout of both ETA and ETB (CD ETA/B KO) were found to hypertension and Na retention that was similar to that seen in CD ET-1 KO mice on a normal or high Na diet, and substantially greater than that in CD ETB KO animals (381). These results were unexpected since CD ETA KO mice had no phenotype. One possible explanation is that both ETA and ETB can exert natriuretic effects, but that ETB are more effective (since ETB are much more prevalent in CD). In this scenario, absence of CD ETA may be obscured by abundant ETB, while absence of ETB may be particularly compensated for by upregulation of ETA (this has not been specifically investigated). Another possibility is that CD ET receptors may undergo homo- and hetero-dimerization with potential functional implications. For example, one might envision that ETB homodimers and ETA/B heterodimers normally exist since the ETB isoform predominates, however in CD ETB KO, ETA homodimers may form and exert a natriuretic effect. Resolution of these possibilities will be quite challenging, but represents an exciting new avenue for investigation into how ET receptors operate.
6. Mechanism of ET regulation of collecting duct Na transport
Several kinases have been implicated in ET-1 inhibition of CD NaCl transport (Figure 7). PKC may play a role in ET-1 inhibition of AVP-stimulated chloride transport in the rat CCD (448), however is does not appear to mediate ET-1 effects on ENaC in this nephron segment (40). ET-1 inhibition of ENaC was dependent upon Src kinase activity in 3T3 cells transfected with the three ENaC isoforms (135). In addition, ETB-mediated inhibition of ENaC open channel probability in isolated split-open CCD required Src kinase and MAPK1/2 (40), while ET-1 increases MAPK activity in rat OMCD and IMCD (442). Thus, ET-1 inhibition of CD ENaC is due, at least partly, to ETB activation with resultant involvement of Src and MAP kinases. In this regard, it is relevant to note that MAPK-mediated ENaC phosphorylation reduces both ENaC activity and number (32, 56, 109, 395). Recent studies using cultured CD cells led credence to the notion that ET-1 can reduced ENaC apical membrane expression in that long-term ET-1 exposure reduced ENaC channel number through enhanced ß1Pix binding to 14-3-3ß, preventing 14-3-3ß interaction with the ubiquitin ligase Nedd4-2, and thus enhancing ENaC internalization and degradation (323).
Nitric oxide also mediates, at least in part, ET-1 inhibition of CD Na reabsorption. Nitric oxide has been demonstrated to inhibit Cl transport in TAL (330), reduce Na flux in CCD (316), and decrease ENaC activity in cultured CD cells (154). IMCD from ETB-deficient rats have decreased NOS activity (436), while ETB activation increases NOS1-dependent NO production and cGMP accumulation in rat IMCD cells (416). Further, ET-1 increased NOS1 protein expression in IMCD3 cells (possibly via ETA) (421), while ETA or ETB blockade reduces NOS3 mRNA and protein expression in rat IMCD cells (515). The most direct evidence for a role for NO in mediating ET-1 natriuretic actions comes from studies using CD ET-1 KO mice (381). These animals had reduced NO metabolite excretion during normal or high Na intake as well as during graded increases in renal perfusion pressure; this was associated with reduced inner medullary NOS1 and NOS3 activities. Importantly, inhibition of NO formation greatly increased BP in control animals, but had a minimal effect on BP in CD ET-1 KO mice; in essence, the difference in BP between the two groups was eliminated. In agreement with these findings, the natriuretic response to intramedullary infusion of an ETB agonist was reduced by NOS1 inhibition (299). Thus, these studies strongly indicate that NO is a key mediator of ET-1-induced natriuresis; how this occurs remains to be determined.
Initial studies suggested that prostaglandins might be involved in the natriuretic response to ET-1, however subsequent studies have not supported this notion. Cyclooxygenase blockade was originally shown to abolish ET-1 inhibition of Na/K ATPase activity in rabbit IMCD (520), while ET-1 was found to stimulate rabbit and mouse IMCD PGE2 production (via ETB and COX2) (221, 520). In contrast, generalized COX or COX2-specific inhibition did not alter Na retention or BP in CD ET-1 KO mice (131). In addition, CD ET-1 KO actually had increased IMCD PGE2 levels, i.e., COX activity appears to be upregulated. Hence, eicosanoids do not appear to be central in mediating the natriuretic effects of ET in the CD.
7. Paracrine effects of collecting duct-derived ET
Since the CD produces very large amounts of ET-1, the potential exists for CD-derived ET-1 to exert paracrine effects. While a number of obvious possibilities exist, including modulation of regional blood flow or transport functions in neighboring tubules, this has not been definitively shown. However, there is some data to suggest the interesting possibility that CD-derived ET-1 may modulate renin production. CD ET-1 KO mice, despite being volume expanded and hypertensive, have plasma renin activity and aldosterone levels that are not different from controls (4). These mice have renal renin contents similar to controls, while their hypertension is significantly abrogated by either AII or aldosterone blockade (130). Thus, the hypertension in CD ET-1 KO animals may be partly due to failure to suppress renin production. Since ET-1 is well described to inhibit renal renin production (3, 100, 244, 263, 288, 297, 358, 359, 373, 385, 388, 427), and since CCD and connecting segment are adjacent to the juxtaglomerular apparatus and afferent arteriole (355, 464), the possibility exists that CD-derived ET-1 exerts a paracrine inhibitory effect on renal renin production.
V. ET regulation of water transport
A. Overview of ET and water transport
Intravascularly administered ET increases urinary water excretion, even if given at doses that reduce RBF (137, 197, 364, 383). ETB-specific agonists also increase urine flow, although interpretation of these studies is confounded by the renal vasodilatory effects of these agents (65, 281, 519). In addition, water loading increases human and experimental animal urinary ET-1 excretion (220, 282, 503, 521). Thus, the renal response to water loading is increased ET-1 synthesis which, in turn, can exert a diuretic effect.
The evidence is compelling that ET inhibits water reabsorption in the CD. ET-1 dose-dependently decreases AVP-stimulated water (but not urea) transport in rat CCD and IMCD (90, 296, 309, 448). Mice lacking ET-1 in the CD have altered water metabolism, including reduced diuresis after acute water loading, decreased plasma AVP concentration under euvolemic conditions, and exaggerated responsiveness to exogenous administered AVP (increased urine osmolality, AQP2 mRNA and AQP2 phosphorylation) (128). Thus, absence of CD ET-1 augments AVP responsiveness, indicating that endogenous CD ET-1 functions as a physiologic inhibitor of AVP-induced water retention.
B. Signaling pathways in ET regulation of water transport
ET inhibition of AVP-stimulated water transport in the CD is partly due to reduced adenylyl cyclase activity; ET inhibits AVP-stimulated adenylyl cyclase-dependent cAMP accumulation in rat and/or porcine CCD, OMCD and IMCD (90, 221, 284, 296, 309, 431, 448, 449) (Figure 7). ET-1 may exert this cAMP-inhibiting effect in an autocrine manner since anti-ET-1 antibodies enhanced AVP-stimulated cAMP accumulation in IMCD cells (218). Further, ET-1 does not inhibit dibutyryl cAMP-stimulated water transport in the IMCD (309), indicating that the peptide’s effect depends upon adenylyl cyclase activity. CD isolated from CD ET-1 KO mice have increased cAMP accumulation in response to AVP or forskolin (128) and this is associated with increased expression of adenylyl cyclases 5 and/or 6 (414). Thus, there is compelling evidence that CD ET-1 acts as a physiologic inhibitor of AVP-stimulated adenylyl cyclase activity in the CD.
The diuretic effect of ET-1 on the CD is primarily due to activation of ETB; ETB-specific agonists inhibit AVP-induced cAMP accumulation in rat IMCD, while ETB-, but not ETA-, specific antagonists, block ET-1’s effect (90, 221, 502). Notably, mice with CD-specific ETA deletion have increased plasma AVP levels, increased ability to eliminate a water load, and reduced AVP- and forskolin-stimulated cAMP accumulation (the latter in acutely isolated IMCD) (132). Since these mice express only ETB in the CD, this raises the possibility that enhanced ETB binding by ET-1 (since ETA are absent) promotes a diuresis. In addition, these mouse studies suggest that CD ETA may exert an antidiuretic effect, however more direct evidence of this is needed. Finally, the diuretic effect of ET-1 on the CD may be mitigated by AVP itself; AVP reduces ETB affinity for ET-1 in rat CCD (via a PKA) (430).
The inhibitory effect of ET-1 on cAMP accumulation is due, at least in part, to inhibitory G proteins, PKC and changes in intracellular Ca2+(Figure 7). Pertussis toxin prevents the inhibitory effect of ET-1 on AVP-induced cAMP and water transport in the IMCD, implicating Gi in this process (221, 296). These effects of ET-1 on AVP responsiveness are dependent upon PKC and are associated with increased inositol phosphates in CD cells (221, 296, 449, 450, 501, 502). In turn, ET-induced increases in [Ca2+]i are likely involved in this process. The data on ET-1 actions on intracellular Ca2+ in CD cells is somewhat conflicted, however this likely reflects ET-1 modulation of multiple regulatory pathways. In general, ET-1 seems to cause an initial rapid rise, follow by a sustained elevation, in CD [Ca2+]I (284, 302). The initial increase in [Ca2+]I is probably related, at least in part, to release of Ca2+ from cell stores. While initial studies showed that the rapid and transient increase in [Ca2+]I depended upon extracellular Ca2+ entry (302), subsequent studies found that extracellular Ca2+ entry was uninvolved (235, 296). In contrast, the prolonged ET-1-induced increase in [Ca2+]I most likely depends upon extracellular Ca2+ entry which is partly mediated by dihydropyridine-sensitive Ca2+ channels (235, 243). Initial studies found no evidence for dependence upon dihydropyridine-sensitive Ca2+ channels (302), however subsequent studies indicated that the sustained increase in [Ca2+]I was blocked by nifedipine or similar agents (235, 243, 296). These increases in [Ca2+]I may play a role in ET-1 inhibition of water transport since increased [Ca2+]I has been demonstrated to reduce AVP-stimulated adenylyl cyclase activity in rat IMCD (via PLC-induced activation of PKC) (437). However, the component of ET-1-augmented [Ca2+]I that involves dihydropyridine-sensitive Ca2+ entry may not be involved in regulation of water transport since inhibition of these channels did not change AVP-induced cAMP accumulation in rat CD (449, 450).
While ET-1 can stimulate CD production of prostaglandins and NO, and these substances have been shown to modify AVP actions, there is no evidence to date that they are involved in ET-1 regulation of water transport. Inhibition of COX does not change ET-1 effects on AVP-stimulated cAMP content in cortical through inner medullary CD (221, 449, 450). In addition, neither NOS blockade nor factors that augment NO production (NO donors, L-arginine, NADPH, or tetahydrobiopterin) altered ET-1 inhibition of AVP-stimulated cAMP accumulation in the IMCD (416).
VI. ET and acid/base transport
A. Overview of ET and acid/base transport
Published data to date support the notion that renal ET-1 helps mediate increased kidney acid (H+) excretion in response to a systemic H+ challenge, but does not measurably contribute to H+ excretion under “control” conditions. Because diets in industrialized societies are typically acid inducing (353), “control” conditions typical of humans in industrialized societies might be one in which ET-mediated kidney H+ excretion is the basal state. Additionally, ET-1 mediates increased kidney acidification in experimental models of chronic kidney disease with reduced GFR (488), a syndrome with a significant and increasing prevalence. Furthermore, data from animal (327) and more recently from human (328) studies suggest that increased kidney ET-1 action induced by metabolic acidosis associated with reduced GFR contributes to progressive GFR decline in some types of progressive nephropathies. Hence, understanding the ET-mediated contribution to kidney acidification has important physiologic, pathophysiologic, and possibly clinical relevance.
B. Overview of the renal response to a systemic H+ challenge
The routine H+ challenge to systemic acid-base homeostasis for most humans is H+ derived from liver metabolism of dietary protein (252, 353). Metabolism of sulfur- and phosphate-containing amino acids yields H+ and most diets in industrialized societies include dietary protein with a high proportion of such amino acids (353). This metabolically produced, “fixed” H+ might increase body fluid [H+] and induce a physiologic response designed to restore optimum [H+]. Two responses designed to maintain and/or restore optimal body fluid [H+] are 1) H+ buffering (251) in which body buffers bind added H+ to minimize the [H+] increase that would otherwise occur, and; 2) “fixed” H+ excretion, predominantly by kidneys (146, 252). Because H+-titrated buffers less effectively buffer subsequently added H+, buffer-bound H+ must eventually be excreted to regenerate body buffers and restore buffering capacity. Consequently, all added H+ must be excreted to restore acid-base homeostasis. Thus, ET-mediated kidney H+ excretion might routinely contribute to the H+ challenge induced by our industrialized society diets.
Most experimental models exploring the effect of an H+ challenge on systemic acid-base balance use H+ challenges that are in excess of that routinely encountered by animals or humans. Such studies show an important role for enhanced proximal tubule acidification in mediating kidney H+ excretion (39). The Na+/H+ exchanger type 3 (NHE3) is the major luminal H+ transporter in the proximal tubule, while more modest contributions are made by the H+-ATPase (340). Large H+ loads lead to increased activity of both NHE3 (340) and H+-ATPase (52) in the proximal tubule. Similarly, these large H+ loads increase acidification in the thick ascending limb of the loop of Henle (42). Because NHE is the major luminal H+ transporter in the loop of Henle with smaller contributions by H+-ATPase (42), the acidification increase in response to this large H+ challenge presumably includes stimulated NHE activity. Finally, these large H+ loads administered systemically enhance distal nephron acidification (18, 256, 275) that is mediated by enhanced activity of NHE2 (275), H+-ATPase (276), and H+, K+-ATPase (400). In short, large systemic H+ loads lead to increased acidification in most acidifying nephron segments that have been studied.
Animals and humans rarely face H+ challenges of the magnitude used in most experimental models. The more routine H+ challenge faced by humans is much more modest and is associated with little to no measurable changes in plasma acid-base parameters (245). More modest H+ challenges that substantially increase urine net H+ excretion do so without measurable changes in plasma acid-base parameters or proximal nephron acidification, but do increase distal nephron acidification (487, 488, 490). Furthermore, unlike the mineral salts typically used to induce H+ challenges in experimental models, the H+ challenge faced by humans is typically provided by H+-producing dietary protein. Increased H+-producing dietary protein increases distal nephron acidification (203, 204, 242, 494) that is clearly evident with comparable HCO3 delivered loads (203). Enhanced distal nephron acidification in response to a more physiologic H+ challenge is mediated by both decreased HCO3 secretion and increased H+ secretion whether the challenge is induced by modest NH4Cl ingestion (487, 488, 490) or by H+-producing dietary protein (203, 204, 242, 494). Nevertheless, increased intake of H+-producing dietary protein also increases proximal tubule acidification as indicated by overall recovery of increased filtered HCO3 load mediated by increased whole kidney and single nephron GFR induced by high protein diet (494).
In vivo studies show that a modest and chronic dietary H+ challenge can increase kidney net H+ excretion and distal nephron acidification without measurable changes in plasma acid-base parameters, but with detectable increases in H+ content of the kidney interstitium (487). Such a systemic H+ challenge also increases renal ET-1 protein (262, 488) and its mRNA (203, 262). This increase in kidney ET-1 is detectable within the interstitium (203), however the cellular source of this ET is not clear. As has been discussed previously, virtually every cell type within the kidney can synthesize and release ET-1. Furthermore, endothelial and epithelial cells likely release the majority of their ET-1 abluminally, thereby facilitating autocrine and paracrine effects within the kidney (467).
C. Adrenal cortex ET production and its role in kidney acidification
Acid-stimulated ET secretion from non-kidney tissue might directly or indirectly enhance kidney acidification in response to an H+ challenge. ET-stimulated aldosterone secretion (14, 79, 307) enhances distal nephron acidification induced by H+-producing dietary protein (204). In addition, dietary mineral H+ (379) and H+-producing dietary protein (393) increase plasma aldosterone. Furthermore, an H+ extracellular environment increases aldosterone secretion by adrenocortical cells in vitro (237). The adrenal cortex synthesizes ET (14) that stimulates secretion of mineralocorticoids (14, 79) and glucocorticoids (79). Because glucocorticoids (9, 26) and mineralocorticoids (94) stimulate H+ secretion in kidney tubule epithelium, adrenal cortical ET might indirectly increase kidney acidification through increased mineralocorticoid and glucocorticoid secretion.
D. ET effects on acidification
1. Cells
ET increases intracellular pH of many cell types in vitro including skin fibroblasts (124), platelets (451), vascular smooth muscle (172), glomerular mesangial (407), cardiac myocyte (470) and kidney epithelial cells (469). ET increases Na+/H+ antiporter activity in kidney epithelial membrane vesicles (93) and cortical slices (144) and is the cell membrane H+ transporter that is most consistently influenced by ET in these indicated cell types.
2. Renal tubules
In addition to enhancing cell membrane H+ transport, ET enhances acidification across kidney tubule epithelia. ET mediates enhanced proximal tubule acidification in chronic metabolic acidosis induced by NH4Cl loading through stimulation of ETB but appears not to mediate proximal tubule acidification in non-H+-loaded animals (246, 247). ET, via ETB activation, mediates enhanced distal nephron acidification induced by dietary H+ loading with NH4+ salts (488) and H+-producing dietary protein (203, 204, 242, 494). ET also stimulates H+ secretion in alpha intercalated cells and decreases HCO3 secretion in beta-intercalated cells of the rabbit cortical collecting duct in an in vitro model of metabolic acidosis (455). The latter studies support that these effects of ET are direct rather than acting through other mediators. In addition, exogenous ET stimulates distal nephron acidification in vivo (484). As observed in the proximal tubule, ET appears not to mediate basal distal nephron acidification in control animals (203, 204, 488). In the loop of Henle, ET stimulates local NO release (329) and NO inhibits thick ascending limb Na+/H+ exchange (127), suggesting that ET indirectly inhibits acidification in this segment. Because ET-induced NO action on thick ascending limb Na+/H+ exchange activity might more importantly inhibit NaCl reabsorption in this nephron segment (330), this indirect action of ET action in the thick ascending limb would increase distal nephron Na+ delivery with an anticipated increase in distal nephron Na+/H+ exchange activity (479), enhancing distal nephron acidification. Consequently, the net effect of ET is increased proximal and distal nephron acidification.
E. Direct mechanisms by which H+-induced ET activity increases kidney acidification
ET increases proximal tubule acidification in H+-challenged animals and cells primarily if not exclusively through enhanced activity of NHE3 (59, 246, 247), the major H+ transporter in the proximal tubule (340). ET-1-mediated stimulated NHE3 activity in opossum kidney cells (a proximal tubule cell line) appears to act through ETB, but not ETA (60). The ETB effect was mediated by the COOH-terminal tail and the second intracellular loop of ETB (248), and involved tyrosine kinase pathways (59). ETB stimulation of NHE3 activity is Ca2+- and Rho kinase-dependent, requires phosphorylation and exocytosis of NHE3 into the apical membrane (339), and might involve Gi-mediated inhibition of cAMP accumulation (320). Because ET increases Na+/H+ exchange in the distal tubule and NHE2 appears to be the major apical NHE isoform responsible for luminal H+ secretion in this segment (472), ET appears to increase NHE2 activity. As noted, ET stimulates H+ secretion in alpha intercalated cells (455) whose luminal acidification is mediated predominantly by H+-ATPase (453, 478). These in vitro studies (455) suggest that ET can stimulate H+-ATPase activity without acting through other mediators. On the other hand, ET might indirectly stimulate distal nephron H+-ATPase in vivo (203, 204) through ET-induced stimulation of aldosterone secretion that in turn increases distal nephron H+-ATPase activity (204). Whether ET stimulates H+-ATPase activity in vivo directly and/or through other mediators awaits clarification.
F. Indirect mechanisms by which H+-induced ET activity increases kidney acidification
Because many cytokines influence distal nephron acidification and ET influences levels of many of these cytokines, ET might affect kidney acidification indirectly as well as directly. As discussed earlier, ET increases distal nephron H+-ATPase activity in vivo through stimulated aldosterone secretion (204). Of the three major distal nephron H+ transporters (Na+/H+ exchanger, H+-ATPase, and H+, K+-ATPase) (113), dietary H+ leads to ET-mediated stimulated activity of Na+/H+ exchange (246, 247) and H+-ATPase (203, 204). Dietary H+-induced, ETmediated distal nephron acidification appears not to be mediated by stimulated H+, K+-ATPase activity (204). Although K+ depletion increases H+, K+-ATPase activity (94, 489), stimulated H+, K+-ATPase in chronic metabolic alkalosis associated with K+ depletion appears not to be mediated by ET (489). In addition, ET might indirectly enhance kidney acidification through ET-stimulated secretion of mineralocorticoids and glucocorticoids by the adrenal cortex (26, 79) that stimulates H+-ATPase (26) and Na+/H+ exchange (9, 26), respectively, in kidney tubules as discussed earlier.
Reduced HCO3 secretion contributes importantly to enhanced acidification induced by chronic dietary ingestion of mineral H+ (488, 490) and H+-producing dietary protein (203, 204, 494). The phenomenon of reduced HCO3 delivery to the terminal distal nephron leads to increased net H+ excretion (113) but this phenomenon also enhances NH4+ secretion (211) and permits secreted H+ to titrate non-HCO3 buffers and thereby constitute net H+ excretion rather than HCO3 recovery (39). ET reduces distal nephron HCO3 secretion (that reduces distal nephron acidification) (203, 204, 494) and does so indirectly through stimulated NO production (494). Other studies show that NO stimulates overall acidification in both the proximal (471) and distal (454) nephron in other settings.
G. ET interaction with other mediators of kidney tubule acidification
Other hormones/cytokines modify kidney tubule acidification but few if any have been studied to determine if and/or how any of them interrelate with ET to modify kidney tubule acidification. AII stimulates luminal Na+/H+ exchange and basolateral Na+/HCO3 co-transport (133) as well as stimulates H+-ATPase (466) in the proximal tubule, increasing acidification in this nephron segment. Whether stimulation of Na+/H+ exchange by AII and ET-1 is additive has not been reported in kidney epithelia. Furthermore, AII stimulates distal nephron H+-ATPase (258, 259) thereby increasing acidification in this nephron segment as well. Because AII stimulates ET-1 secretion in kidney mesangial cells (222), some AII effects on acidification might be mediated through ET-1. On the other hand, cAMP stimulates cortical collecting duct HCO3 secretion, thereby decreasing acidification in this nephron segment (390). Because cAMP inhibits cytokine-stimulated ET-1 secretion in kidney mesangial cells (223), cAMP might decrease this effect of ET to decrease distal nephron HCO3 secretion (494). The net cAMP effect would be reduced distal nephron acidification.
Figure 8 outlines a proposed cascade by which ET increases kidney acidification in response to an H+ challenge:
Figure 8.
Role of the renal ET system in control of urinary acid excretion. Dietary acid intake stimulates renal and possibly adrenal ET-1 production. Renal ET-1 increases proximal tubule H+ secretion and distal nephron H+ secretion and bicarbonate reabsorption. Adrenal ET-1 increases aldosterone which stimulates distal nephron H+ secretion. Please see text for definitions of abbreviations.
H. ET role in enhanced kidney acidification associated with pathophysiologic conditions
Enhanced distal nephron H+ secretion that “maintains” chronic metabolic alkalosis is mediated by kidney ET (485, 486, 491). Conversely, it is reduction of this enhanced distal nephron H+ secretion (rather than an induced increase in distal nephron HCO3 secretion) that “corrects” this disturbed distal nephron acidification in this setting (486). Because K+ depletion is important in the maintenance of chronic metabolic alkalosis (486) and because ET mediates enhanced NHE3 activity in an autocrine fashion in proximal tubule epithelium exposed to an acid environment in vitro (10), K+ depletion appears to be an important component of this effect. Indeed, K+ repletion in chronic alkalosis reduces the associated increased urine ET-1 excretion (486), a surrogate of kidney ET-1 production (483), which is characteristic of this disorder (489).
The reduced functioning nephron mass of CKD challenges remaining functioning nephrons to excrete metabolically produced H+ despite their reduced number. Animals with reduced kidney mass have increased proximal (271) and distal (241, 259, 488) nephron acidification in vivo. Animals with CKD due to reduced nephron mass also have increased urine ET-1 excretion (28, 488), consistent with increased endogenous kidney ET-1 production (483, 488). ET mediates enhanced distal nephron acidification in remnant kidneys (488), an experimental model of CKD.
I. Possible pathophysiologic role of ET in syndromes with enhanced kidney acidification
Animals with chronic metabolic alkalosis develop fibrosis with subsequent calcium deposition in kidney parenchyma (257). In addition, experimental animals with reduced kidney mass develop glomerulosclerosis with tubulo-interstitial fibrosis (141). ET increases kidney matrix production and fibrosis in vitro (369) and might mediate glomerulosclerosis in vivo (354). Consequently, increased ET activity in these and possibly other settings might contribute to associated progressive kidney injury through ET-mediated glomerulosclerosis and fibrosis. Indeed, ET mediates 1) tubulo-interstitial injury in animals with intact nephron that ingest an H+-inducing diet (492), 2) GFR decline induced by metabolic acidosis in animals with reduced nephron mass (327); and 3) GFR decline in animals with less severely reduced nephron mass without metabolic acidosis but with H+ retention (493). Consistent with these animal studies, amelioration of the metabolic acidosis of humans with low GFR using dietary alkali reduced parameters of kidney injury, slowed GFR decline, and reduced kidney ET production (328). These recent studies suggest strategies designed to prevent or ameliorate the untoward actions of increased kidney ET activity induced by chronic metabolic acidosis and/or by chronic dietary H+ will help to slow or stop the associated progressive kidney injury.
VII. ET and the renin-angiotensin system
Renin Release
In vitro studies have shown that ET-1 can inhibit renin release from juxtaglomerular cells through a Ca2+ dependent mechanism (280, 348, 426). Consistent with these observations are studies in anesthetized dogs showing that intravenous infusion of ET-1 reduces renin release (263, 317). It appears as though the intrarenal baroreceptor dominates over the direct actions of ET-1 given that higher doses of ET-1 that increase renal vascular resistance, and thus reduce glomerular pressure, actually increase renin release.
ET-1 may also attenuate renin release stimulated by other factors such as isoproterenol and cAMP (244, 288), most likely through ETB-dependent NO release (3, 359, 385). However, the physiological interplay between ET-1 and NO to control renin release has yet to be clarified. This may be due to the conflicting roles proposed for NO more than anything. NO directly stimulates renin release from isolated juxtaglomerular preparations, although NO-dependent vascular relaxation may effectively increase pressure reaching the glomerulus, which would inhibit renin release via the intrarenal baroreceptor (391). In isolated afferent arterioles, inhibition of NO synthase reduces renin release (445). Addition of the ET receptor antagonist, bosentan, reversed the effect of NOS inhibition suggesting that NO functions to inhibit the effects of ET-1 on renin secretion.
The influence of ET-1 on juxtaglomerular cells to attenuate renin secretion is consistent with the general function of ET-1 and ETB to promote sodium excretion, although as described above, ETA appears to function in opposition. Since renin secretion is influenced by many factors, including renal perfusion pressure, sodium intake, and sympathetic nerve activity, the physiological role of ET-1 on renin secretion has yet to be resolved.
Based on current knowledge, we hypothesize that endogenous ET-1 stimulated during high salt intake will attenuate renin release stimulated by other factors such as sympathetic nerve activity. This hypothesis is supported by long-term studies in rats and humans demonstrating increased plasma renin activity following ET receptor blockade (238, 388).
Angiotensin II and ET-1
These vasoactive peptides share many common features in that both are small peptides that activate G-protein coupled receptors, they exert dominant vasoconstrictor effects, and their activities are modulated by changes in dietary salt intake. In addition, there are a number of direct interactions between these systems. AII stimulates release of ET-1 and increases mRNA expression in a variety of cell types, including endothelial cells (97, 98, 180), vascular smooth muscle cells (356) and cardiomyocytes (188). ETA blockade attenuates the vasoconstrictor effects of AII in isolated vascular preparations (53, 475).
Blockade of ET receptors in vivo also inhibits the acute vasoconstrictor response to AII in several vascular beds including the kidney (22, 357, 482). The ability of ET antagonists, either ETA selective or combined ETA/ETB, to inhibit the in vivo actions of AII is most likely attributable to the AII-dependent release of ET-1 from vascular endothelium that in turn activates ETA to produce vasoconstriction. However, AII-induced constriction is much more rapid and shorter-lived compared to ET-1-dependent effects and so a more complicated relationship is likely. Indeed, there have been suggestions that ETA and AT1 receptors may heterodimerize to create an interplay in post-receptor signaling. ET-1 also appears to contribute to the hypertension produced by chronic elevations in AII as again evidenced by the ability of ET antagonists to lower blood pressure in these models (24, 72, 157, 346). The degree of hypertension produced by chronic AII infusion is salt-dependent. Although we know that the ET system is activated by a high salt diet, the potential interaction between AII and ET-1 to influence sodium excretion has yet to be resolved.
VIII. ET and renal nerves
The renorenal reflex involves increased renal pelvic pressure activating nerves in the renal pelvic wall which cause a reflex fall in efferent renal sympathetic nerve activity, stimulating a diuresis and natriuresis (232, 234). ETB activation in Schwann cells surrounding renal afferent nerves can stimulate noradrenergic mediated release of PGE2, which in turn induces the release of substance P, leading to increased renal afferent nerve activity (231, 233). ETB blockade in the renal pelvis in rats fed a high Na diet reduced renal afferent nerve activity in response to increased pelvic pressure (231). Hence, at least during high Na intake, ET-1, via ETB, may augment renal mechanosensory nerve activity, thereby increasing the renorenal reflex response and stimulating Na excretion.
While ETB has been clearly implicated in modulating the renorenal reflex, the role of renal pelvis ETA is less clear. In contrast to ETB, ETA are found in smooth muscle of the pelvic wall and not on nerve tissue (233). ETA can enhance smooth muscle contraction, an effect that should increase renal afferent nerve activity (233). However, during low Na intake, ETA, via unclear mechanisms, suppresses renal afferent nerve activity in response to increased pelvic pressure (229, 233). One way ETA may act is through interaction with AII. Similar to ETA activation, AII inhibits the renorenal reflex; the AII effect may be mediated by ETA since: 1) AII effects are not additive with ETA; and 2) ETA blockade prevents AII inhibition of renal nerve activity as well as AII suppression of PGE2-induced cAMP accumulation and substance P release (229-231). How ETA and AII interact is unclear as well as whether this has physiological relevance.
IX. Renal ET and hypertension
The powerful vasoconstrictor actions of ET-1 make it easy to propose this system as an important contributor to hypertension. Indeed, there are numerous reports that ET-1 production is elevated in animal models and humans (previously reviewed in (177)). The role for ET-1 is complicated by the opposing actions of ETA and ETB and the difficulty of studying a peptide that functions as an autocrine and paracrine factor. Soon after the development of ETA and ETA/ETB antagonists, it became evident that blood pressure lowering actions were observed only in salt-dependent models such as in mineralocorticoid and AII-dependent models as well as the Dahl salt-sensitive rat (177, 331). ET receptor blockade has no effect in other models such as the SHR and the 2-kidney, 1-clip Goldblatt hypertensive rat (260, 261).
Initial studies examining the influence of chronic ET-1 infusion in an attempt to duplicate a model of hypertension also yielded a less than simplistic mechanism of vasoconstriction dependent hypertension. Intravenous infusion of ET-1 for 7 days in Sprague-Dawley rats results in hypertension only when rats are maintained on a high salt intake (95, 295). Similarly, mice expressing a transgene for preproET-1 targeted to the endothelium have normal blood pressures (12), but become hypertensive when maintained on a high salt diet (11).
Pharmacological blockade of ETB or genetic mutation of ETB increases endogenous ET-1 levels, and therefore, increases ETA activation (126, 336). The resulting hypertension can be inhibited with an ETA antagonist and produces a consistent level of hypertension even in animals on a normal salt diet. These findings suggest that ETB normally functions to prevent ETA-dependent hypertension. However, hypertension produced by reduced ETB activity is not simply due to reduced ET-1 clearance since the elevation in blood pressure is highly dependent upon salt intake consistent with salt-dependent increases in ET-1 production and/or activity (126, 336).
Intrarenal production of ET-1 has been difficult to use to assess the role of ET-1 in hypertension due to the contrasting actions of ETA and ETB in the vascular versus tubular system. Increased ET-1 or ET receptor expression may occur in hypertension, but could represent an effort to increase ETB activity, increase sodium and water excretion, and thus lower arterial pressure. On the other hand, increased ETA activity in hypertension could reduce renal blood flow and GFR, and potentially lead to retention of extracellular fluid volume and thus hypertension. Likewise, we know that urinary ET-1 arises from intrarenal sources and can be increased in hypertension, but whether this is spill-over from overproduction of vascular ET-1, or a consequence of renal tubular ET-1 contributing to the natriuretic actions of the peptide, remains to be determined.
In human hypertension, there are reports of increased or no change in plasma ET-1 (7, 34, 165, 195, 253, 254, 310, 418). As in animal studies, it is not known whether increases in plasma ET-1 represent increased production or reduced clearance of the peptide. The variability in findings could be attributable to several reasons, including the antibody used in the assay, the genetic heterogeneity of the subject population, the level of dietary salt intake, the degree of stress during blood sampling, and any number of other factors. One aspect that appears fairly clear is that plasma ET-1 is elevated in African Americans compared to age-matched Caucasians (102, 108, 452). It is uncertain whether this is related to earlier observations that African Americans have a higher incidence of salt-dependent hypertension (149, 477).
Both ETA and ETA/B antagonists can lower systemic arterial pressure in a wide range of subjects with hypertension (82, 177, 206). Their use has been approved specifically for pulmonary hypertension. To date, there has been only one published phase III study for resistant hypertension (476). Although this initial study demonstrated clear efficacy, the company terminated their development of this compound for apparent lack of efficacy in a second unpublished trial. The reasons for the disparate results in the two trials have not been adequately explained. For a more thorough presentation or discussion of the use of ET antagonists in human hypertension, the reader is referred to other excellent reviews (1, 82, 83, 206, 331).
X. Summary and Conclusion
ET-1 is produced by, and acts upon, virtually every cell type in the kidney. It is not surprising, therefore, that ET-1 exerts multiple effects on the kidney. The major physiologic effects of renal ET-1 are regulation of renal hemodynamics and control of urinary Na, water and acid excretion. In this final section, we provide an overview of the importance of the renal ET system with regard to each of the above physiologic functions.
ET-1 regulation of water transport is the least complex renal physiologic regulatory pathway involving this peptide. High water intake increases renal (probably mainly CD) ET-1 production. ET-1 inhibits, most likely through an autocrine pathway involving ETB, AVP-stimulated cAMP accumulation, leading to reduced AQP2 water channel insertion into the apical membrane and inhibition of water reabsorption. Elimination of CD ET-1 causes impaired excretion of an acute water load, implicating this system in the physiologic regulation of the renal response to enhanced water intake.
ET-1 modulation of renal Na handling involves multiple cells types and includes tubular and vascular elements (Figure 9). Within the nephron, high Na intake increases TAL and CD ET-1 production. TAL-derived ET-1 activates autocrine ETB leading to increased NO production and inhibition of Cl- transport. CD-derived ET-1 activates ETB, which, through a variety of signaling mechanisms, decreases both ENaC open probability and number. ET-1 from both TAL and CD may act in a paracrine manner to increase medullary blood flow through dilation of vasa recta. The effects of high Na intake on PT ET-1 production are uncertain, however it is possible that autocrine inhibition of Na/K ATPase activity by ET-1 in the PT could contribute to the natriuretic response. High Na intake-stimulated renal ET-1 production is also likely to reduce juxtaglomerular apparatus renin production, although the cellular source of such ET-1 is uncertain. Finally, renal ET-1 may affect renal pelvis mechanosensory nerve activity during high Na intake leading to enhancement of the renorenal reflex. Taken together, these effects of renal ET-1 are of fundamental importance in reducing blood pressure and promoting a natriuresis in response to elevated Na intake.
Figure 9.
Integrated renal response to high Na intake. High Na intake increases renal ET-1 synthesis, particularly in the nephron. This can reduced tubule Na reabsorption by inhibiting Na transport mechanisms in the proximal tubule, thick ascending limb and collecting duct. ET-1, most likely derived from the medullary collecting duct and/or medullary thick ascending limb, may also increase medullary blood flow through vasodilation of vasa recta.
From the above discussion, it is apparent that, in general, ETB activation has a net antihypertensive and natriuretic effect through renal vasodilation, reduction of renin release, and direct inhibition of nephron Na reabsorptive mechanisms. In addition, collecting duct ETB activation inhibits AVP-stimulated water reabsorption. In contrast, ETA activation leads to reductions in RBF and GFR associated with increases in arterial pressure; tubule ETA modulation of Na and water transport requires clarification. Alterations in renal ET-1 production and/or ET receptor activity may be involved in the pathogenesis of salt-sensitive hypertension. Further studies on how the renal ET-1 system is regulated, and exerts its effects on renal hemodynamics and tubular Na and water transport processes, will likely shed substantial light on normal and disordered regulation of arterial pressure.
Renal ET-1 is also involved in the control of acid/base balance. Acidemia stimulates renal ET-1 production, although the precise cellular sources are uncertain, ET-1 than acts upon the proximal tubule (via ETB) to increase NHE activity, while it stimulates distal nephron proton secretion and bicarbonate reabsorption. While this effect of academia on renal ET-1 production helps maintain acid/base homeostasis, it may also contribute to acidosis-associated progression of chronic kidney disease.
In conclusion, the renal ET-1 is fundamentally important in normal and disordered regulation of renal hemodynamics and multiple tubule transport processes. Continued studies are needed to not only better define how renal ET-1 and its receptors function in health and disease, but to potentially direct future therapies that target specific components in the renal ET system.
Acknowledgments
Research by the authors that was discussed in this review was supported by National Institutes of Health grants HL74167 (D.M.P. and E.W.I.), HL60653 (D.M.P.), HL69999 (D.M.P.), DK96392 (D.E.K.), and DK44628 (E.W.I.). The authors would like to express their appreciation to the MCG Medical Illustrations Graduate Program Class of 2011 for their help in creating the figures for this chapter: William Andrews (faculty), Josh Bird, Sarah Brooks, Delia Dykes, Emily Dyson, Paul Kim, Alex McCain, Colby Polonsky, Veneliza Salcedo, and Carly Trowbridge.
References
- 1.Abassi Z, Ellahham S, Winaver J, Hoffman A. The intrarenal endothelin system and hypertension. News Physiol Sci. 2001;16:152–156. doi: 10.1152/physiologyonline.2001.16.4.152. [DOI] [PubMed] [Google Scholar]
- 2.Abassi ZA, Tate JE, Golomb E, Keiser HR. Role of neutral endopeptidase in the metabolism of endothelin. Hypertension. 1992;20:89–95. doi: 10.1161/01.hyp.20.1.89. [DOI] [PubMed] [Google Scholar]
- 3.Ackermann M, Ritthaler T, Riegger G, Kurtz A, Kramer BK. Endothelin inhibits cAMP-induced renal release from isolated renal juxtaglomerular cells. Journal of Cardiovascular Pharmacology. 1995;26:S135–S137. [PubMed] [Google Scholar]
- 4.Ahn D, Ge Y, Stricklett PK, Gill P, Taylor D, Hughes AK, Yanagisawa M, Miller L, Nelson RD, Kohan DE. Collecting duct-specific knockout of endothelin-1 causes hypertension and sodium retention. J Clin Invest. 2004;114:504–511. doi: 10.1172/JCI21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akabane S, Yoshimi H, Yoshida K, Kawano Y, Ashida T, Kinoshita O, Matsushima Y, Kawamura M, Imanishi M, Kuramochi M, Omae T. Differential effects of endothelin-1 and big endothelin on canine kidneys. J Cardiovasc Pharmacol. 1991;17(Suppl. 7):S302–S304. doi: 10.1097/00005344-199100177-00086. [DOI] [PubMed] [Google Scholar]
- 6.Albrightson CR, Pullen M, Wu H-L, Dytko G, Hersh LB, Nambi P. Thrombin-mediated down-regulation of endothelin receptors in mesangial cells coincides with the downregulation of neutral endopeptidase activity. Mol Pharmacol. 1995;47:1156–1163. [PubMed] [Google Scholar]
- 7.Allen SW, Chatfield BA, Koppenhafer SA, Schaffer MS, Wolfe RR, Abman SH. Circulating immunoreactive endothelin-1 in children with pulmonary hypertension. Association with acute hypoxic pulmonary vasoreactivity. Am Rev Respir Dis. 1993;148:519–522. doi: 10.1164/ajrccm/148.2.519. [DOI] [PubMed] [Google Scholar]
- 8.Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- 9.Ambuhl PM, Yang X, Peng Y, Preisig PA, Moe OW, Alpern RJ. Glucocorticoids enhance acid activation of the Na+/H+ exchanger 3 (NHE3) J Clin Invest. 1999;103:429–435. doi: 10.1172/JCI2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amemiya M, Mori H, Imamura S, Toyoda A, Funayama I, Asano Y, Kusano E, Tabei K. Stimulation of NHE3 in OKP cells by an autocrine mechanism. Nephron Exp Nephrol. 2004;96:e23–30. doi: 10.1159/000075573. [DOI] [PubMed] [Google Scholar]
- 11.Amiri F, Ko EA, Javeshghani D, Reudelhuber TL, Schiffrin EL. Deleterious combined effects of salt-loading and endothelial cell restricted endothelin-1 overexpression on blood pressure and vascular function in mice. J Hypertens. 28:1243–1251. doi: 10.1097/HJH.0b013e328338bb8b. [DOI] [PubMed] [Google Scholar]
- 12.Amiri F, Virdis A, Neves MF, Iglarz M, Seidah NG, Touyz RM, Reudelhuber TL, Schiffrin EL. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110:2233–2240. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 13.Andersen LJ, Norsk P, Johansen LB, Christensen P, Engstrom T, Bie P. Osmoregulatory control of renal sodium excretion after sodium loading in humans. Am J Physiol. 1998;275:R1833–1842. doi: 10.1152/ajpregu.1998.275.6.R1833. [DOI] [PubMed] [Google Scholar]
- 14.Andreis PG, Tortorella C, Malendowicz LK, Nussdorfer GG. Endothelins stimulate aldosterone secretion from dispersed rat adrenal zona glomerulosa cells, acting through ETB receptors coupled with the phospholipase C-dependent signaling pathway. Peptides. 2001;22:117–122. doi: 10.1016/s0196-9781(00)00363-6. [DOI] [PubMed] [Google Scholar]
- 15.Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- 16.Arendshorst WJ, Thai TL. Regulation of the renal microcirculation by ryanodine receptors and calcium-induced calcium release. Curr Opin Nephrol Hypertens. 2009;18:40–49. doi: 10.1097/MNH.0b013e32831cf5bd. [DOI] [PubMed] [Google Scholar]
- 17.Arinami T, Ishikawa M, Inoue A, Yanagisawa M, Masaki T, Yoshida MC, Hamaguchi H. Chromosomal assignments of the human endothelin family genes: the endothelin-1 gene(EDN1) to 6p23-p24, the endothelin-2 gene(EDN2) to Ip34, and the endothelin-3 gene(EDN3) to 20q13.2-q13.3. Am J Hum Genet. 1991;48:990–996. [PMC free article] [PubMed] [Google Scholar]
- 18.Atkins JL, Burg MB. Bicarbonate transport by isolated perfused rat collecting ducts. Am J Physiol. 1985;249:F485–489. doi: 10.1152/ajprenal.1985.249.4.F485. [DOI] [PubMed] [Google Scholar]
- 19.Backer A, Bokemeyer D, Kramer HJ. Endothelin synthesis and receptors in porcine kidney. Acta Physiol Scand. 2001;171:105–112. doi: 10.1046/j.1365-201X.2001.00789.x. [DOI] [PubMed] [Google Scholar]
- 20.Badr KF, Murray JJ, Breyer MD, Takahashi K, Inagami T, Harris RC. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. Elucidation of signal transduction pathways. J Clin Invest. 1989;83:336–342. doi: 10.1172/JCI113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey M, Haton C, Orea V, Sassard J, Bailly C, Unwin R, Imbert-Teboul M. ETA receptor-mediated Ca2+ signaling in thin descending limbs of Henle’s loop: impariment in genetic hypertension. Kidney Int. 2003;63:1276–1284. doi: 10.1046/j.1523-1755.2003.00880.x. [DOI] [PubMed] [Google Scholar]
- 22.Balakrishnan SM, Wang HD, Gopalakrishnan V, Wilson TW, McNeill JR. Effect of an endothelin antagonist on hemodynamic responses to angiotensin II. Hypertension. 1996;28:806–809. doi: 10.1161/01.hyp.28.5.806. [DOI] [PubMed] [Google Scholar]
- 23.Baldi E, Dunn MJ. Endothelin binding and receptor down regulation in rat glomerular mesangial cells. J Pharmacol Exp Ther. 1991;256:581–586. [PubMed] [Google Scholar]
- 24.Ballew JR, Fink GD. Role of endothelin ETB receptor activation in angiotensin II-induced hypertension: effects of salt intake. Am J Physiol Heart Circ Physiol. 2001;281:H2218–2225. doi: 10.1152/ajpheart.2001.281.5.H2218. [DOI] [PubMed] [Google Scholar]
- 25.Barton M. Reversal of proteinuric renal disease and the emerging role of endothelin. Nat Clin Pract Nephrol. 2008;4:490–501. doi: 10.1038/ncpneph0891. [DOI] [PubMed] [Google Scholar]
- 26.Baum M, Moe OW, Gentry DL, Alpern RJ. Effect of glucocorticoids on renal cortical NHE-3 and NHE-1 mRNA. Am J Physiol. 1994;267:F437–442. doi: 10.1152/ajprenal.1994.267.3.F437. [DOI] [PubMed] [Google Scholar]
- 27.Beck K-F, Mohaupt MG, Sterzel RB. Endothelin-1 inhibits cytokine-stimulated transcription of inducible nitric oxide synthase in glomerular mesangial cells. Kidney Int. 1995;48:1893–1899. doi: 10.1038/ki.1995.488. [DOI] [PubMed] [Google Scholar]
- 28.Benigni A, Perico N, Gaspari F, Zoja C, Bellizzi I, Gabanelli M, Remuzzi G. Increased renal endothelin production in rats with reduced renal mass. Am J Physiol. 1991;260:F331–F339. doi: 10.1152/ajprenal.1991.260.3.F331. [DOI] [PubMed] [Google Scholar]
- 29.Bloom ITM, Bentley FR, Wilson MA, Garrison RN. In vivo effects of endothelin on the renal microcirculation. J Surg Res. 1993;54:274–280. doi: 10.1006/jsre.1993.1043. [DOI] [PubMed] [Google Scholar]
- 30.Boesen EI. Endothelin ETB receptor heterodimerization: beyond the ETA receptor. Kidney Int. 2008;74:693–694. doi: 10.1038/ki.2008.324. [DOI] [PubMed] [Google Scholar]
- 31.Boesen EI, Pollock DM. Acute increases of renal medullary osmolality stimulate endothelin release from the kidney. Am J Physiol Renal Physiol. 2007;292:F185–191. doi: 10.1152/ajprenal.00021.2006. [DOI] [PubMed] [Google Scholar]
- 32.Booth RE, Tong Q, Medina J, Snyder PM, Patel P, Stockand JD. A region directly following the second transmembrane domain in gamma ENaC is required for normal channel gating. J Biol Chem. 2003;278:41367–41379. doi: 10.1074/jbc.M305400200. [DOI] [PubMed] [Google Scholar]
- 33.Bousso-Mittler D, Galron R, Sokolovsky M. Endothelin/sarafotoxin receptor heterogeneity: evidence for different glycosylation in receptors from different tissues. Biochem Biophys Res Commun. 1991;178:921–926. doi: 10.1016/0006-291x(91)90979-h. [DOI] [PubMed] [Google Scholar]
- 34.Bragulat E, de la Sierra A, Antonio MT, Jimenez W, Urbano-Marquez A, Coca A. Effect of salt intake on endothelium-derived factors in a group of patients with essential hypertension. Clin Sci (Lond) 2001;101:73–78. [PubMed] [Google Scholar]
- 35.Brodsky S, Abassi Z, Wessale J, Ramadan R, Winaver J, Hoffman A. Effects of A-192621.1, a specific endothelin-B antagonist, on intrarenal hemodynamic responses to endothelin-1. J Cardiovasc Pharmacol. 2000;36(Suppl 1):S311–S313. doi: 10.1097/00005344-200036051-00090. [DOI] [PubMed] [Google Scholar]
- 36.Brooks DP, DePalma PD, Pullen M, Nambi P. Characterization of canine renal endothelin receptor subtypes and their function. J Pharmacol Exp Ther. 1994;268:1091–1097. [PubMed] [Google Scholar]
- 37.Brunner F, Bras-Silva C, Cerdeira AS, Leite-Moreira AF. Cardiovascular endothelins: Essential regulators of cardiovascular homeostasis. Pharmacol Ther. 2006;111:508–531. doi: 10.1016/j.pharmthera.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Bruzzi I, Corna D, Zoja C, Orisio S, Schiffrin EL, Cavallotti D, Remuzzi G, Benigni A. Time course and localization of endothelin-1 gene expression in a model of renal disease progression. Am J Pathol. 1997;151:1241–1247. [PMC free article] [PubMed] [Google Scholar]
- 39.Buerkert J, Martin D, Trigg D. Segmental analysis of the renal tubule in buffer production and net acid formation. Am J Physiol. 1983;244:F442–454. doi: 10.1152/ajprenal.1983.244.4.F442. [DOI] [PubMed] [Google Scholar]
- 40.Bugaj V, Pochynyuk O, Mironova E, Vandewalle A, Medina JL, Stockand JD. Regulation of the epithelial Na+ channel by endothelin-1 in rat collecting duct. Am J Physiol Renal Physiol. 2008;295:F1063–1070. doi: 10.1152/ajprenal.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cairns HS, Rogerson ME, Fairbanks LD, Neild GH, Westwick J. Endothelin induces an increase in renal vascular resistance and a fall in glomerular filtration rate in the rabbit isolated perfused kidney. Br J Pharmacol. 1989;98:155–160. doi: 10.1111/j.1476-5381.1989.tb16876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capasso G, Unwin R, Rizzo M, Pica A, Giebisch G. Bicarbonate transport along the loop of Henle: molecular mechanisms and regulation. J Nephrol. 2002;15(Suppl 5):S88–96. [PubMed] [Google Scholar]
- 43.Carmines PK, Mitchell KD, Navar LG. Effects of calcium antagonists on renal hemodynamics and glomerular function. Kidney Int Suppl. 1992;36:S43–48. [PubMed] [Google Scholar]
- 44.Carmines PK, Navar LG. Disparate effects of Ca channel blockade on afferent and efferent arteriolar responses to ANG II. Am J Physiol. 1989;256:F1015–1020. doi: 10.1152/ajprenal.1989.256.6.F1015. [DOI] [PubMed] [Google Scholar]
- 45.Casellas D, Carmines PK, Navar LG. Microvascular reactivity of in vitro blood perfused juxtamedullary nephrons from rats. Kidney Int. 1985;28:752–759. doi: 10.1038/ki.1985.194. [DOI] [PubMed] [Google Scholar]
- 46.Casellas D, Navar LG. In vitro perfusion of juxtamedullary nephrons in rats. Am J Physiol. 1984;246:F349–358. doi: 10.1152/ajprenal.1984.246.3.F349. [DOI] [PubMed] [Google Scholar]
- 47.Cavarape A, Bartoli E. Effects of BQ-123 on systemic and renal hemodynamic responses to endothelin-1 in the rat split hydronephrotic kidney. J Hypertens. 1998;16:1449–1458. doi: 10.1097/00004872-199816100-00008. [DOI] [PubMed] [Google Scholar]
- 48.Cavarape A, Endlich N, Assaloni R, Bartoli E, Steinhausen M, Parekh N, Endlich K. Rho-kinase inhibition blunts renal vasoconstriction induced by distinct signaling pathways in vivo. J Am Soc Nephrol. 2003;14:37–45. doi: 10.1097/01.asn.0000039568.93355.85. [DOI] [PubMed] [Google Scholar]
- 49.Cazaubon S, Chaverot N, Romero I, Girault J, Adamson P, Strosberg A, Couraud P. Growth factor activity of endothelin-1 in primary astrocytes mediated by adhesion-dependent and -independent pathways. J Neurosci. 1997;17:6203–6212. doi: 10.1523/JNEUROSCI.17-16-06203.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cernacek P, Legault L, Stewart DJ, Levy M. Specific endothelin binding sites in renal medullary collecting duct cells: lack of interaction with ANP binding and cGMP signalling. Can J Physiol Pharmacol. 1992;70:1167–1174. doi: 10.1139/y92-162. [DOI] [PubMed] [Google Scholar]
- 51.Chahdi A, Sorokin A. Endothelin-1 induces p66Shc activation through EGF receptor transactivation: Role of beta(1)Pix/Galpha(i3) interaction. Cell Signal. 2010;22:325–329. doi: 10.1016/j.cellsig.2009.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chambrey R, Paillard M, Podevin RA. Enzymatic and functional evidence for adaptation of the vacuolar H(+)-ATPase in proximal tubule apical membranes from rats with chronic metabolic acidosis. J Biol Chem. 1994;269:3243–3250. [PubMed] [Google Scholar]
- 53.Chen L, McNeill JR, Wilson TW, Gopalakrishnan V. Heterogeneity in vascular smooth muscle responsiveness to angiotensin II. Role of endothelin. Hypertension. 1995;26:83–88. doi: 10.1161/01.hyp.26.1.83. [DOI] [PubMed] [Google Scholar]
- 54.Chen M, Todd-Turla K, Wang W-H, Cao X, Smart A, Brosius FC, Killen PD, Keiser JA, Briggs JP, Schnermann J. Endothlein-1 mRNa in glomerular and epithelial cells of kidney. Am J Physiol. 1993;265:F542–F550. doi: 10.1152/ajprenal.1993.265.4.F542. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, Berrou J, Nguyen G, Sraer J-D, Rondeau E. Endothelin-1 induces rapid and long lasting internalization of the thrombin receptor in human glomerular epithelial cells. Biochem Biophys Res Com. 1995;217:445–451. doi: 10.1006/bbrc.1995.2796. [DOI] [PubMed] [Google Scholar]
- 56.Chigaev A, Lu G, Shi H, Asher C, Xu R, Latter H, Seger R, Garty H, Reuveny E. In vitro phosphorylation of COOH termini of the epithelial Na(+) channel and its effects on channel activity in Xenopus oocytes. Am J Physiol Renal Physiol. 2001;280:F1030–1036. doi: 10.1152/ajprenal.2001.280.6.F1030. [DOI] [PubMed] [Google Scholar]
- 57.Chou SY, Porush JG. Renal actions of endothelin-1 and endothelin-3: interactions with the prostaglandin system and nitric oxide. Am J Kidney Dis. 1995;26:116–123. doi: 10.1016/0272-6386(95)90164-7. [DOI] [PubMed] [Google Scholar]
- 58.Chow LH, Subramanian S, Nuovo GJ, Miller F, Nord EP. Endothelin receptor mRNA expression in renal medulla identified by in situ PT-PCR. Am J Physiol. 1995;269:F449–F457. doi: 10.1152/ajprenal.1995.269.3.F449. [DOI] [PubMed] [Google Scholar]
- 59.Chu T-S, Peng Y, Cano A, Yanagisawa M, Alpern RJ. Endothelin B receptor activates NHE-3 by a Ca2+-dependent pathway in OKP cells. J Clin Invest. 1996;97:1454–1462. doi: 10.1172/JCI118567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chu TS, Cano A, Yanagisawa M, Allpern RJ. Endothelin activates the Na/H antiporter in OKP cells stably transfected with the ETB receptor cDNA. J Am Soc Nephrol. 1993;4:250. [Google Scholar]
- 61.Chu TS, Tsuganezawa H, Peng Y, Cano A, Yanagisawa M, Alpern RJ. Role of tyrosine kinase pathways in ETB receptor activation of NHE3. Am J Physiol. 1996;271:C763–771. doi: 10.1152/ajpcell.1996.271.3.C763. [DOI] [PubMed] [Google Scholar]
- 62.Chu TS, Wu MS, Hsiech BS. Urinary endothelin-1 in patients with renal disease. J Formos Med Assoc. 1998;97:667–672. [PubMed] [Google Scholar]
- 63.Chun M, Lin HY, Henis YI, Lodish HF. Endothelin-induced endocytosis of cell surface ETA receptors. J Biol Chem. 1995;270:10855–10860. doi: 10.1074/jbc.270.18.10855. [DOI] [PubMed] [Google Scholar]
- 64.Claria J, Jimenez W, Villa GLA, Asbert M, Castro A, Llibre J, Arroyo V, Rivera F. Effects of endothelin on renal hemodynamics and segemental sodium handling in conscious rats. Acta Physiol Scand. 1991;141:305–308. doi: 10.1111/j.1748-1716.1991.tb09085.x. [DOI] [PubMed] [Google Scholar]
- 65.Clavell AL, Stingo AJ, Margulies KB, Brandt RR, Burnett JC., Jr Role of endothelin receptor subtypes in the in vivo regulation of renal function. Am J Physiol. 1995;268:F455–F460. doi: 10.1152/ajprenal.1995.268.3.F455. [DOI] [PubMed] [Google Scholar]
- 66.Clozel M, Löffler B-M, Breu V, Hilfiger L, Maire J-P, Butscha B. Downregulation of endothelin receptors by autocrine production of endothelin-1. Am J Physiol. 1993;265:C188–C192. doi: 10.1152/ajpcell.1993.265.1.C188. [DOI] [PubMed] [Google Scholar]
- 67.Collino F, Bussolati B, Gerbaudo E, Marozio L, Pelissetto S, Benedetto C, Camussi G. Preeclamptic sera induce nephrin shedding from podocytes through endothelin-1 release by endothelial glomerular cells. Am J Physiol Renal Physiol. 2008;294:F1185–1194. doi: 10.1152/ajprenal.00442.2007. [DOI] [PubMed] [Google Scholar]
- 68.Coroneos EJ, Kester M, Maclouf J, Thomas P, Dunn MJ. Calcium-regulated protein tyrosine phosphorylation is required for endothelin-1 to induce prostaglandin endoperoxide synthase-2 mRNA expression and protein synthesis in mesangial cells. J Am Soc Nephrol. 1997;8:1080–1090. doi: 10.1681/ASN.V871080. [DOI] [PubMed] [Google Scholar]
- 69.Cuzzola F, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Cataliotti A, Stancanelli B, Malatino LS, Bellanuova I, Ferri C, Galletti F, Filigheddu F, Glorioso N, Strazzullo P, Zoccali C. Urinary adrenomedullin is related to ET-1 and salt intake in patients with mild essential hypertension. Am J Hypertens. 2001;14:224–230. doi: 10.1016/s0895-7061(00)01265-6. [DOI] [PubMed] [Google Scholar]
- 70.Cybulsky AV, Stewart DJ, Cybulsky MI. Glomerular epithelial cells produce endothelin-1. J Am Soc Nephrol. 1993;3:1398–1404. doi: 10.1681/ASN.V371398. [DOI] [PubMed] [Google Scholar]
- 71.D’Orleans-Juste P, Plante M, Honore JC, Carrier E, Labonte J. Synthesis and degradation of endothelin-1. Can J Physiol Pharmacol. 2003;81:503–510. doi: 10.1139/y03-032. [DOI] [PubMed] [Google Scholar]
- 72.d’Uscio LV, Moreau P, Shaw S, Takase H, Barton M, Luscher TF. Effects of chronic ETA-receptor blockade in angiotensin II-induced hypertension. Hypertension. 1997;29:435–441. doi: 10.1161/01.hyp.29.1.435. [DOI] [PubMed] [Google Scholar]
- 73.da Silva RF, Chambaz C, Stergiopulos N, Hayoz D, Silacci P. Transcriptional and post-transcriptional regulation of preproendothelin-1 by plaque-prone hemodynamics. Atherosclerosis. 2007;194:383–390. doi: 10.1016/j.atherosclerosis.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 74.Dancu MB, Berardi DE, Vanden Heuvel JP, Tarbell JM. Asynchronous shear stress and circumferential strain reduces endothelial NO synthase and cyclooxygenase-2 but induces endothelin-1 gene expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2004;24:2088–2094. doi: 10.1161/01.ATV.0000143855.85343.0e. [DOI] [PubMed] [Google Scholar]
- 75.Davenport AP, Kuc RE, Hoskins SL, Karet FE, Fitzgerald F. [125I]-PD151242: a selective ligand for endothelin ETA receptors in human kidney which localizes to renal vasculature. Br J Pharmacol. 1994;113:1303–1310. doi: 10.1111/j.1476-5381.1994.tb17140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.de Jesus Ferreira M, Bailly C. Luminal and basolateral endothelin inhibit chloride reabsorption in the mouse thick ascending limb via a Ca(2+)-independent pathway. J Physiol. 1997;505:749–758. doi: 10.1111/j.1469-7793.1997.749ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.De Nucci G, Thomas R, D’Orleans-Juste P, Antunes E, Walder C, Warner TD, Vane JR. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dean R, Zhuo J, Alcorn D, Casley D, Mendelsohn FAO. Cellular distribution of 125Iendothelin- 1 binding in rat kidney following in vivo labeling. Am J Physiol. 1994;267:F845–F852. doi: 10.1152/ajprenal.1994.267.5.F845. [DOI] [PubMed] [Google Scholar]
- 79.Delarue C, Conlon JM, Remy-Jouet I, Fournier A, Vaudry H. Endothelins as local activators of adrenocortical cells. J Mol Endocrinol. 2004;32:1–7. doi: 10.1677/jme.0.0320001. [DOI] [PubMed] [Google Scholar]
- 80.Denton K, Shweta A, Finkelstein L, Flower R, Evans R. Effect of endothelin-1 on regional kidney blood flow and renal arteriole calibre in rabbits. Clin Exp Pharmacol Physiol. 2004;31:494–501. doi: 10.1111/j.1440-1681.2004.04036.x. [DOI] [PubMed] [Google Scholar]
- 81.Denton KM, Anderson WP. Vascular actions of endothelin in the rabbit kidney. Clin Exp Pharmacol Physiol. 1990;17:861–872. doi: 10.1111/j.1440-1681.1990.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 82.Dhaun N, Goddard J, Kohan DE, Pollock DM, Schiffrin EL, Webb DJ. Role of endothelin-1 in clinical hypertension: 20 years on. Hypertension. 2008;52:452–459. doi: 10.1161/HYPERTENSIONAHA.108.117366. [DOI] [PubMed] [Google Scholar]
- 83.Dhaun N, Lilitkarntakul P, Macintyre IM, Muilwijk E, Johnston NR, Kluth DC, Webb DJ, Goddard J. Urinary endothelin-1 in chronic kidney disease and as a marker of disease activity in lupus nephritis. Am J Physiol Renal Physiol. 2009;296:F1477–1483. doi: 10.1152/ajprenal.90713.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Disashi T, Nonoguchi H, Iwaoka T, Naomi S, Nakayama Y, Shimada K, Tanzawa K, Tomita K. Endothelin converting enzyme-1 gene expression in the kidney of spontaneously hypertensive rats. Hypertension. 1997;30:1591–1597. doi: 10.1161/01.hyp.30.6.1591. [DOI] [PubMed] [Google Scholar]
- 85.Dorado F, Velasco S, Esparis-Ogando A, Pericacho M, Pandiella A, Silva J, Lopez-Novoa JM, Rodriguez-Barbero A. The mitogen-activated protein kinase Erk5 mediates human mesangial cell activation. Nephrol Dial Transplant. 2008;23:3403–3411. doi: 10.1093/ndt/gfn333. [DOI] [PubMed] [Google Scholar]
- 86.Douglas SA, Ohlstein EH. Signal transduction mechanisms mediating the vascular actions of endothelin. J Vasc Res. 1997;34:152–164. doi: 10.1159/000159219. [DOI] [PubMed] [Google Scholar]
- 87.Douthwaite JA, Lees DM, Corder R. A role for increased mRNA stability in the induction of endothelin-1 synthesis by lipopolysaccharide. Biochem Pharmacol. 2003;66:589–594. doi: 10.1016/s0006-2952(03)00336-8. [DOI] [PubMed] [Google Scholar]
- 88.Dulin N, Sorokin A, Reed E, Elliott S, Kehrl J, Dunn M. RGS3 inhibits G protein-mediated signaling via translocation to the membrane and binding to Galpha. Mol Cell Biol. 1999;19:714–723. doi: 10.1128/mcb.19.1.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Edwards RM, Pullen M, Nambi P. Activation of endothelin ETB receptors increases glomerular cGMP via an L-arginine-dependent pathway. Am J Physiol. 1992;263:F1020–1025. doi: 10.1152/ajprenal.1992.263.6.F1020. [DOI] [PubMed] [Google Scholar]
- 90.Edwards RM, Stack EJ, Pullen M, Nambi P. Endothelin inhibits vasopressin action in rat inner medullary collecting duct via the ETB receptor. J Pharmacol Exp Ther. 1993;267:1028–1033. [PubMed] [Google Scholar]
- 91.Edwards RM, Trizna W. Characterization of 125I-endothelin-1 binding to rat and rabbit renal microvasculature. J Pharmacol Exp Ther. 1995;274:1064–1089. [PubMed] [Google Scholar]
- 92.Edwards RM, Trizna W, Ohlstein EH. Renal microvascular effects of endothelin. Am J Physiol. 1990;259:F217–F221. doi: 10.1152/ajprenal.1990.259.2.F217. [DOI] [PubMed] [Google Scholar]
- 93.Eiam-Ong S, Hilden SA, King AJ, Johns CA, Madias NE. Endothelin-1 stimulates the Na+/H+ and Na+/HCO3- transporters in rabbit renal cortex. Kidney Int. 1992;42:18–24. doi: 10.1038/ki.1992.255. [DOI] [PubMed] [Google Scholar]
- 94.Eiam-Ong S, Kurtzman NA, Sabatini S. Regulation of collecting tubule adenosine triphosphatases by aldosterone and potassium. J Clin Invest. 1993;91:2385–2392. doi: 10.1172/JCI116471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Elmarakby AA, Dabbs Loomis E, Pollock JS, Pollock DM. ETA receptor blockade attenuates hypertension and decreases reactive oxygen species in ETB receptor-deficient rats. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S7–10. doi: 10.1097/01.fjc.0000166205.66555.40. [DOI] [PubMed] [Google Scholar]
- 96.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. TNF-alpha inhibition reduces renal injury in DOCA-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Emori T, Hirata Y, Ohta K, Kanno K, Eguchi S, Imai T, Shichiri M, Marumo F. Cellular mechanism of endothelin-1 release by angiotensin and vasopressin. Hypertension. 1991;18:165–170. doi: 10.1161/01.hyp.18.2.165. [DOI] [PubMed] [Google Scholar]
- 98.Emori T, Hirata Y, Ohta K, Shichiri M, Marumo F. Secretory mechanism of immunoreactive endothelin in cultured bovine endothelial cells. Biochem Biophys Res Commun. 1989;160:93–100. doi: 10.1016/0006-291x(89)91625-2. [DOI] [PubMed] [Google Scholar]
- 99.Emoto N, Yanagisawa M. Endothelin-converting enzyme-2 is a membrane-bound phosphoramidon-sensitive metalloprotease with acidic pH optimum. J Biol Chem. 1995;270:15262–15268. doi: 10.1074/jbc.270.25.15262. [DOI] [PubMed] [Google Scholar]
- 100.Endemann D, Schweda F, Stubanus M, Ittner KP, Fischereder M, Kammerl MC, Kramer BK. Naftidrofuryl exerts antiserotonergic but no endothelin-receptor blocking effects in AS4.1 cells, juxtaglomerular cells and isolated perfused rat kidneys. J Cardiovasc Pharmacol. 2002;39:1–8. doi: 10.1097/00005344-200201000-00001. [DOI] [PubMed] [Google Scholar]
- 101.Endlich K, Hoffend J, Steinhausen M. Localization of endothelin ETA and ETB receptor-mediated constriction in the renal microcirculation of rats. J Physiol. 1996;497(Pt 1):211–218. doi: 10.1113/jphysiol.1996.sp021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ergul S, Parish DC, Puett D, Ergul A. Racial differences in plasma endothelin-1 concentrations in individuals with essential hypertension. Hypertension. 1996;28:652–655. doi: 10.1161/01.hyp.28.4.652. [DOI] [PubMed] [Google Scholar]
- 103.Escalante B, McGiff J, Oyekan A. Role of cytochrome P-450 arachidonate metabolites in endothelin signaling in rat proximal tubule. Am J Physiol. 2002;282:F144–F150. doi: 10.1152/ajprenal.0064.2001. [DOI] [PubMed] [Google Scholar]
- 104.Evangelista S, Maggi CA, Castellucci A. Effect of endothelin (ET)-1, ET-3, and ET-(16-21) on the isolated and perfused rat kidney from normotensive and spontaneously hypertensive rats. Jpn J Pharmacol. 1992;59:239–241. doi: 10.1254/jjp.59.239. [DOI] [PubMed] [Google Scholar]
- 105.Evans NJ, Walker JW. Sustained Ca2+ signaling and delayed internalization associated with endothelin receptor heterodimers linked through a PDZ finger. Can J Physiol Pharmacol. 2008;86:526–535. doi: 10.1139/Y08-050. [DOI] [PubMed] [Google Scholar]
- 106.Evans R, Madden A, Oliver J, Lewis T. Effects of ETA- and ETB- receptor antagonists on regional kidney blood flow, and response to intravenous endothelin-1, in anaesthetized rabbits. J Hypertens. 2001;19:1789–1799. doi: 10.1097/00004872-200110000-00013. [DOI] [PubMed] [Google Scholar]
- 107.Evans RG, Eppel GA, Anderson WP, Denton KM. Mechanisms underlying the differential control of blood flow in the renal medulla and cortex. J Hypertens. 2004;22:1439–1451. doi: 10.1097/01.hjh.0000133744.85490.9d. [DOI] [PubMed] [Google Scholar]
- 108.Evans RR, Phillips BG, Singh G, Bauman JL, Gulati A. Racial and gender differences in endothelin-1. Am J Cardiol. 1996;78:486–488. doi: 10.1016/s0002-9149(96)00344-x. [DOI] [PubMed] [Google Scholar]
- 109.Falin RA, Cotton CU. Acute downregulation of ENaC by EGF involves the PY motif and putative ERK phosphorylation site. J Gen Physiol. 2007;130:313–328. doi: 10.1085/jgp.200709775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fattal I, Abassi Z, Ovcharenko E, Shimada K, Takahashi M, Hoffman A, Winaver J. Effect of dietary sodium intake on the expression of endothelin-converting enzyme in the renal medulla. Nephron Physiol. 2004;98:89–96. doi: 10.1159/000081557. [DOI] [PubMed] [Google Scholar]
- 111.Fellner SK, Arendshorst W. Endothelin-A and -B receptors, superoxide, and Ca2+ signaling in afferent arterioles. Am J Physiol Renal Physiol. 2007;292:F175–184. doi: 10.1152/ajprenal.00050.2006. [DOI] [PubMed] [Google Scholar]
- 112.Fellner SK, Arendshorst WJ. Endothelin A and B receptors of preglomerular vascular smooth muscle cells. Kidney Int. 2004;65:1810–1817. doi: 10.1111/j.1523-1755.2004.00579.x. [DOI] [PubMed] [Google Scholar]
- 113.Fernandez R, Lopes MJ, de Lira RF, Dantas WF, Cragoe Junior EJ, Malnic G. Mechanism of acidification along cortical distal tubule of the rat. Am J Physiol. 1994;266:F218–226. doi: 10.1152/ajprenal.1994.266.2.F218. [DOI] [PubMed] [Google Scholar]
- 114.Ferri C, Bellini C, Desideri G, Mazzocchi C, De Siati L, Santucci A. Elevated plasma and urinary endothelin-I levels in human salt-sensitive hypertension. Clin Sci. 1997;93:35–41. doi: 10.1042/cs0930035. [DOI] [PubMed] [Google Scholar]
- 115.Foschi M, Sorokin A, Pratt P, McGinty A, La Villa G, Franchi F, Dunn M. PreproEndothelin-1 expression in human mesangial cells: evidence for a p38 mitogen-activated protein kinase/protein kinases-C-dependent mechanism. J Am Soc Nephrol. 2001;12:1137–1150. doi: 10.1681/ASN.V1261137. [DOI] [PubMed] [Google Scholar]
- 116.Francis B, Abassi Z, Heyman S, Winaver J, Hoffman A. Differential regulation of ETA and ETB in the renal tissue of rats with compensated and decompensated heart failure. J Cardiovasc Pharmacol. 2004;44:S362–S365. doi: 10.1097/01.fjc.0000166302.56184.fa. [DOI] [PubMed] [Google Scholar]
- 117.Frank K, Zeier M, Gross ML, Waldherr R, Ritz E, Amann K. Comprehensive immunohistological analysis of the endothelin system in human kidney grafts. Nephrol Dial Transplant. 2006;21:1365–1372. doi: 10.1093/ndt/gfk087. [DOI] [PubMed] [Google Scholar]
- 118.Freed MI, Thompson KA, Wilson DE, Etheredge R, Jorkasky DK. Endothelin receptor antagonism does not alter renal hemodynamic responses or urinary sodium excretion in healthy humans. J Am Soc Nephrol. 1996;7:1580. [Google Scholar]
- 119.Fretschner M, Endlich K, Gulbins E, Lang RE, Schlottmann K, Steinhausen M. Effects of endothelin on the renal microcirculation of the split hydronephrotic rat kidney. Renal Physiol Biochem. 1991;14:112–127. doi: 10.1159/000173394. [DOI] [PubMed] [Google Scholar]
- 120.Fukunaga M, Fujiwara Y, Ochi S, Yokoyama K, Shoji T, Fukuhara Y, Orita Y, Kamada T, Badr KF, Ueda N. Mechanism of induction of prostaglandin E2 production by endothelin 1 in cultured rat mesangial cells. Exp Nephrol. 1996;4:340–349. [PubMed] [Google Scholar]
- 121.Furuya S, Naruse S, Nakayama T, Nokihara K. Effect and distribution of intravenously injected 125I-endothelin-1 in rat kidney and lung examined by electron microscopic radioautography. Anat Embryol (Berl) 1992;185:87–96. doi: 10.1007/BF00213604. [DOI] [PubMed] [Google Scholar]
- 122.Gallego MS, Ling BN. Regulation of amiloride-sensitive sodium channels by endothelin-1 in distal nephron cells. Am J Physiol. 1996;271:F451–F460. doi: 10.1152/ajprenal.1996.271.2.F451. [DOI] [PubMed] [Google Scholar]
- 123.Garcia NH, Garvin JL. Endothelin’s biphasic effect on fluid absorption in the proximal straight tubule. J Clin Invest. 1994;93:2572–2577. doi: 10.1172/JCI117268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gardner JP, Maher E, Aviv A. Calcium mobilization and Na+/H+ antiport activation by endothelin in human skin fibroblasts. FEBS Lett. 1989;256:38–42. doi: 10.1016/0014-5793(89)81713-2. [DOI] [PubMed] [Google Scholar]
- 125.Gariepy C, Wang Y, Nguyen N, Li S, Yanagisawa M. Salt-sensitive hypertension in ETB-deficient rats is not due to mechanisms intrinsic to the kidney. Seventh International Conference on Endothelin; Edinburgh, Scotland. 2001. p. O46. [Google Scholar]
- 126.Gariepy CE, Ohuchi T, Williams SC, Richardson JA, Yanagisawa M. Salt-sensitive hypertension in endothelin-B receptor-deficient rats. J Clin Invest. 2000;105:925–933. doi: 10.1172/JCI8609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Garvin J, Sanders K. Endothelin inhibits fluid and bicarbonate transport in part by reducing Na+/K+ ATPase activity in the rat proximal straight tubule. J Am Soc Nephrol. 1991;2:976–982. doi: 10.1681/ASN.V25976. [DOI] [PubMed] [Google Scholar]
- 128.Ge Y, Ahn D, Stricklett PK, Hughes AK, Yanagisawa M, Verbalis JG, Kohan DE. Collecting duct-specific knockout of endothelin-1 alters vasopressin regulation of urine osmolality. Am J Physiol Renal Physiol. 2005;288:F912–920. doi: 10.1152/ajprenal.00432.2004. [DOI] [PubMed] [Google Scholar]
- 129.Ge Y, Bagnall A, Stricklett PK, Strait K, Webb DJ, Kotelevtsev Y, Kohan DE. Collecting duct-specific knockout of the endothelin B receptor causes hypertension and sodium retention. Am J Physiol Renal Physiol. 2006;291:F1274–1280. doi: 10.1152/ajprenal.00190.2006. [DOI] [PubMed] [Google Scholar]
- 130.Ge Y, Huang Y, Kohan DE. Role of the renin-angiotensin-aldosterone system in collecting duct-derived endothelin-1 regulation of blood pressure. Can J Physiol Pharmacol. 2008;86:329–336. doi: 10.1139/Y08-028. [DOI] [PubMed] [Google Scholar]
- 131.Ge Y, Strait KA, Stricklett PK, Yang T, Kohan DE. Role of prostaglandins in collecting duct-derived endothelin-1 regulation of blood pressure and water excretion. Am J Physiol Renal Physiol. 2007;293:F1805–1810. doi: 10.1152/ajprenal.00307.2007. [DOI] [PubMed] [Google Scholar]
- 132.Ge Y, Stricklett PK, Hughes AK, Yanagisawa M, Kohan DE. Collecting duct-specific knockout of the endothelin A receptor alters renal vasopressin responsiveness, but not sodium excretion or blood pressure. Am J Physiol Renal Physiol. 2005;289:F692–698. doi: 10.1152/ajprenal.00100.2005. [DOI] [PubMed] [Google Scholar]
- 133.Geibel J, Giebisch G, Boron WF. Angiotensin II stimulates both Na(+)-H+ exchange and Na+/HCO3- cotransport in the rabbit proximal tubule. Proc Natl Acad Sci U S A. 1990;87:7917–7920. doi: 10.1073/pnas.87.20.7917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gellai M, DeWolf R, Pullen M, Nambi P. Distribution and functional role of renal ET receptor subtypes in normotensive and hypertensive rats. Kidney Int. 1994;46:1287–1294. doi: 10.1038/ki.1994.396. [DOI] [PubMed] [Google Scholar]
- 135.Gilmore ES, Stutts MJ, Milgram SL. SRC family kinases mediate epithelial Na+ channel inhibition by endothelin. J Biol Chem. 2001;276:42610–42617. doi: 10.1074/jbc.M106919200. [DOI] [PubMed] [Google Scholar]
- 136.Girchev R, Backer A, Markova P, Kramer H. Impaired response of the denervated kidney to endothelin receptor blockade in normotensive and spontaneously hypertensive rats. Kidney Int. 2004;65:982–989. doi: 10.1111/j.1523-1755.2004.00483.x. [DOI] [PubMed] [Google Scholar]
- 137.Goetz K, Wang BC, Leadley RJ, Zhu JL, Madwed J, Bie P. Endothelin and sarafotoxin produce dissimilar effects on renal blood flow, but both block the antidiuretic effects of vasopressin. Proc Soc Exp Biol Med. 1989;191:425–427. doi: 10.3181/00379727-191-4-rc1. [DOI] [PubMed] [Google Scholar]
- 138.Goruppi S, Bonventre J, Kyriakis J. Signaling pathways and late-onset gene induction associated with renal mesangial cell hypertrophy. EMBO J. 2002;216:5427–5436. doi: 10.1093/emboj/cdf535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gregan B, Jurgensen J, Papsdorf G, Furkert J, Schaefer M, Beyermann M, Rosenthal W, Oksche A. Ligand-dependent differences in the internalization of endothelin A and endothelin B receptor heterodimers. J Biol Chem. 2004;279:27679–27687. doi: 10.1074/jbc.M403601200. [DOI] [PubMed] [Google Scholar]
- 140.Gregan B, Schaefer M, Rosenthal W, Oksche A. Fluorescence resonance energy transfer analysis reveals the existence of endothelin-A and endothelin-B receptor homodimers. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S30–33. doi: 10.1097/01.fjc.0000166218.35168.79. [DOI] [PubMed] [Google Scholar]
- 141.Grond J, Beukers JY, Schilthuis MS, Weening JJ, Elema JD. Analysis of renal structural and functional features in two rat strains with a different susceptibility to glomerular sclerosis. Lab Invest. 1986;54:77–83. [PubMed] [Google Scholar]
- 142.Gulbins E, Hoffend J, Zou AP, Dietrich MS, Schlottmann K, Cavarape A, Steinhausen M. Endothelin and endothelium-derived relaxing factor control of basal renovascular tone in hydronephrotic rat kidneys. J Physiol. 1993;469:571–582. doi: 10.1113/jphysiol.1993.sp019830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gumz ML, Popp MP, Wingo CS, Cain BD. Early transcriptional effects of aldosterone in a mouse inner medullary collecting duct cell line. Am J Physiol Renal Physiol. 2003;285:F664–673. doi: 10.1152/ajprenal.00353.2002. [DOI] [PubMed] [Google Scholar]
- 144.Guntupalli J, DuBose TDJ. Effects of endothelin on rat renal proximal tubule Na+-Pi contransport and Na+/H+ exchange. Am J Physiol. 1994;266:F658–F666. doi: 10.1152/ajprenal.1994.266.4.F658. [DOI] [PubMed] [Google Scholar]
- 145.Guo X, Yang T. Endothelin B receptor antagonism in the rat renal medulla reduces urine flow rate and sodium excretion. Exp Biol Med (Maywood) 2006;231:1001–1005. [PubMed] [Google Scholar]
- 146.Harrington JT, Lemann J., Jr The metabolic production and disposal of acid and alkali. Med Clin North Am. 1970;54:1543–1554. [PubMed] [Google Scholar]
- 147.Harris PJ, Zhuo J, Mendelsohn FAO, Skinner SL. Haemodynamic and renal tubular effects of low doses of endothelin in anesthetized rats. J Physiol. 1991;433:25–39. doi: 10.1113/jphysiol.1991.sp018412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Harrison VJ, Barnes K, turner AJ, Wood E, Corder R, Vane JR. Identification of endothelin 1 and big endothelin 1 in secretory vesicles isolated from bovine aortic endothelial cells. PNAS. 1995;92:6344–6348. doi: 10.1073/pnas.92.14.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Harshfield GA, Alpert BS, Pulliam DA, Willey ES, Somes GW, Stapelton FB. Sodium excretion and racial differences in ambulatory blood pressure patterns. Hypertension. 1991;18:813–818. doi: 10.1161/01.hyp.18.6.813. [DOI] [PubMed] [Google Scholar]
- 150.Hatae N, Aksentijevich N, Zemkova HW, Kretschmannova K, Tomic M, Stojilkovic SS. Cloning and functional identification of novel endothelin receptor type A isoforms in pituitary. Mol Endocrinol. 2007;21:1192–1204. doi: 10.1210/me.2006-0343. [DOI] [PubMed] [Google Scholar]
- 151.Haug C, Linder TM, Schmid-Kotsas A, Hetzel S, Ernst F, Gruenert A, Jehle PM. Inhibitory effect of epidermal growth factor and hepatocyte growth factor on endothelin-1 release by rabbit proximal tubule cells. J Cardiovasc Pharmacol. 2000;36:S248–251. doi: 10.1097/00005344-200036051-00073. [DOI] [PubMed] [Google Scholar]
- 152.Hayashi K, Loutzenhiser R, Epstein M. Direct evidence that thromboxane mimetic U44069 preferentially constricts the afferent arteriole. J Am Soc Nephrol. 1997;8:25–31. doi: 10.1681/ASN.V8125. [DOI] [PubMed] [Google Scholar]
- 153.Heller J, Kramer HJ, Horacek V. Action of endothelin-1 on glomerular haemodynamics in the dog: lack of direct effects on glomerular ultrafiltration coefficient. Clin Sci (Lond) 1996;90:385–391. doi: 10.1042/cs0900385. [DOI] [PubMed] [Google Scholar]
- 154.Helms MN, Yu L, Malik B, Kleinhenz DJ, Hart CM, Eaton DC. Role of SGK1 in nitric oxide inhibition of ENaC in Na+-transporting epithelia. Am J Physiol Cell Physiol. 2005;289:C717–726. doi: 10.1152/ajpcell.00006.2005. [DOI] [PubMed] [Google Scholar]
- 155.Hercule H, Oyekan A. Renal cytochrome p450 oxygenases and preglomerular vascular response to arachidonic acid and endothelin-1 following ischemia/reperfusion. J Pharmacol Exp Ther. 2002;302:717–724. doi: 10.1124/jpet.302.2.717. [DOI] [PubMed] [Google Scholar]
- 156.Hercule H, Oyekan A. Role of NO and cytochrome P-450-derived eicosanoids in ET-1-induced changes in intrarenal hemodynamics in rats. Am J Physiol. 2000;279:R2132–R2141. doi: 10.1152/ajpregu.2000.279.6.R2132. [DOI] [PubMed] [Google Scholar]
- 157.Herizi A, Jover B, Bouriquet N, Mimran A. Prevention of the cardiovascular and renal effects of angiotensin II by endothelin blockade. Hypertension. 1998;31:10–14. doi: 10.1161/01.hyp.31.1.10. [DOI] [PubMed] [Google Scholar]
- 158.Herman WH, Emancipator SN, Rhoten RL, Simonson MS. Vascular and glomerular expression of endothelin-1 in normal human kidney. Am J Physiol. 1998;275:F8–17. doi: 10.1152/ajprenal.1998.275.1.F8. [DOI] [PubMed] [Google Scholar]
- 159.Herrera M, Garvin J. A high-salt diet stimulates thick ascending limb eNOS expression by raising medullary osmolality and increasing release of endothelin-1. Am J Physiol. 2005;288:F58–F64. doi: 10.1152/ajprenal.00209.2004. [DOI] [PubMed] [Google Scholar]
- 160.Herrera M, Garvin J. Endothelin stimulates endothelial nitric oxide synthase expression in the thick ascending limb. Am J Physiol. 2004;287:F231–F235. doi: 10.1152/ajprenal.00413.2003. [DOI] [PubMed] [Google Scholar]
- 161.Herrera M, Hong NJ, Ortiz PA, Garvin JL. Endothelin-1 inhibits thick ascending limb transport via Akt-stimulated nitric oxide production. J Biol Chem. 2009;284:1454–1460. doi: 10.1074/jbc.M804322200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Hirahashi J, Nakaki T, Hishikawa K, Marumo T, Yasumori T, Hayashi M, Suzuki H, Saruta T. Endothelin-1 inhibits induction of nitric oxide synthase and GTP cyclohydrolase I in rat mesangial cells. Pharmacology. 1996;53:241–249. doi: 10.1159/000139436. [DOI] [PubMed] [Google Scholar]
- 163.Hoffman A, Abassi ZA, Brodsky S, Ramadan R, Winaver J. Mechanisms of big endothelin-I-induced diuresis and natriuresis. Hypertension. 2000;35:732–739. doi: 10.1161/01.hyp.35.3.732. [DOI] [PubMed] [Google Scholar]
- 164.Hoffman A, Grossman E, Goldstein DS, Gill JR, Jr, Keiser HR. Urinary excretion rate of endothelin-1 in patients with essential hypertension and salt sensitivity. Kidney Int. 1993;45:556–560. doi: 10.1038/ki.1994.72. [DOI] [PubMed] [Google Scholar]
- 165.Hoffman A, Grossman E, Goldstein DS, Gill JRJ, Keiser HR. Urinary excretion rate of endothelin-1 in patients with essential hypertension and salt sensitivity. Kidney Int. 1994;45:556–560. doi: 10.1038/ki.1994.72. [DOI] [PubMed] [Google Scholar]
- 166.Honing M, Hijmering M, Ballard D, Yang Y, Padley R, Morrison P, Rabelink T. Selective ETA receptor antagonism with ABT-627 attenuates all renal effects of endothelin in humans. J Am Soc Nephrol. 2000;11:1498–1504. doi: 10.1681/ASN.V1181498. [DOI] [PubMed] [Google Scholar]
- 167.Horie M, Uchida S, Yanagisawa M, Matsushita Y, Kurokawa K, Ogata E. Mechanisms of endothelin-1 mRNA and peptides induction by TGF-beta and TPA in MDCK cells. J Cardiovasc Pharmacol. 1991;17(Suppl 7):S222–225. doi: 10.1097/00005344-199100177-00064. [DOI] [PubMed] [Google Scholar]
- 168.Hosoda K, Nakao K, Tamura N, Arai H, Ogawa Y, Suga S, Nakanishi S, Imura H. Organization, structure, chromosomal assignment, and expression of the gene encoding the human endothelin-A receptor. J Biol Chem. 1992;267:18797–18804. [PubMed] [Google Scholar]
- 169.Hovater MB, Olteanu D, Hanson EL, Cheng NL, Siroky B, Fintha A, Komlosi P, Liu W, Satlin LM, Bell PD, Yoder BK, Schwiebert EM. Loss of apical monocilia on collecting duct principal cells impairs ATP secretion across the apical cell surface and ATP-dependent and flow-induced calcium signals. Purinergic Signal. 2008;4:155–170. doi: 10.1007/s11302-007-9072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hsieh TJ, Lin SR, Lee YJ, Shin SJ, Lai YH, Hsu CH, Tsai JH. Increased renal medullary endothelin-1 synthesis in prehypertensive DOCA- and salt-treated rats. Am J Physiol Renal Physiol. 2000;279:F112–121. doi: 10.1152/ajprenal.2000.279.1.F112. [DOI] [PubMed] [Google Scholar]
- 171.Hua H, Munk S, Whiteside CI. Endothelin-1 activates mesangial cell ERK1/2 via EGF-receptor transactivation and caveolin-1 interaction. Am J Physiol Renal Physiol. 2003;284:F303–312. doi: 10.1152/ajprenal.00127.2002. [DOI] [PubMed] [Google Scholar]
- 172.Hubel CA, Highsmith RF. Endothelin-induced changes in intracellular pH and Ca2+ in coronary smooth muscle: role of Na(+)-H+ exchange. Biochem J. 1995;310(Pt 3):1013–1020. doi: 10.1042/bj3101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hughes AK, Cline RC, Kohan DE. Alterations in renal endothelin production in the spontaneously hypertensive rat. Hypertension. 1992;20:666–673. doi: 10.1161/01.hyp.20.5.666. [DOI] [PubMed] [Google Scholar]
- 174.Hughes AK, Padilla E, Kutchera WA, Michael JR, Kohan DE. Endothelin-1 induction of cyclooxygenase-2 expression in rat mesangial cells. Kidney Int. 1995;47:53–61. doi: 10.1038/ki.1995.6. [DOI] [PubMed] [Google Scholar]
- 175.Hughes AK, Stricklett PK, Kishore BK, Kohan DE. Adenosine triphosphate inhibits endothelin-1 production by rat inner medullary collecting duct cells. Exp Biol Med (Maywood) 2006;231:1006–1009. [PubMed] [Google Scholar]
- 176.Hwang YS, Hsieh TJ, Lee YJ, Tsai JH. Circadian rhythm of urinary endothelin-1 excretion in mild hypertensive patients. Am J Hypertens. 1998;11:1344–1351. doi: 10.1016/s0895-7061(98)00170-8. [DOI] [PubMed] [Google Scholar]
- 177.Iglarz M, Schiffrin EL. Role of endothelin-1 in hypertension. Curr Hypertens Rep. 2003;5:144–148. doi: 10.1007/s11906-003-0071-4. [DOI] [PubMed] [Google Scholar]
- 178.Ikeda M, Kohno M, Takeda T. Endothelin production in cultured mesangial cells of spontaneously hypertensive rats. Hypertension. 1995;25:1196–1201. doi: 10.1161/01.hyp.25.6.1196. [DOI] [PubMed] [Google Scholar]
- 179.Ikeda S, Emoto N, Alimsardjono H, Yokoyama M, Matsuo M. Molecular isolation and characterization of novel four subisoforms of ECE-2. Biochem Biophys Res Commun. 2002;293:421–426. doi: 10.1016/S0006-291X(02)00252-8. [DOI] [PubMed] [Google Scholar]
- 180.Imai T, Hirata Y, Emori T, Yanagisawa M, Masaki T, Marumo F. Induction of endothelin-1 gene by angiotensin and vasopressin in endothelial cells. Hypertension. 1992;19:753–757. doi: 10.1161/01.hyp.19.6.753. [DOI] [PubMed] [Google Scholar]
- 181.Imamura T, Huang J, Dalle S, Ugi S, Usui I, Luttrell L, Miller W, Lefkowitz R, Olefsky J. beta -Arrestin-mediated recruitment of the Src family kinase Yes mediates endothelin-1-stimulated glucose transport. J Biol Chem. 2001;276:43663–43667. doi: 10.1074/jbc.M105364200. [DOI] [PubMed] [Google Scholar]
- 182.Imig JD. Eicosanoid regulation of the renal vasculature. Am J Physiol Renal Physiol. 2000;279:F965–981. doi: 10.1152/ajprenal.2000.279.6.F965. [DOI] [PubMed] [Google Scholar]
- 183.Imig JD, Pham BT, LeBlanc EA, Reddy KM, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension. 2000;35:307–312. doi: 10.1161/01.hyp.35.1.307. [DOI] [PubMed] [Google Scholar]
- 184.Inoue A, Yanagisawa M, Takuwa Y, Mitsui Y, Kobayashi M, Masaki T. The human preproendothelin-1 gene. J Biol Chem. 1989;264:14954–14959. [PubMed] [Google Scholar]
- 185.Inscho EW, Cook AK, Webb RC, Jin LM. Rho-kinase inhibition reduces pressure-mediated autoregulatory adjustments in afferent arteriolar diameter. Am J Physiol Renal Physiol. 2009;296:F590–597. doi: 10.1152/ajprenal.90703.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Inscho EW, Imig JD, Cook AK, Pollock DM. ETA and ETB receptors differentially modulate afferent and efferent arteriolar responses to endothelin. Br J Pharmacol. 2005;146:1019–1026. doi: 10.1038/sj.bjp.0706412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ishii K, Warner TD, Sheng H, Murad F. Endothelin increases cyclic GMP levels in LLC-PK1 porcine kidney epithelial cells via formation of an endothelium-derived relaxing factor-like substance. J Pharmacol Exp Ther. 1991;259:1102–1108. [PubMed] [Google Scholar]
- 188.Ito H, Hirata Y, Adachi S, Tanaka M, Tsujino M, Koike A, Nogami A, Murumo F, Hiroe M. Endothelin-1 is an autocrine/paracrine factor in the mechanism of angiotensin II-induced hypertrophy in cultured rat cardiomyocytes. J Clin Invest. 1993;92:398–403. doi: 10.1172/JCI116579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Itoh K, Kase R, Shimmoto M, Satake A, Sakuraba H, Suzuki Y. Protective protein as an endogenous endothelin degradation enzyme in human tissues. J Biol Chem. 1995;270:515–518. doi: 10.1074/jbc.270.2.515. [DOI] [PubMed] [Google Scholar]
- 190.Iwasaki S, Homma T, Matsuda Y, Kon V. Endothelin receptor subtype B mediates autoinduction of endothelin-1 in rat mesangial cells. J Biol Chem. 1995;270:6997–7003. doi: 10.1074/jbc.270.12.6997. [DOI] [PubMed] [Google Scholar]
- 191.Jackman HL, Morris PW, Deddish PA, Skidgel RA, Erdos EG. Inactivation of endothelin I by deamidase (lysosomal protective protein) J Biol Chem. 1992;267:2872–2875. [PubMed] [Google Scholar]
- 192.Jackson RW, Treiber FA, Harshfield GA, Waller JL, Pollock JS, Pollock DM. Urinary excretion of vasoactive factors are correlated to sodium excretion. Am J Hypertens. 2001;14:1003–1006. doi: 10.1016/s0895-7061(01)02169-0. [DOI] [PubMed] [Google Scholar]
- 193.Jacques D, Sader S, Perreault C, Abdel-Samad D, Jules F, Provost C. NPY, ET-1, and Ang II nuclear receptors in human endocardial endothelial cells. Can J Physiol Pharmacol. 2006;84:299–307. doi: 10.1139/y05-158. [DOI] [PubMed] [Google Scholar]
- 194.Jaffer FE, Knauss TC, Poptic E, Abboud HE. Endothelin stimulates PDGF secretion in cultured human mesangial cells. Kidney Int. 1990;38:1193–1198. doi: 10.1038/ki.1990.333. [DOI] [PubMed] [Google Scholar]
- 195.Januszewicz A, Lapinski M, Symonides B, Dabrowska E, Kuch-Wocial A, Trzepla E, Ignatowska-Switalska H, Wocial B, Chodakowska J, Januszewicz W. Elevated endothelin-1 plasma concentration in patients with essential hypertension. J Cardiovasc Risk. 1994;1:81–85. [PubMed] [Google Scholar]
- 196.Just A, Whitten CL, Arendshorst WJ. Reactive oxygen species participate in acute renal vasoconstrictor responses induced by ETA and ETB receptors. Am J Physiol Renal Physiol. 2008;294:F719–728. doi: 10.1152/ajprenal.00506.2007. [DOI] [PubMed] [Google Scholar]
- 197.Kamphuis C, Yates NA, McDaugall JG. Differential blockade of the renal vasoconstrictor and iduretic responses to endothelin-1 by endothelin antagonist. Clin Exper Pharmacol Physiol. 1994;21:329–333. doi: 10.1111/j.1440-1681.1994.tb02522.x. [DOI] [PubMed] [Google Scholar]
- 198.Karet FE, Davenport AP. Localization of endothelin peptides in human kidney. Kidney Int. 1996;49:382–387. doi: 10.1038/ki.1996.56. [DOI] [PubMed] [Google Scholar]
- 199.Karet FE, Kuc RE, Davenport AP. Novel ligands BQ123 and BQ3020 characterize endothelin receptor subtypes ETA and ETB in human kidney. Kidney Int. 1993;44:36–42. doi: 10.1038/ki.1993.210. [DOI] [PubMed] [Google Scholar]
- 200.Kasinath BS, Fried TA, Davalath S, Marsden PA. Glomerular epithelial cells synthesize endothelin peptides. Am J Pathol. 1992;141:279–283. [PMC free article] [PubMed] [Google Scholar]
- 201.Katoh T, Chang H, Uchida S, Okuda T, Kurokawa K. Direct effects of endothelin in the rat kidney. Am J Physiol. 1990;258:F397–F402. doi: 10.1152/ajprenal.1990.258.2.F397. [DOI] [PubMed] [Google Scholar]
- 202.Kawabata M, Han WH, Ise T, Kobayashi K, Takabatake T. Role of endogenous endothelin and nitric oxide in tubuloglomerular feedback. Kidney Int Suppl. 1996;55:S135–137. [PubMed] [Google Scholar]
- 203.Khanna A, Simoni J, Hacker C, Duran MJ, Wesson DE. Increased endothelin activity mediates augmented distal nephron acidification induced by dietary protein. J Am Soc Nephrol. 2004;15:2266–2275. doi: 10.1097/01.ASN.0000138233.78329.4E. [DOI] [PubMed] [Google Scholar]
- 204.Khanna A, Simoni J, Wesson DE. Endothelin-induced increased aldosterone activity mediates augmented distal nephron acidification as a result of dietary protein. J Am Soc Nephrol. 2005;16:1929–1935. doi: 10.1681/ASN.2004121054. [DOI] [PubMed] [Google Scholar]
- 205.King AJ, Brenner BM, Anderson S. Endothelin: a potent renal and systemic vasoconstrictor peptide. Am J Physiol. 1989;256:F1051–F1058. doi: 10.1152/ajprenal.1989.256.6.F1051. [DOI] [PubMed] [Google Scholar]
- 206.Kirkby NS, Hadoke PW, Bagnall AJ, Webb DJ. The endothelin system as a therapeutic target in cardiovascular disease: great expectations or bleak house? Br J Pharmacol. 2008;153:1105–1119. doi: 10.1038/sj.bjp.0707516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kirton CA, Loutzenhiser R. Alterations in basal protein kinase C activity modulate renal afferent arteriolar myogenic reactivity. Am J Physiol. 1998;275:H467–475. doi: 10.1152/ajpheart.1998.275.2.H467. [DOI] [PubMed] [Google Scholar]
- 208.Kitamura K, Shiraishi N, Singer W, Handlogten M, Tomita K, Miller R. Endothelin-B receptors activate Galpha13. Am J Physiol. 1999;276:C930–C937. doi: 10.1152/ajpcell.1999.276.4.C930. [DOI] [PubMed] [Google Scholar]
- 209.Kitamura K, Tanaka T, Kato J, Eto T, Tanaka K. Regional distribution of immunoreactive endothelin in porcine tissue: abundance in inner medulla of kidney. Biochem Biophys Res Commun. 1989;161:348–352. doi: 10.1016/0006-291x(89)91603-3. [DOI] [PubMed] [Google Scholar]
- 210.Kitamura K, Tanaka T, Kato J, Qgawa Y, Eto T, Tanaka K. Immunoreactive endothelin in rat kidney inner medulla: marked decrease in spontaneously hypertensive rats. Biochem Biophys Res Commun. 1989;162:38–44. doi: 10.1016/0006-291x(89)91958-x. [DOI] [PubMed] [Google Scholar]
- 211.Knepper MA, Packer R, Good DW. Ammonium transport in the kidney. Physiol Rev. 1989;69:179–249. doi: 10.1152/physrev.1989.69.1.179. [DOI] [PubMed] [Google Scholar]
- 212.Kohan D. Endothelins in the normal and diseased kidney. Am J Kid Dis. 1997;29:2–26. doi: 10.1016/s0272-6386(97)90004-4. [DOI] [PubMed] [Google Scholar]
- 213.Kohan DE. Endothelin production by human inner medullary collecting duct cells. J Am Soc Nephrol. 1993;3:1719–1721. doi: 10.1681/ASN.V3101719. [DOI] [PubMed] [Google Scholar]
- 214.Kohan DE. Endothelin synthesis by rabbit renal tubule cells. Am J Physiol. 1991;261:F221–F226. doi: 10.1152/ajprenal.1991.261.2.F221. [DOI] [PubMed] [Google Scholar]
- 215.Kohan DE. Production of endothelin-1 by rat mesangial cells: regulation by tumor necrosis factor. J Lab Clin Med. 1992;119:477–484. [PubMed] [Google Scholar]
- 216.Kohan DE, Fiedorek FTJ. Endothelin synthesis by rat inner medullary collecting duct cells. J Am Soc Nephrol. 1991;2:150–155. doi: 10.1681/ASN.V22150. [DOI] [PubMed] [Google Scholar]
- 217.Kohan DE, Hughes AK, Perkins SP. Characterization of endothelin receptors in the inner medullary collecting duct of the rat. J Biol Chem. 1992;267:12336–12340. [PubMed] [Google Scholar]
- 218.Kohan DE, Padilla E. Endothelin-1 is an autocrine factor in rat inner medullary collecting ducts. Am J Physiol. 1992;263:F607–F612. doi: 10.1152/ajprenal.1992.263.4.F607. [DOI] [PubMed] [Google Scholar]
- 219.Kohan DE, Padilla E. Endothelin-1 production by rat inner medullary collecting ducts: effect of nitric oxide, cGMP, and immune cytokines. Am J Physiol. 1994;266:F291–F297. doi: 10.1152/ajprenal.1994.266.2.F291. [DOI] [PubMed] [Google Scholar]
- 220.Kohan DE, Padilla E. Osmolar regulation of endothelin-1 production by rat inner medullary collecting duct. J Clin Invest. 1993;91:1235–1240. doi: 10.1172/JCI116286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kohan DE, Padilla E, Hughes AK. Endothelin B receptor mediates ET-1 effects on cAMP and PGE2 accumulation in rat IMCD. Am J Physiol. 1993;265:F670–F676. doi: 10.1152/ajprenal.1993.265.5.F670. [DOI] [PubMed] [Google Scholar]
- 222.Kohno M, Horio T, Ikeda M, Yokokawa K, Fukui T, Yasunari K, Kurihara N, Takeda T. Angiotensin II stimulates endothelin-1 secretion in cultured rat mesangial cells. Kidney Int. 1992;42:860–866. doi: 10.1038/ki.1992.361. [DOI] [PubMed] [Google Scholar]
- 223.Kohno M, Ikeda M, Johchi M, Horio T, Yasunari K, Kurihara N, Takeda T. Interaction of PDGF and natriuretic peptides on mesangial cell proliferation and endothelin secretion. Am J Physiol. 1993;265:E673–679. doi: 10.1152/ajpendo.1993.265.5.E673. [DOI] [PubMed] [Google Scholar]
- 224.Kohno M, Murakawa K, Yasunari K, Yokokawa K, Horio T, Kurihara N, Takeda T. Prolonged blood pressure elevation after endothelin administration in bilaterally nephrectomized rats. Metabolism. 1989;38:712–713. doi: 10.1016/0026-0495(89)90054-1. [DOI] [PubMed] [Google Scholar]
- 225.Kohno M, Yokokawa K, Mandal AK, Horio T, Yasunari K, Takeda T. Cardiac natriuretic peptides inhibit cyclosporine-induced production of endothelin in cultured rat mesangial cells. Metabolism. 1995;44:404–409. doi: 10.1016/0026-0495(95)90174-4. [DOI] [PubMed] [Google Scholar]
- 226.Kohzuki M, Johnston CI, Abe K, Chai SY, Casley DJ, Yasujima M, Yoshinaga K, Mendelsohn FA. In vitro autoradiographic endothelin-1 binding sites and sarafotoxin S6B binding sites in rat tissues. Clin Exp Pharmacol Physiol. 1991;18:509–515. doi: 10.1111/j.1440-1681.1991.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 227.Kon V, Badr KF. Biological actions and pathophysiologic significance of endothelin in the kidney. Kidney Int. 1991;40:1–12. doi: 10.1038/ki.1991.172. [DOI] [PubMed] [Google Scholar]
- 228.Kon V, Yoshioka T, Fogo A, Ichikawa I. Glomerular actions of endothelin in vivo. J Clin Invest. 1989;83:1762–1767. doi: 10.1172/JCI114079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kopp UC, Cicha MZ, Smith LA. Activation of endothelin-a receptors contributes to angiotensin-induced suppression of renal sensory nerve activation. Hypertension. 2007;49:141–147. doi: 10.1161/01.HYP.0000249634.46212.7b. [DOI] [PubMed] [Google Scholar]
- 230.Kopp UC, Cicha MZ, Smith LA. Angiotensin blocks substance P release from renal sensory nerves by inhibiting PGE2-mediated activation of cAMP. Am J Physiol Renal Physiol. 2003;285:F472–483. doi: 10.1152/ajprenal.00399.2002. [DOI] [PubMed] [Google Scholar]
- 231.Kopp UC, Cicha MZ, Smith LA. Differential effects of endothelin on activation of renal mechanosensory nerves: stimulatory in high-sodium diet and inhibitory in low-sodium diet. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1545–1556. doi: 10.1152/ajpregu.00878.2005. [DOI] [PubMed] [Google Scholar]
- 232.Kopp UC, Cicha MZ, Smith LA. Endogenous angiotensin modulates PGE(2)-mediated release of substance P from renal mechanosensory nerve fibers. Am J Physiol Regul Integr Comp Physiol. 2002;282:R19–30. doi: 10.1152/ajpregu.2002.282.1.R19. [DOI] [PubMed] [Google Scholar]
- 233.Kopp UC, Grisk O, Cicha MZ, Smith LA, Steinbach A, Schluter T, Mahler N, Hokfelt T. Dietary sodium modulates the interaction between efferent renal sympathetic nerve activity and afferent renal nerve activity: role of endothelin. Am J Physiol Regul Integr Comp Physiol. 2009;297:R337–351. doi: 10.1152/ajpregu.91029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Kopp UC, Olson LA, DiBona GF. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am J Physiol. 1984;246:F67–77. doi: 10.1152/ajprenal.1984.246.1.F67. [DOI] [PubMed] [Google Scholar]
- 235.Korbmacher C, Boulpaep EL, Giebisch G, Geibel J. Endothelin increases [Ca2+]i in M-1 mouse cortical collecting duct cells by a dual mechanism. Am J Physiol. 1993;265:C349–C357. doi: 10.1152/ajpcell.1993.265.2.C349. [DOI] [PubMed] [Google Scholar]
- 236.Kramer H, Hashemi T, Backer A, Bokemeyer D. Hyperosmolality Induced by Betaine or Urea Stimulates Endothelin Synthesis by Differential Activation of ERK and p38 MAP Kinase in MDCK Cells. Kidney Blood Press Res. 2002;25:65–70. doi: 10.1159/000063510. [DOI] [PubMed] [Google Scholar]
- 237.Kramer RE, Robinson TV, Schneider EG, Smith TG. Direct modulation of basal and angiotensin II-stimulated aldosterone secretion by hydrogen ions. J Endocrinol. 2000;166:183–194. doi: 10.1677/joe.0.1660183. [DOI] [PubMed] [Google Scholar]
- 238.Krum H, Viskoper RJ, Lacourciere Y, Budde M, Charlon V. The effect of an endothelin-receptor antagonist, bosentan, on blood pressure in patients with essential hypertension. Bosentan Hypertension Investigators. N Engl J Med. 1998;338:784–790. doi: 10.1056/NEJM199803193381202. [DOI] [PubMed] [Google Scholar]
- 239.Kuc R, Davenport AP. Comparison of endothelin-A and endothelin-B receptor distribution visualized by radioligand binding versus immunocytochemical localization using subtype selective antisera. J Cardiovasc Pharmacol. 2004;44(Suppl 1):S224–226. doi: 10.1097/01.fjc.0000166260.35099.d5. [DOI] [PubMed] [Google Scholar]
- 240.Kuc RE, Davenport AP. Endothelin-A-receptors in human aorta and pulmonary arteries are downregulated in patients with cardiovascular disease: an adaptive response to increased levels of endothelin-1? J Cardiovasc Pharmacol. 2000;36:S377–379. doi: 10.1097/00005344-200036051-00109. [DOI] [PubMed] [Google Scholar]
- 241.Kunau RT, Jr, Walker KA. Distal tubular acidification in the remnant kidney. Am J Physiol. 1990;258:F69–74. doi: 10.1152/ajprenal.1990.258.1.F69. [DOI] [PubMed] [Google Scholar]
- 242.Kunau RT, Jr, Walker KA. Total CO2 absorption in the distal tubule of the rat. Am J Physiol. 1987;252:F468–473. doi: 10.1152/ajprenal.1987.252.3.F468. [DOI] [PubMed] [Google Scholar]
- 243.Kurokawa K, Yoshitomi K, Ikeda M, Uchida S, Naruse M, Imai M. Regulation of cortical collecting duct function: effect of endothelin. Am Heart J. 1993;125:582–588. doi: 10.1016/0002-8703(93)90207-p. [DOI] [PubMed] [Google Scholar]
- 244.Kurtz A, Kaissling B, Busse R, Baier W. Endothelial cells modulate renin secretion from isolated mouse juxtaglomerular cells. J Clin Invest. 1991;88:1147–1154. doi: 10.1172/JCI115415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kurtz I, Maher T, Hulter HN, Schambelan M, Sebastian A. Effect of diet on plasma acid-base composition in normal humans. Kidney Int. 1983;24:670–680. doi: 10.1038/ki.1983.210. [DOI] [PubMed] [Google Scholar]
- 246.Laghmani K, Preisig P, Alpern R. The role of endothelin in proximal tubule proton secretion and the adaptation to a chronic metabolic acidosis. J Nephrol. 2002;15(Suppli 5):S75–S87. [PubMed] [Google Scholar]
- 247.Laghmani K, Preisig P, Moe O, Yangisawa M, Alpern R. Endothelin-1/endothelin-B receptor-mediated increase in NHE3 activity in chronic metabolic acidosis. J Clin Invest. 2001;107:1563–1569. doi: 10.1172/JCI11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Laghmani K, Sakamoto A, Yanagisawa M, Preisig PA, Alpern RJ. A consensus sequence in the endothelin-B receptor second intracellular loop is required for NHE3 activation by endothelin-1. Am J Physiol Renal Physiol. 2005;288:F732–739. doi: 10.1152/ajprenal.00300.2004. [DOI] [PubMed] [Google Scholar]
- 249.Lanese DM, Yuan BH, McMurtry IF, Conger JD. Comparative sensitivities of isolated rat renal arterioles to endothelin. Am J Physiol. 1992;263:F894–899. doi: 10.1152/ajprenal.1992.263.5.F894. [DOI] [PubMed] [Google Scholar]
- 250.Largo R, Gomez-Garre D, Liu XH, Alonso J, Blanco J, Plaza JJ, Egido J. Endothelin-1 upregulation in the kidney of uninephrectomized spontaneously hypertensive rats and its modification by the angiotensin-converting enzyme inhibitor quinapril. Hypertension. 1997;29:1178–1185. doi: 10.1161/01.hyp.29.5.1178. [DOI] [PubMed] [Google Scholar]
- 251.Lemann J, Jr, Litzow JR, Lennon EJ. The effects of chronic acid loads in normal man: further evidence for the participation of bone mineral in the defense against chronic metabolic acidosis. J Clin Invest. 1966;45:1608–1614. doi: 10.1172/JCI105467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Lennon EJ, Lemann J, Jr, Litzow JR. The effects of diet and stool composition on the net external acid balance of normal subjects. J Clin Invest. 1966;45:1601–1607. doi: 10.1172/JCI105466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Letizia C, Cerci S, De Ciocchis A, D’Ambrosio C, Scuro L, Scavo D. Plasma endothelin-1 levels in normotensive and borderline hypertensive subjects during a standard cold pressor test. J Hum Hypertens. 1995;9:903–907. [PubMed] [Google Scholar]
- 254.Letizia C, Iannaccone A, Cerci S, Santi G, Cilli M, Coassin S, Pannarale MR, Scavo D. Circulating endothelin-1 in non-insulin-dependent diabetic patients with retinopathy. Horm Metab Res. 1997;29:247–251. doi: 10.1055/s-2007-979030. [DOI] [PubMed] [Google Scholar]
- 255.Lev S, Moreno H, Martinez R, Canoll P, Peles E, Musacchio J, Plowman G, Rudy B, Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca(2+)-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 256.Levine DZ. An in vivo microperfusion study of distal tubule bicarbonate reabsorption in normal and ammonium chloride rats. J Clin Invest. 1985;75:588–595. doi: 10.1172/JCI111735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Levine DZ. Single-nephron studies: implications for acid-base regulation. Kidney Int. 1990;38:744–761. doi: 10.1038/ki.1990.267. [DOI] [PubMed] [Google Scholar]
- 258.Levine DZ, Iacovitti M, Buckman S, Harrison V. In vivo modulation of rat distal tubule net HCO3 flux by VIP, isoproterenol, angiotensin II, and ADH. Am J Physiol. 1994;266:F878–883. doi: 10.1152/ajprenal.1994.266.6.F878. [DOI] [PubMed] [Google Scholar]
- 259.Levine DZ, Iacovitti M, Buckman S, Hincke MT, Luck B, Fryer JN. ANG II-dependent HCO3- reabsorption in surviving rat distal tubules: expression/activation of H(+)-ATPase. Am J Physiol. 1997;272:F799–808. doi: 10.1152/ajprenal.1997.272.6.F799. [DOI] [PubMed] [Google Scholar]
- 260.Li JS, Knafo L, Turgeon A, Garcia R, Schiffrin EL. Effect of endothelin antagonism on blood pressure and vascular structure in renovascular hypertensive rats. Am J Physiol. 1996;271:H88–93. doi: 10.1152/ajpheart.1996.271.1.H88. [DOI] [PubMed] [Google Scholar]
- 261.Li JS, Schiffrin EL. Effect of chronic treatment of adult spontaneously hypertensive rats with an endothelin receptor antagonist. Hypertension. 1995;25:495–500. doi: 10.1161/01.hyp.25.4.495. [DOI] [PubMed] [Google Scholar]
- 262.Licht C, Laghmani K, Yanagisawa M, Preisig PA, Alpern RJ. An autocrine role for endothelin-1 in the regulation of proximal tubule NHE3. Kidney Int. 2004;65:1320–1326. doi: 10.1111/j.1523-1755.2004.00506.x. [DOI] [PubMed] [Google Scholar]
- 263.Lin H, Sangmal M, Smith MJ, Jr, Young DB. Effect of endothelin-1 on glomerular hydraulic pressure and renin release in dogs. Hypertension. 1993;21:845–851. doi: 10.1161/01.hyp.21.6.845. [DOI] [PubMed] [Google Scholar]
- 264.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005 doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 265.López-Farré A, Gómez-Garre D, Bernabeu F, Montanes I, Millas I, López-Novoa JM. Renal effects and mesangial cell contraction induced by endothelin are mediated by PAF. Kidney Int. 1991;39:624–630. doi: 10.1038/ki.1991.74. [DOI] [PubMed] [Google Scholar]
- 266.Loutzenhiser K, Loutzenhiser R. Angiotensin II-induced Ca(2+) influx in renal afferent and efferent arterioles: differing roles of voltage-gated and store-operated Ca(2+) entry. Circ Res. 2000;87:551–557. doi: 10.1161/01.res.87.7.551. [DOI] [PubMed] [Google Scholar]
- 267.Loutzenhiser R, Chilton L, Trottier G. Membrane potential measurements in renal afferent and efferent arterioles: actions of angiotensin II. Am J Physiol. 1997;273:F307–314. doi: 10.1152/ajprenal.1997.273.2.F307. [DOI] [PubMed] [Google Scholar]
- 268.Loutzenhiser R, Epstein M, Hayashi K, Horton C. Direct visualization of effects of endothelin on the renal microvasculature. Am J Physiol. 1990;258:F61–F68. doi: 10.1152/ajprenal.1990.258.1.F61. [DOI] [PubMed] [Google Scholar]
- 269.Loutzenhiser R, Hayashi K, Epstein M. Divergent effects of KCl-induced depolarization on afferent and efferent arterioles. Am J Physiol. 1989;257:F561–564. doi: 10.1152/ajprenal.1989.257.4.F561. [DOI] [PubMed] [Google Scholar]
- 270.MacDonald JA, Borman MA, Muranyi A, Somlyo AV, Hartshorne DJ, Haystead TA. Identification of the endogenous smooth muscle myosin phosphatase-associated kinase. Proc Natl Acad Sci U S A. 2001;98:2419–2424. doi: 10.1073/pnas.041331498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Maddox DA, Horn JF, Famiano FC, Gennari FJ. Load dependence of proximal tubular fluid and bicarbonate reabsorption in the remnant kidney of the Munich-Wistar rat. J Clin Invest. 1986;77:1639–1649. doi: 10.1172/JCI112481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Madeddu P, Troffa C, Glorioso N, Pazzola A, Soro A, Manunta P, Tonolo G, Demontis MP, Varoni MV, Anania V. Effect of endothelin on regional hemodynamics and renal function in awake normotensive rats. J Cardiovasc Pharmacol. 1989;14:818–825. doi: 10.1097/00005344-198912000-00004. [DOI] [PubMed] [Google Scholar]
- 273.Majowicz MP, Gonzalez Bosc LV, Albertoni Borghese MF, Delgado MF, Ortiz MC, Sterin Speziale N, Vidal NA. Atrial natriuretic peptide and endothelin-3 target renal sodium-glucose cotransporter. Peptides. 2003;24:1971–1976. doi: 10.1016/j.peptides.2003.07.030. [DOI] [PubMed] [Google Scholar]
- 274.Malatino LS, Bellanuova I, Cataliotti A, Cuzzola F, Mallamaci F, Tripepi G, Parlongo S, Cutrupi S, Mangiafico RA, Ferri C, Galletti F, Gloriso N, Strazzullo P, Zoccali C. Renal endothelin-1 is linked to changes in urinary salt and volume in essential hypertension. J Nephrol. 2000;13:178–184. [PubMed] [Google Scholar]
- 275.Malnic G, Fernandez R, Cassola AC, Barreto-Chaves ML, de Souza MO, Aires Mde M. Mechanisms and regulation of H+ transport in distal tubule epithelial cells. Wien Klin Wochenschr. 1997;109:429–434. [PubMed] [Google Scholar]
- 276.Malnic G, Fernandez R, Lopes MJ. The role of the distal nephron in the regulation of acid-base equilibrium by the kidney. Braz J Med Biol Res. 1994;27:831–850. [PubMed] [Google Scholar]
- 277.Marchetti J, Meneton P, Lebrun F, Bloch-Faure M, Rajerison RM. The parietal sheet of Bowman’s capsule of rat renal glomerulus: a target of endothelin and PAF. Am J Physiol. 1995;268:F1053–F1061. doi: 10.1152/ajprenal.1995.268.6.F1053. [DOI] [PubMed] [Google Scholar]
- 278.Masaki T. Historical review: Endothelin. Trends Pharmacol Sci. 2004;25:219–224. doi: 10.1016/j.tips.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 279.Masaki T, Kimura S, Yanagisawa M, Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation. 1991;84:1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- 280.Matsumura Y, Nakase K, Ikegawa R, Hayashi K, Ohyama T, Morimoto S. The endothelium-derived vasoconstrictor peptide endothelin inhibits renin release in vitro. Life Sci. 1989;44:149–157. doi: 10.1016/0024-3205(89)90533-x. [DOI] [PubMed] [Google Scholar]
- 281.Matsuo G, Matsummura Y, Tadano K, Hashimoto T, Morimoto S. Effects of sarafotoxin S6c on renal haemodynamics and urine formation in anaesthetized dogs. Clin Exp Pharmacol Physiol. 1997;24:487–491. doi: 10.1111/j.1440-1681.1997.tb01232.x. [DOI] [PubMed] [Google Scholar]
- 282.Mattyus I, Zimmerhack LB, Schwarz A, Hentschel M, Brandis M, Milteny M, Tulassay T. Renal excretion of endothelin in children is influenced by age and diuresis. Acta Pædiatr. 1994;83:468–472. doi: 10.1111/j.1651-2227.1994.tb13061.x. [DOI] [PubMed] [Google Scholar]
- 283.Mawji IA, Robb GB, Tai SC, Marsden PA. Role of the 3’-untranslated region of human endothelin-1 in vascular endothelial cells. Contribution to transcript lability and the cellular heat shock response. J Biol Chem. 2004;279:8655–8667. doi: 10.1074/jbc.M312190200. [DOI] [PubMed] [Google Scholar]
- 284.Migas I, Bäcker A, Meyer-Lehnert H, Kramer HJ. Endothelin synthesis by porcine inner medullary collecting duct cells. Am J Hyperten. 1995;8:748–752. doi: 10.1016/0895-7061(95)00115-6. [DOI] [PubMed] [Google Scholar]
- 285.Miller RL, Kohan DE. Hypoxia regulates endothelin-1 production by the inner medullary collecting duct. J Lab Clin Med. 1998;131:45–48. doi: 10.1016/s0022-2143(98)90076-2. [DOI] [PubMed] [Google Scholar]
- 286.Modesti P, Cecioni I, Migliorini A, Naldoni A, Costoli A, Vanni S, Serneri G. Increased renal endothelin formation is associated with sodium retention and increased free water clearance. Am J Physiol. 1998;275:H1070–H1077. doi: 10.1152/ajpheart.1998.275.3.H1070. [DOI] [PubMed] [Google Scholar]
- 287.Modesti PA, Cecioni I, Costoli A, Poggesi L, Galanti G, Serneri GG. Renal endothelin in heart failure and its relation to sodium excretion. Am Heart J. 2000;140:617–622. doi: 10.1067/mhj.2000.109917. [DOI] [PubMed] [Google Scholar]
- 288.Moe O, Tejedor A, Campbell WB, Alpern R, Henrich WL. Effects of endothelin on in vitro renin secretion. Am J Physiol. 1991;260:E521–E525. doi: 10.1152/ajpendo.1991.260.4.E521. [DOI] [PubMed] [Google Scholar]
- 289.Montero A, Rodriguez-Barbero A, Lopez-Novoa JM. A role for platelet-activating factor in endothelin-1-induced rat mesangial cell proliferation. Eur J Pharmacol. 1993;243:235–240. doi: 10.1016/0014-2999(93)90180-p. [DOI] [PubMed] [Google Scholar]
- 290.Morgera S, Schlenstedt J, Hambach P, Giessing M, Deger S, Hocher B, Neumayer H. Combined ETA/ETB receptor blockade of human peritoneal mesothelial cells inhibits collagen I RNA synthesis. Kidney Int. 2003;64:2033–2040. doi: 10.1046/j.1523-1755.2003.00320.x. [DOI] [PubMed] [Google Scholar]
- 291.Moridaira K, Nodera M, Sato G, Yanagisawa H. Detection of prepro-ET-1 mRNA in normal rat kidney by in situ RT-PCR. Nephron Exp Nephrol. 2003;95:e55–61. doi: 10.1159/000073672. [DOI] [PubMed] [Google Scholar]
- 292.Morigi M, Buelli S, Angioletti S, Zanchi C, Longaretti L, Zoja C, Galbusera M, Gastoldi S, Mundel P, Remuzzi G, Benigni A. In response to protein load podocytes reorganize cytoskeleton and modulate endothelin-1 gene: implication for permselective dysfunction of chronic nephropathies. Am J Pathol. 2005;166:1309–1320. doi: 10.1016/S0002-9440(10)62350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Morigi M, Buelli S, Zanchi C, Longaretti L, Macconi D, Benigni A, Moioli D, Remuzzi G, Zoja C. Shigatoxin-induced endothelin-1 expression in cultured podocytes autocrinally mediates actin remodeling. Am J Pathol. 2006;169:1965–1975. doi: 10.2353/ajpath.2006.051331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Morimoto T, Liu W, Woda C, Carattino MD, Wei Y, Hughey RP, Apodaca G, Satlin LM, Kleyman TR. Mechanism underlying flow stimulation of sodium absorption in the mammalian collecting duct. Am J Physiol Renal Physiol. 2006;291:F663–669. doi: 10.1152/ajprenal.00514.2005. [DOI] [PubMed] [Google Scholar]
- 295.Mortensen LH, Fink GD. Salt-dependency of endothelin-induced, chronic hypertension in conscious rats. Hypertension. 1992;19:549–554. doi: 10.1161/01.hyp.19.6.549. [DOI] [PubMed] [Google Scholar]
- 296.Nadler SP, Zimplemann JA, Hebert RL. Endothelin inhibits vasopressin-stimulated water permeability in rat terminal inner medullary collecting duct. J Clin Invest. 1992;90:1458–1466. doi: 10.1172/JCI116013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Naess PA, Christensen G, Kiil F. Inhibitory effect of endothelin on renin release in dog kidneys. Acta Physiol Scand. 1993;148:131–136. doi: 10.1111/j.1748-1716.1993.tb09542.x. [DOI] [PubMed] [Google Scholar]
- 298.Nakano D, Pollock DM. Contribution of endothelin A receptors in endothelin 1- dependent natriuresis in female rats. Hypertension. 2009;53:324–330. doi: 10.1161/HYPERTENSIONAHA.108.123687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Nakano D, Pollock JS, Pollock DM. Renal medullary ETB receptors produce diuresis and natriuresis via NOS1. Am J Physiol Renal Physiol. 2008;294:F1205–1211. doi: 10.1152/ajprenal.00578.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Nambi P, Pullen M, Wu H, Aiyar N, Ohlstein EH, Edwards RM. Identification of endothelin receptor subtypes in human renal cortex and medulla using subtypes-selective ligands. Endocrinology. 1992;131:1081–1086. doi: 10.1210/endo.131.3.1324149. [DOI] [PubMed] [Google Scholar]
- 301.Nambi P, Wu HL, Pullen M, Aiyar N, Bryan H, Elliott J. Identification of endothelin receptor subtypes in rat kidney cortex using subtype-selective ligands. Mol Pharmacol. 1992;42:336–339. [PubMed] [Google Scholar]
- 302.Naruse M, Uchida S, Ogata E, Kurokawa K. Endothelin-1 increases cell calcium in mouse collecting tubule cells. Am J Physiol. 1991;261:F720–F725. doi: 10.1152/ajprenal.1991.261.4.F720. [DOI] [PubMed] [Google Scholar]
- 303.Navar L, Arendshorst W, Pallone T, Inscho E, Imig J, Bell P. The Renal Microcirculation. In: Tuma RF, D W, L K, editors. Handbook of Physiology, Microcirculation. San Diego: Elsevier; 2008. pp. 550–683. [Google Scholar]
- 304.Neuser D, Zaiss S, Stasch J. Endothelin receptors in cultured renal epithelial cells. Eur J Pharmacol. 1990;176:241–243. doi: 10.1016/0014-2999(90)90536-f. [DOI] [PubMed] [Google Scholar]
- 305.Nir A, Clavell AL, Heublein D, Aarhus LL, Burnett JCJ. Acute hypoxia and endogenous renal endothelin. J Am Soc Nephrol. 1994;4:1920–1924. doi: 10.1681/ASN.V4111920. [DOI] [PubMed] [Google Scholar]
- 306.Nobiling R, Buhrle CP, Hackenthal E, Helmchen U, Steinhausen M, Whalley A, Taugner R. Ultrastructure, renin status, contractile and electrophysiological properties of the afferent glomerular arteriole in the rat hydronephrotic kidney. Virchows Arch A Pathol Anat Histopathol. 1986;410:31–42. doi: 10.1007/BF00710903. [DOI] [PubMed] [Google Scholar]
- 307.Nussdorfer GG, Rossi GP, Belloni AS. The role of endothelins in the paracrine control of the secretion and growth of the adrenal cortex. Int Rev Cytol. 1997;171:267–308. doi: 10.1016/s0074-7696(08)62590-5. [DOI] [PubMed] [Google Scholar]
- 308.Ohta K, Hirata Y, Imai T, Kanno K, Emori T, Shichiri M, Marumo F. Cytokine-induced release of endothelin-1 from porcine renal epithelial cell line. Biochem Biophys Res Commun. 1990;169:578–584. doi: 10.1016/0006-291x(90)90370-3. [DOI] [PubMed] [Google Scholar]
- 309.Oishi R, Nonoguchi H, Tomita K, Marumo F. Endothelin-1 inhibits AVP-stimulated osmotic water permeability in rat medullary collecting duct. Am J Physiol. 1991;261:F951–F956. doi: 10.1152/ajprenal.1991.261.6.F951. [DOI] [PubMed] [Google Scholar]
- 310.Oishi S, Sasaki M, Sato T. Elevated immunoreactive endothelin levels in patients with pheochromocytoma. Am J Hypertens. 1994;7:717–722. doi: 10.1093/ajh/7.8.717. [DOI] [PubMed] [Google Scholar]
- 311.Omatsu S, Tomoyoshi T. Immunohistochemical study on endothelin in rat kidney. Hinyokika Kiyo. 1997;43:109–114. [PubMed] [Google Scholar]
- 312.Ominato M, Satoh T, Katz AI. Endothelins inhibit Na-K-ATPase activity in proximal tubules: studies of mechanisms. J Am Soc Nephrol. 1994;5:588. [Google Scholar]
- 313.Ong AC, Jowett TP, Moorhead JF, Owen JS. Human high density lipoproteins stimulate endothelin-1 release by cultured human renal proximal tubular cells. Kidney Int. 1994;46:1315–1321. doi: 10.1038/ki.1994.400. [DOI] [PubMed] [Google Scholar]
- 314.Ong ACM, Jowett TP, Firth JD, Burton S, Karet FE, Fine LG. An endothelin-1 mediated autocrine growth loop involved in human renal tubular regeneration. Kidney Int. 1995;48:390–401. doi: 10.1038/ki.1995.307. [DOI] [PubMed] [Google Scholar]
- 315.Orth SR, Amann K, Gehlen F, Unger L, Wagner J, Raschack M, Ritz E. Adult human mesangial cells (HMCs) express endothelin-B-receptors which mediate endothelin-1-induced cell growth. J Cardiovasc Pharmacol. 2000;36:S232–237. doi: 10.1097/00005344-200036051-00069. [DOI] [PubMed] [Google Scholar]
- 316.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol. 2002;282:F777–784. doi: 10.1152/ajprenal.00334.2001. [DOI] [PubMed] [Google Scholar]
- 317.Otsuka A, Mikami H, Katahira K, Tsunetoshi T, Minamitani K, Ogihara T. Changes in plasma renin activity and aldosterone concentration in response to endothelin injection in dogs. Acta Endocrinol (Copenh) 1989;121:361–364. doi: 10.1530/acta.0.1210361. [DOI] [PubMed] [Google Scholar]
- 318.Owada A, Tomita K, Terada Y, Sakamoto H, Nonguchi H, Marumo F. Endothelin (ET)-3 stimulates cyclic guanosine 3’,5’-monophosphate production via ETB receptor by producing nitric oxide in isolated rat glomerulus, and in cultured rat mesangial cells. J Clin Invest. 1994;93:556–563. doi: 10.1172/JCI117007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Oyekan A, Balazy M, McGiff JC. Renal oxygenases: differential contribution to vasoconstriction induced by ET-1 and ANG II. Am J Physiol. 1997;273:R293–300. doi: 10.1152/ajpregu.1997.273.1.R293. [DOI] [PubMed] [Google Scholar]
- 320.Ozaki S, Ihara M, Saeki T, Fukami T, Ishikawa K, Yano M. Endothelin ETB receptors couple to two distinct signaling pathways in porcine kidney epithelial LLC-PK1 cells. J Pharmacol Exp Ther. 1994;270:1035–1040. [PubMed] [Google Scholar]
- 321.Ozawa Y, Hasegawa T, Tsuchiya K, Yoshizumi M, Tamaki T. Effect of endothelin-1 (1-31) on the renal resistance vessels. J Med Invest. 2003;50:87–94. [PubMed] [Google Scholar]
- 322.Pallone TL, Zhang Z, Rhinehart K. Physiology of the renal medullary microcirculation. Am J Physiol Renal Physiol. 2003;284:F253–266. doi: 10.1152/ajprenal.00304.2002. [DOI] [PubMed] [Google Scholar]
- 323.Pavlov TS, Chahdi A, Ilatovskaya DV, Levchenko V, Vandewalle A, Pochynyuk O, Sorokin A, Staruschenko A. Endothelin-1 inhibits the epithelial Na+ channel through betaPix/14-3-3/Nedd4-2. J Am Soc Nephrol. 2010;21:833–843. doi: 10.1681/ASN.2009080885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Perico N, Cornejo RP, Benigni A, Malanchini B, Ladny JR, Remuzzi G. Endothelin induces diuresis and natriuresis in the rat by acting on proximal tubular cells through a mechanism mediated by lipoxygenase products. J Am Soc Nephrol. 1991;2:57–69. doi: 10.1681/ASN.V2157. [DOI] [PubMed] [Google Scholar]
- 325.Pernow J, Franco-Cereceda A, Matran R, Lundberg JM. Effect of endothelin-1 on regional vascular resistances in the pig. J Cardiovasc Pharmacol. 1989;13(Suppli. 5):S205–S206. doi: 10.1097/00005344-198900135-00058. [DOI] [PubMed] [Google Scholar]
- 326.Pfitzer G. Invited review: regulation of myosin phosphorylation in smooth muscle. J Appl Physiol. 2001;91:497–503. doi: 10.1152/jappl.2001.91.1.497. [DOI] [PubMed] [Google Scholar]
- 327.Phisitkul S, Hacker C, Simoni J, Tran RM, Wesson DE. Dietary protein causes a decline in the glomerular filtration rate of the remnant kidney mediated by metabolic acidosis and endothelin receptors. Kidney Int. 2008;73:192–199. doi: 10.1038/sj.ki.5002647. [DOI] [PubMed] [Google Scholar]
- 328.Phisitkul S, Khanna A, Simoni J, Broglio K, Sheather S, Rajab MH, Wesson DE. Amelioration of metabolic acidosis in patients with low GFR reduced kidney endothelin production and kidney injury, and better preserved GFR. Kidney Int. 77:617–623. doi: 10.1038/ki.2009.519. [DOI] [PubMed] [Google Scholar]
- 329.Plato C, Garvin J. Nitric oxide, endothelin and nephron transport: potential interactions. Clin Exp Pharmacol Physiol. 1999;26:262–268. doi: 10.1046/j.1440-1681.1999.03028.x. [DOI] [PubMed] [Google Scholar]
- 330.Plato C, Pollock D, Garvin J. Endothelin inhibits thick ascending limb chloride flux via ETB receptor-mediated NO release. Am J Physiol. 2000;279:F326–F333. doi: 10.1152/ajprenal.2000.279.2.F326. [DOI] [PubMed] [Google Scholar]
- 331.Pollock DM. Renal endothelin in hypertension. Curr Opin Nephrol Hypertens. 2000;9:157–164. doi: 10.1097/00041552-200003000-00010. [DOI] [PubMed] [Google Scholar]
- 332.Pollock DM, Jenkins JM, Cook AK, Imig JD, Inscho EW. L-type calcium channels in the renal microcirculatory response to endothelin. Am J Physiol Renal Physiol. 2005;288:F771–777. doi: 10.1152/ajprenal.00315.2004. [DOI] [PubMed] [Google Scholar]
- 333.Pollock DM, Opgenorth TJ. Comparison of the hemodynamic effects of endothelin-1 and big endothelin-1 in the rat. Biochem Biophys Res Commun. 1991;179:1122–1126. doi: 10.1016/0006-291x(91)91936-7. [DOI] [PubMed] [Google Scholar]
- 334.Pollock DM, Opgenorth TJ. ETA receptor-mediated responses to endothelin-1 and big endothelin-1 in the rat kidney. Br J Pharmacol. 1994;111:729–732. doi: 10.1111/j.1476-5381.1994.tb14798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Pollock DM, Opgenorth TJ. Evidence for endothelin-induced renal vasoconstriction independent of ETA receptor activation. Am J Physiol. 1993;264:R222–R226. doi: 10.1152/ajpregu.1993.264.1.R222. [DOI] [PubMed] [Google Scholar]
- 336.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am J Physiol Renal Physiol. 2001;281:F144–150. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 337.Praetorius H, Spring K. The renal cell primary cilium functions as a flow sensor. Curr Opin Nephrol Hypertens. 2003;12:517–520. doi: 10.1097/00041552-200309000-00006. [DOI] [PubMed] [Google Scholar]
- 338.Praetorius HA, Spring KR. Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol. 2001;184:71–79. doi: 10.1007/s00232-001-0075-4. [DOI] [PubMed] [Google Scholar]
- 339.Preisig PA. The acid-activated signaling pathway: starting with Pyk2 and ending with increased NHE3 activity. Kidney Int. 2007;72:1324–1329. doi: 10.1038/sj.ki.5002543. [DOI] [PubMed] [Google Scholar]
- 340.Preisig PA, Ives HE, Cragoe EJ, Jr, Alpern RJ, Rector FC., Jr Role of the Na+/H+ antiporter in rat proximal tubule bicarbonate absorption. J Clin Invest. 1987;80:970–978. doi: 10.1172/JCI113190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Pupilli C, Brunori M, Misciglia N, Selli C, Ianni L, Yanagisawa M, Mannelli M, Serio M. Presence and distribution of endothelin-1 gene expression in human kidney. Am J Physiol. 1994;267:F679–F687. doi: 10.1152/ajprenal.1994.267.4.F679. [DOI] [PubMed] [Google Scholar]
- 342.Pupilli C, Romagnani P, Lasagni L, Bellini F, Misciglia N, Emoto N, Yanagisawa M, Rizzo M, Mannelli M, Serio M. Localization of endothelin-converting enzyme-1 in human kidney. Am J Physiol. 1997;273:F749–756. doi: 10.1152/ajprenal.1997.273.5.F749. [DOI] [PubMed] [Google Scholar]
- 343.Qiu C, Baylis C. Endothelin and angiotensin mediate most glomerular responses to nitric oxide inhibition. Kidney Int. 1999;55:2390–2396. doi: 10.1046/j.1523-1755.1999.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Qiu C, Samsell L, Baylis C. Actions of endogenous endothelin on glomerular hemodynamics in the rat. Am J Physiol. 1995;269:R469–473. doi: 10.1152/ajpregu.1995.269.2.R469. [DOI] [PubMed] [Google Scholar]
- 345.Rabelink TJ, Kaasiager KAH, Boer P, Stroes EG, Braam B, Koomans HA. Effects of endothelin-1 on renal function in humans: Implications for physiology and pathophysiology. Kidney Int. 1994;46:376–381. doi: 10.1038/ki.1994.284. [DOI] [PubMed] [Google Scholar]
- 346.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension. 1997;30:29–34. doi: 10.1161/01.hyp.30.1.29. [DOI] [PubMed] [Google Scholar]
- 347.Rajapakse NW, Oliver JJ, Evans RG. Nitric oxide in responses of regional kidney blood flow to vasoactive agents in anesthetized rabbits. J Cardiovasc Pharmacol. 2002;40:210–219. doi: 10.1097/00005344-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 348.Rakugi H, Nakamaru M, Saito H, Higaki J, Ogihara T. Endothelin inhibits renin release from isolated rat glomeruli. Biochem Biophys Res Commun. 1988;155:1244–1247. doi: 10.1016/s0006-291x(88)81273-7. [DOI] [PubMed] [Google Scholar]
- 349.Rascher W, Gyodi G, Worgall S, Sulyok E. Effect of sodium chloride supplementation on urinary sodium excretion in premature infants. J Pediatr. 1994;125:793–797. doi: 10.1016/s0022-3476(94)70080-x. [DOI] [PubMed] [Google Scholar]
- 350.Rebibou JM, He CJ, Delarue F, Peraldi MN, Adida C, Rondeau E, Sraer JD. Functional endothelin-1 receptors on human glomerular podocytes and mesangial cells. Nephrol Dial Transplant. 1992;7:288–292. doi: 10.1093/oxfordjournals.ndt.a092130. [DOI] [PubMed] [Google Scholar]
- 351.Redmond EM, Cahill PA, Hodges R, Zhang S, Sitzmann JV. Regulation of endothelin receptors by nitric oxide in cultured rat vascular smooth muscle cells. J Cell Physiol. 1996;166:469–479. doi: 10.1002/(SICI)1097-4652(199603)166:3<469::AID-JCP1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 352.Redmond EM, Cahill PA, Sitzmann JV. Flow-mediated regulation of endothelin receptors in cocultured vascular smooth muscle cells: an endothelium-dependent effect. J Vasc Res. 1997;34:425–435. doi: 10.1159/000159253. [DOI] [PubMed] [Google Scholar]
- 353.Remer T. Influence of nutrition on acid-base balance--metabolic aspects. Eur J Nutr. 2001;40:214–220. doi: 10.1007/s394-001-8348-1. [DOI] [PubMed] [Google Scholar]
- 354.Remuzzi G. Role of endothelin in the development of glomerulosclerosis. Kidney Blood Press Res. 1996;19:182–183. doi: 10.1159/000174070. [DOI] [PubMed] [Google Scholar]
- 355.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int. 2007;71:1116–1121. doi: 10.1038/sj.ki.5002190. [DOI] [PubMed] [Google Scholar]
- 356.Resink TJ, Hahn AW, Scott-Burden T, Powell J, Weber E, Buhler FR. Inducible endothelin mRNA expression and peptide secretion in cultured human vascular smooth muscle cells. Biochem Biophys Res Commun. 1990;168:1303–1310. doi: 10.1016/0006-291x(90)91171-n. [DOI] [PubMed] [Google Scholar]
- 357.Riggleman A, Harvey J, Baylis C. Endothelin mediates some of the renal actions of acutely administered angiotensin II. Hypertension. 2001;38:105–109. doi: 10.1161/01.hyp.38.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Ritthaler T, Della Bruna R, Kramer BK, Kurtz A. Endothelins inhibit cyclic-AMP induced renin gene expression in cultured mouse juxtaglomerular cells. Kidney Int. 1996;50:108–115. doi: 10.1038/ki.1996.293. [DOI] [PubMed] [Google Scholar]
- 359.Ritthaler T, Scholz H, Ackermann M, Riegger G, Kurtz A, Kramer BK. Effects of endothelins on renin secretion from isolated mouse renal justaglomerular cells. Am J Physiol. 1995;268:F39–F45. doi: 10.1152/ajprenal.1995.268.1.F39. [DOI] [PubMed] [Google Scholar]
- 360.Roczniak A, Burns KD. Nitric oxide stimulates guanylate cyclase and regulates sodium transport in rabbit proximal tubule. Am J Physiol. 1996;270:F106–115. doi: 10.1152/ajprenal.1996.270.1.F106. [DOI] [PubMed] [Google Scholar]
- 361.Rodriguez-Pascual F, Redondo-Horcajo M, Magan-Marchal N, Lagares D, Martinez-Ruiz A, Kleinert H, Lamas S. Glyceraldehyde-3-phosphate dehydrogenase regulates endothelin-1 expression by a novel, redox-sensitive mechanism involving mRNA stability. Mol Cell Biol. 2008;28:7139–7155. doi: 10.1128/MCB.01145-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Romano G, Giagu P, Favret G, Bartoli E. Effect of endothelin 1 on proximal reabsorption and tubuloglomerular feedback. Kidney Blood Press Res. 2000;23:360–365. doi: 10.1159/000025984. [DOI] [PubMed] [Google Scholar]
- 363.Rossi GP, Belloni AS, Nussdorfer GG, Pessina AC. Endothelin-1 and the adrenal gland. J Cardiovasc Pharmacol. 2000;35:S17–20. doi: 10.1097/00005344-200000002-00005. [DOI] [PubMed] [Google Scholar]
- 364.Rossi NF. Effect of endothelin-3 on vasopressin release in vitro and water excretion in vivo in Long-Evans rats. J Physiol. 1993;461:501–511. doi: 10.1113/jphysiol.1993.sp019525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Rossi NF. Regulation of vasopressin secretion by ETA and ETB receptors in compartmentalized rat hypothalamo-neurohypophysial explants. Am J Physiol Endocrinol Metab. 2004;286:E535–541. doi: 10.1152/ajpendo.00344.2003. [DOI] [PubMed] [Google Scholar]
- 366.Rossi NF, O’Leary DS, Scislo TJ, Caspers ML, Chen H. Central endothelin 1 regulation of arterial pressure and arginine vasopressin secretion via the AV3V region. Kidney Int Suppl. 1997;61:S22–26. [PubMed] [Google Scholar]
- 367.Rufanova VA, Alexanian A, Wakatsuki T, Lerner A, Sorokin A. Pyk2 mediates endothelin-1 signaling via p130Cas/BCAR3 cascade and regulates human glomerular mesangial cell adhesion and spreading. J Cell Physiol. 2009;219:45–56. doi: 10.1002/jcp.21649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Rufanova VA, Sorokin A. CrkII associates with BCAR3 in response to endothelin-1 in human glomerular mesangial cells. Exp Biol Med (Maywood) 2006;231:752–756. [PubMed] [Google Scholar]
- 369.Rúiz-Ortega M, Gómez-Garre D, Alcázar R, Palacios I, Bustos C, González S, Plaza JJ, González E, Egido J. Involvement of angiotensin II and endothelin in matrix protein production and renal sclerosis. J Hypertension. 1994;12:551–558. [PubMed] [Google Scholar]
- 370.Ruskoaho H, Leskinen H, Magga J, Taskinen P, Mantymaa P, Vuolteenaho O, Leppaluoto J. Mechanisms of mechanical load-induced atrial natriuretic peptide secretion: role of endothelin, nitric oxide, and angiotensin II. J Mol Med. 1997;75:876–885. doi: 10.1007/s001090050179. [DOI] [PubMed] [Google Scholar]
- 371.Russell FD, Davenport AP. Secretory pathways in endothelin synthesis. Br J Pharmacol. 1999;126:391–398. doi: 10.1038/sj.bjp.0702315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Russell FD, Skepper JN, Davenport AP. Human endothelial cell storage granules: a novel intracellular site for isoforms of the endothelin-converting enzyme. Circ Res. 1998;83:314–321. doi: 10.1161/01.res.83.3.314. [DOI] [PubMed] [Google Scholar]
- 373.Ryan MJ, Black TA, Millard SL, Gross KW, Hajduczok G. Endothelin-1 increases calcium and attenuates renin gene expression in As4.1 cells. Am J Physiol Heart Circ Physiol. 2002;283:H2458–2465. doi: 10.1152/ajpheart.00295.2002. [DOI] [PubMed] [Google Scholar]
- 374.Saito I, Mizuno K, Niimura S, Fukuchi S. Urinary endothelin and sodium excretion in essential hypertension. Nephron. 1993;65:152–153. doi: 10.1159/000187459. [DOI] [PubMed] [Google Scholar]
- 375.Saito M, Homma S, Yamatsu I, Sato M, Ohshima N. Visualization of renal microcirculation in isolated Munich-Wistar rat kidneys: effects of endothelin-1 on renal hemodynamic activity. Jap J Pharmacol. 1994;66:221–229. doi: 10.1254/jjp.66.221. [DOI] [PubMed] [Google Scholar]
- 376.Sakamoto A, Yanagisawa M, Sakurai T, Takuwa Y, Yanagisawa H, Masaki T. Cloning and functional expression of human cDNA for the ETB endothelin receptor. Biochem Biophys Res Commun. 1991;178:656–663. doi: 10.1016/0006-291x(91)90158-4. [DOI] [PubMed] [Google Scholar]
- 377.Sakamoto H, Sasaki S, Nakamura Y, Fushimi K, Marumo F. Regulation of endothelin-1 production in cultured rat mesangial cells. Kidney Int. 1992;41:350–355. doi: 10.1038/ki.1992.48. [DOI] [PubMed] [Google Scholar]
- 378.Sarkis A, Lopez B, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid and epoxyeicosatrienoic acids in hypertension. Curr Opin Nephrol Hypertens. 2004;13:205–214. doi: 10.1097/00041552-200403000-00009. [DOI] [PubMed] [Google Scholar]
- 379.Schambelan M, Sebastian A, Katuna BA, Arteaga E. Adrenocortical hormone secretory response to chronic NH4Cl-induced metabolic acidosis. Am J Physiol. 1987;252:E454–460. doi: 10.1152/ajpendo.1987.252.4.E454. [DOI] [PubMed] [Google Scholar]
- 380.Schiffrin EL, Touyz RM. Vascular biology of endothelin. J Cardiovasc Pharmacol. 1998;32(Suppl 3):S2–13. [PubMed] [Google Scholar]
- 381.Schneider MP, Ge Y, Pollock DM, Pollock JS, Kohan DE. Collecting duct-derived endothelin regulates arterial pressure and Na excretion via nitric oxide. Hypertension. 2008;51:1605–1610. doi: 10.1161/HYPERTENSIONAHA.107.108126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Schneider MP, Inscho EW, Pollock DM. Attenuated vasoconstrictor responses to endothelin in afferent arterioles during a high-salt diet. Am J Physiol Renal Physiol. 2007;292:F1208–1214. doi: 10.1152/ajprenal.00280.2006. [DOI] [PubMed] [Google Scholar]
- 383.Schnermann J, Lorenz JN, Briggs JP, Keiser JA. Induction of water diuresis by endothelin in rats. Am J Physiol. 1992;263:F516–F526. doi: 10.1152/ajprenal.1992.263.3.F516. [DOI] [PubMed] [Google Scholar]
- 384.Schnermann JB, Zhu XL, Shu X, Yang T, Huang YG, Kretzler M, Briggs JP. Regulation of endothelin production and secretion in cultured collecting duct cells by endogenous transforming growth factor-ß. Endocrinol. 1996;137:5000–5008. doi: 10.1210/endo.137.11.8895374. [DOI] [PubMed] [Google Scholar]
- 385.Scholz H, Kramer BK, Hamann M, Gotz KH, Kurtz A. Effects of endothelins on renin secretion from rat kidneys. Acta Physiol Scand. 1995;155:173–182. doi: 10.1111/j.1748-1716.1995.tb09962.x. [DOI] [PubMed] [Google Scholar]
- 386.Schramek H, Gstraunthaler G, Willinger CC, Pfaller W. Hyperosmolality regulates endothelin release by Madin-Darby canine kidney cells. J Am Soc Nephrol. 1993;4:206–213. doi: 10.1681/ASN.V42206. [DOI] [PubMed] [Google Scholar]
- 387.Schramek H, Willinger CC, Gstraunthaler G, Pfaller W. Endothelin-3 modulates glomerular filtration rate in the isolated perfused rat kidney. Renal Physiol Biochem. 1992;15:325–333. doi: 10.1159/000173469. [DOI] [PubMed] [Google Scholar]
- 388.Schricker K, Scholz H, Hamann M, Clozel M, Kramer BK, Kurtz A. Role of endogenous endothelins in the renin system of normal and two-kidney, one clip rats. Hypertension. 1995;25:1025–1029. doi: 10.1161/01.hyp.25.5.1025. [DOI] [PubMed] [Google Scholar]
- 389.Schroeder AC, Imig JD, LeBlanc EA, Pham BT, Pollock DM, Inscho EW. Endothelin-mediated calcium signaling in preglomerular smooth muscle cells. Hypertension. 2000;35:280–286. doi: 10.1161/01.hyp.35.1.280. [DOI] [PubMed] [Google Scholar]
- 390.Schuster VL. Cyclic adenosine monophosphate-stimulated bicarbonate secretion in rabbit cortical collecting tubules. J Clin Invest. 1985;75:2056–2064. doi: 10.1172/JCI111925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 391.Schweda F, Friis U, Wagner C, Skott O, Kurtz A. Renin release. Physiology (Bethesda) 2007;22:310–319. doi: 10.1152/physiol.00024.2007. [DOI] [PubMed] [Google Scholar]
- 392.Schweizer A, Valdenaire O, Nelbock P, Deuschle U, Dumas Milne Edwards JB, Stumpf JG, Loffler BM. Human endothelin-converting enzyme (ECE-1): three isoforms with distinct subcellular localizations. Biochem J. 1997;328(Pt 3):871–877. doi: 10.1042/bj3280871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393.Seney FD, Jr, Marver D. Protein intake and cation transport in the loop of Henle. J Lab Clin Med. 1989;114:587–594. [PubMed] [Google Scholar]
- 394.Serneri GGN, Modesti PA, Cecioni I, Biagini D, Migliorini A, Costoli A, Colella A, Naldoni A, Paoletti P. Plasma endothelin and renal endothelin are two distinct systems involved in volume homeostasis. Am J Physiol. 1995;268:H1829–H1837. doi: 10.1152/ajpheart.1995.268.5.H1829. [DOI] [PubMed] [Google Scholar]
- 395.Shi H, Asher C, Chigaev A, Yung Y, Reuveny E, Seger R, Garty H. Interactions of beta and gamma ENaC with Nedd4 can be facilitated by an ERK-mediated phosphorylation. J Biol Chem. 2002;277:13539–13547. doi: 10.1074/jbc.M111717200. [DOI] [PubMed] [Google Scholar]
- 396.Shichiri M, Hirata Y, Ando K, Emori T, Ohta K, Kimoto S, Ogura M, Inoue A, Marumo F. Plasma endothelin levels in hypertension and chronic renal failure. Hypertens. 1990;15:493–496. doi: 10.1161/01.hyp.15.5.493. [DOI] [PubMed] [Google Scholar]
- 397.Shimada K, Takahashi M, Ikeda M, Tanzawa K. Identification and characterization of two isoforms of an endothelin-converting enzyme-1. FEBS Lett. 1995;371:140–144. doi: 10.1016/0014-5793(95)00886-e. [DOI] [PubMed] [Google Scholar]
- 398.Shyamala V, Moulthrop THM, Stratton-Thomas J, Tekamp-Olson P. Two distinct human endothelin B receptors generated by alternative splicing from a single gene. Cell Mol Biol Res. 1994;40:285–296. [PubMed] [Google Scholar]
- 399.Silldorff EP, Yang S, Pallone TL. Prostaglandin E2 abrogates endothelin-induced vasoconstriction in renal outer meduillary descending vasa recta of the rat. J Clin Invest. 1995;95:2734–2740. doi: 10.1172/JCI117976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Silver RB, Mennitt PA, Satlin LM. Stimulation of apical H-K-ATPase in intercalated cells of cortical collecting duct with chronic metabolic acidosis. Am J Physiol. 1996;270:F539–547. doi: 10.1152/ajprenal.1996.270.3.F539. [DOI] [PubMed] [Google Scholar]
- 401.Simonson M, Dunn MJ. Endothelin-1 stimulates contraction of rat glomerular mesangial cells and potentiates b-adrenergic-mediated cyclic adenosine monophosphate accumulation. J Clin Invest. 1990;85:790–797. doi: 10.1172/JCI114505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Simonson MS. Endothelins: multifunctional renal peptides. Physiol Rev. 1993;73:375–411. doi: 10.1152/physrev.1993.73.2.375. [DOI] [PubMed] [Google Scholar]
- 403.Simonson MS, Dunn MJ. Ca2+ signaling by distinct endothelin peptides in glomerular mesangial cells. Exp Cell Res. 1991;192:148–156. doi: 10.1016/0014-4827(91)90169-u. [DOI] [PubMed] [Google Scholar]
- 404.Simonson MS, Dunn MJ. Cellular signaling by peptides of the endothelin gene family. FASEB J. 1990;4:2989–3000. doi: 10.1096/fasebj.4.12.2168326. [DOI] [PubMed] [Google Scholar]
- 405.Simonson MS, Rooney A. Characterization of endothelin receptors in mesangial cells: Evidence for two functionally distinct endothelin binding sites. Mol Pharmacol. 1994;46:41–50. [PubMed] [Google Scholar]
- 406.Simonson MS, Wann S, Mene P, Dubyak GR, Kester M, Dunn MJ. Endothelin-1 activates the phosphoinositide cascade in cultured glomerular mesangial cells. J Cardiovasc Pharmacol. 1989;13:S80–83. doi: 10.1097/00005344-198900135-00019. discussion S84. [DOI] [PubMed] [Google Scholar]
- 407.Simonson MS, Wann S, Mene P, Dubyak GR, Kester M, Nakazato Y, Sedor JR, Dunn MJ. Endothelin stimulates phospholipase C, Na/H exchange, c-fos expression, and mitogenesis in rat mesangial cells. J Clin Invest. 1989;83:708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol. 2006;290:F1320–1328. doi: 10.1152/ajprenal.00463.2005. [DOI] [PubMed] [Google Scholar]
- 409.Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Sørensen SS, Madsen JK, Pedersen EB. Systemic and renal effect of intravenous infusion of endothelin-1 in healthy human volunteers. Am J Physiol. 1994;266:F411–F418. doi: 10.1152/ajprenal.1994.266.3.F411. [DOI] [PubMed] [Google Scholar]
- 411.Sorokin A, Kohan DE. Physiology and pathology of endothelin-1 in renal mesangium. Am J Physiol Renal Physiol. 2003;285:F579–589. doi: 10.1152/ajprenal.00019.2003. [DOI] [PubMed] [Google Scholar]
- 412.Sorokin A, Kozlowski P, Graves L, Philip A. Protein-tyrosine kinase Pyk2 mediates endothelin-induced p38 MAPK activation in glomerular mesangial cells. J Biol Chem. 2001;276:21521–21528. doi: 10.1074/jbc.M008869200. [DOI] [PubMed] [Google Scholar]
- 413.Steinhausen M, Endlich K. Controversies on glomerular filtration from Ludwig to the present. Pflugers Arch. 1996;432:R73–81. [PubMed] [Google Scholar]
- 414.Strait KA, Stricklett PK, Kohan DE. Altered collecting duct adenylyl cyclase content in collecting duct endothelin-1 knockout mice. BMC Nephrol. 2007;8:8. doi: 10.1186/1471-2369-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Strait KA, Stricklett PK, Kohan JL, Miller MB, Kohan DE. Calcium regulation of endothelin-1 synthesis in rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2007;293:F601–606. doi: 10.1152/ajprenal.00085.2007. [DOI] [PubMed] [Google Scholar]
- 416.Stricklett PK, Hughes AK, Kohan DE. Endothelin-1 stimulates NO production and inhibits cAMP accumulation in rat inner medullary collecting duct through independent pathways. Am J Physiol Renal Physiol. 2006;290:F1315–1319. doi: 10.1152/ajprenal.00450.2005. [DOI] [PubMed] [Google Scholar]
- 417.Stricklett PK, Strait KA, Kohan DE. Novel mechanism for regulation of endothelin synthesis: role of extracellular pH. Cell Physiol Biochem. 2008;21:117–122. doi: 10.1159/000113753. [DOI] [PubMed] [Google Scholar]
- 418.Sudo N, Kamoi K, Ishibashi M, Yamaji T. Plasma endothelin-1 and big endothelin-1 levels in women with pre-eclampsia. Acta Endocrinol (Copenh) 1993;129:114–120. doi: 10.1530/acta.0.1290114. [DOI] [PubMed] [Google Scholar]
- 419.Sugimoto T, Haneda M, Sawano H, Isshiki K, Maeda S, Koya D, Inoki K, Yasuda H, Kashiwagi A, Kikkawa R. Endothelin-1 induces cyclooxygenase-2 expression via nuclear factor of activated T-cell transcription factor in glomerular mesangial cells. J Am Soc Nephrol. 2001;12:1359–1368. doi: 10.1681/ASN.V1271359. [DOI] [PubMed] [Google Scholar]
- 420.Sugiura M, Snajdar RM, Schwartzberg M, Badr KF, Inagami T. Identification of two types of specific endothelin receptors in rat mesangial cell. Biochem Biophys Res Commun. 1989;162:1396–1401. doi: 10.1016/0006-291x(89)90829-2. [DOI] [PubMed] [Google Scholar]
- 421.Sullivan JC, Goodchild TT, Cai Z, Pollock DM, Pollock JS. Endothelin(A) (ET(A)) and ET(B) receptor-mediated regulation of nitric oxide synthase 1 (NOS1) and NOS3 isoforms in the renal inner medulla. Acta Physiol (Oxf) 2007;191:329–336. doi: 10.1111/j.1748-1716.2007.01754.x. [DOI] [PubMed] [Google Scholar]
- 422.Sullivan JC, Wang B, Boesen EI, D’Angelo G, Pollock JS, Pollock DM. Novel use of ultrasound to examine regional blood flow in the mouse kidney. Am J Physiol Renal Physiol. 2009;297:F228–235. doi: 10.1152/ajprenal.00016.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Tack I, Marin Castano E, Pecher C, Praddaude F, Bascands JL, Bompart G, Ader JL, Girolami JP. Endothelin increases NO-dependent cGMP production in isolated glomeruli but not in mesangial cells. Am J Physiol. 1997;272:F31–39. doi: 10.1152/ajprenal.1997.272.1.F31. [DOI] [PubMed] [Google Scholar]
- 424.Tahara A, Tsukada J, Tomura Y, Suzuki T, Yatsu T, Shibasaki M. Effect of YM218, a nonpeptide vasopressin V(1A) receptor-selective antagonist, on rat mesangial cell hyperplasia and hypertrophy. Vascul Pharmacol. 2007;46:463–469. doi: 10.1016/j.vph.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 425.Takabatake T, Ise T, Ohta K, Kobayashi K. Effects of endothelin on renal hemodynamics and tubuloglomerular feedback. Am J Physiol. 1992;263:F103–108. doi: 10.1152/ajprenal.1992.263.1.F103. [DOI] [PubMed] [Google Scholar]
- 426.Takagi M, Matsuoka H, Atarashi K, Yagi S. Endothelin: a new inhibitor of renin release. Biochem Biophys Res Commun. 1988;157:1164–1168. doi: 10.1016/s0006-291x(88)80996-3. [DOI] [PubMed] [Google Scholar]
- 427.Takagi M, Tsukada H, Matsuoka H, Yagi S. Inhibitory effect of endothelin on renin release in vitro. Am J Physiol. 1989;257:E833–E838. doi: 10.1152/ajpendo.1989.257.6.E833. [DOI] [PubMed] [Google Scholar]
- 428.Takeda M, Breyer MD, Noland TD, Homma T, Hoover RL, Inagami T, Kon V. Endothelin-1 receptor antagonist: effects on endothelin- and cyclosporine-treated mesangial cells. Kidney Int. 1992;42:1713–1719. doi: 10.1038/ki.1992.245. [DOI] [PubMed] [Google Scholar]
- 429.Takeda M, Iwasaki S, Hellings SE, Yoshida H, Homma T, Kon V. Divergent expression of EtA and EtB receptors in response to cyclosporine in mesangial cells. Am J Pathol. 1994;144:473–479. [PMC free article] [PubMed] [Google Scholar]
- 430.Takemoto F, Uchida S, Katagirl H, Oka Y, Nakao A, Kurokawa K. Desensitization of endothelin-1 binding by vasopressin via a cAMP-mediated pathway in rat CCD. Am J Physiol. 1995;268:F385–F390. doi: 10.1152/ajprenal.1995.268.3.F385. [DOI] [PubMed] [Google Scholar]
- 431.Takemoto F, Uchida S, Ogata E, Kurokawa K. Endothelin-1 and endothelin-3 binding to rat nephrons. Am J Physiol. 1993;264:F827–F832. doi: 10.1152/ajprenal.1993.264.5.F827. [DOI] [PubMed] [Google Scholar]
- 432.Takenaka T, Epstein M, Forster H, Landry DW, Iijima K, Goligorsky MS. Attenuation of endothelin effects by a chloride channel inhibitor, indanyloxyacetic acid. Am J Physiol. 1992;262:F799–806. doi: 10.1152/ajprenal.1992.262.5.F799. [DOI] [PubMed] [Google Scholar]
- 433.Takenaka T, Forster H, Epstein M. Protein kinase C and calcium channel activation as determinants of renal vasoconstriction by angiotensin II and endothelin. Circ Res. 1993;73:743–750. doi: 10.1161/01.res.73.4.743. [DOI] [PubMed] [Google Scholar]
- 434.Tamas P, Worgall S, Sulyok E, Rascher W. Renal electrolyte and water handling in normal pregnancy: possible role of endothelin-1. Eur J Obstet Gynecol Reprod Biol. 1994;55:89–95. doi: 10.1016/0028-2243(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 435.Taniguchi J, Tsuruoka S, Mizuno A, Sato J, Fujimura A, Suzuki M. TRPV4 as a flow sensor in flow-dependent K+ secretion from the cortical collecting duct. Am J Physiol Renal Physiol. 2007;292:F667–673. doi: 10.1152/ajprenal.00458.2005. [DOI] [PubMed] [Google Scholar]
- 436.Taylor TA, Gariepy CE, Pollock DM, Pollock JS. Gender differences in ET and NOS systems in ETB receptor-deficient rats: effect of a high salt diet. Hypertension. 2003;41:657–662. doi: 10.1161/01.HYP.0000048193.85814.78. [DOI] [PubMed] [Google Scholar]
- 437.Teitelbaum I, Berl T. Increased cytosolic Ca2+ inhibits AVP-stimulated adenylyl cyclase activity in rat IMCT cells by activation of PKC. Am J Physiol. 1994;266:F486–490. doi: 10.1152/ajprenal.1994.266.3.F486. [DOI] [PubMed] [Google Scholar]
- 438.Telemaque-Potts S, Kuc RE, Maguire JJ, Ohlstein E, Yanagisawa M, Davenport AP. Elevated systemic levels of endothelin-1 and blood pressure correlate with blunted constrictor responses and downregulation of endothelin(A), but not endothelin(B), receptors in an animal model of hypertension. Clin Sci (Lond) 2002;103(Suppl 48):357S–362S. doi: 10.1042/CS103S357S. [DOI] [PubMed] [Google Scholar]
- 439.Terada Y, Tomita K, Nonoguchi H, Marumo F. Different localization of two types of endothelin receptor mRNA in microdissected rat nephron segments using reverse transcription and polymerase chain reaction assay. J Clin Invest. 1992;90:107–112. doi: 10.1172/JCI115822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 440.Terada Y, Tomita K, Nonoguchi H, Yang T, Marumo F. Different localization and regulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J Clin Invest. 1993;92:2339–2345. doi: 10.1172/JCI116838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Terada Y, Tomita K, Nonoguchi H, Yang T, Marumo F. Expression of endothelin-3 mRNA along rat nephron segments using polymerase chain reaction. Kidney Int. 1993;44:1273–1280. doi: 10.1038/ki.1993.379. [DOI] [PubMed] [Google Scholar]
- 442.Terada Y, Yamada T, Takayama M, Nonoguchi H, Sasaki S, Tomita K, Marumo F. Presence and regulation of Raf-1-K (Kinase), MAPK-K, MAP-K, and S6-K in rat nephron segments. J Am Soc Nephrol. 1995;6:1565–1577. doi: 10.1681/ASN.V661565. [DOI] [PubMed] [Google Scholar]
- 443.Thai TL, Arendshorst WJ. ADP-ribosyl cyclase and ryanodine receptors mediate endothelin ETA and ETB receptor-induced renal vasoconstriction in vivo. Am J Physiol Renal Physiol. 2008;295:F360–368. doi: 10.1152/ajprenal.00512.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Thai TL, Churchill GC, Arendshorst WJ. NAADP receptors mediate calcium signaling stimulated by endothelin-1 and norepinephrine in renal afferent arterioles. Am J Physiol Renal Physiol. 2009;297:F510–516. doi: 10.1152/ajprenal.00116.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Tharaux PL, Dussaule JC, Pauti MD, Vassitch Y, Ardaillou R, Chatziantoniou C. Activation of renin synthesis is dependent on intact nitric oxide production. Kidney Int. 1997;51:1780–1787. doi: 10.1038/ki.1997.245. [DOI] [PubMed] [Google Scholar]
- 446.Todd-Turla KM, Chen M, Kretzler M, Rose D, Smart A, Yu T, Zhu X, Fehes-Toth G, Briggs JP, Schnermann J. Synthesis and polarized secretion of endothelin in a cortical collecting duct cell line (M1) Hypertens. 1994;24:408. doi: 10.1152/ajprenal.1996.271.2.F330. [DOI] [PubMed] [Google Scholar]
- 447.Todd-Turla KM, Zhu XL, Shu X, Chen M, Yu T, Smart A, Killen PD, Fejes-Toth G, Briggs JP, Schnermann JB. Synthesis and secretion of endothelin in a cortical collecting duct cell line. Am J Physiol. 1996;271:F330–F339. doi: 10.1152/ajprenal.1996.271.2.F330. [DOI] [PubMed] [Google Scholar]
- 448.Tomita K, Nonguchi H, Terada Y, Marumo F. Effects of ET-1 on water and chloride transport in cortical collecting ducts of the rat. Am J Physiol. 1993;264:F690–F696. doi: 10.1152/ajprenal.1993.264.4.F690. [DOI] [PubMed] [Google Scholar]
- 449.Tomita K, Nonoguchi H, Marumo F. Effects of endothelin on peptide-dependent cyclic adenosine monophosphate accumulation along the nephron segments of the rat. J Clin Invest. 1990;85:2014–2018. doi: 10.1172/JCI114667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 450.Tomita K, Nonoguchi H, Marumo F. Inhibition of fluid transport by endothelin through protein kinase C in collecting duct of rats. Basel: Karger; 1991. [DOI] [PubMed] [Google Scholar]
- 451.Touyz RM, Lariviere R, Schiffrin EL. Endothelin influences pHi of human platelets through protein kinase C mediated pathways. Thromb Res. 1995;78:55–65. doi: 10.1016/0049-3848(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 452.Treiber FA, Jackson RW, Davis H, Pollock JS, Kapuku G, Mensah GA, Pollock DM. Racial differences in endothelin-1 at rest and in response to acute stress in adolescent males. Hypertension. 2000;35:722–725. doi: 10.1161/01.hyp.35.3.722. [DOI] [PubMed] [Google Scholar]
- 453.Tsuruoka S, Schwartz GJ. Adaptation of rabbit cortical collecting duct HCO3- transport to metabolic acidosis in vitro. J Clin Invest. 1996;97:1076–1084. doi: 10.1172/JCI118500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Tsuruoka S, Schwartz GJ, Wakaumi M, Nishiki K, Yamamoto H, Purkerson JM, Fujimura A. Nitric oxide production modulates cyclosporin A-induced distal renal tubular acidosis in the rat. J Pharmacol Exp Ther. 2003;305:840–845. doi: 10.1124/jpet.102.048207. [DOI] [PubMed] [Google Scholar]
- 455.Tsuruoka S, Watanabe S, Purkerson JM, Fujimura A, Schwartz GJ. Endothelin and nitric oxide mediate adaptation of the cortical collecting duct to metabolic acidosis. Am J Physiol Renal Physiol. 2006;291:F866–873. doi: 10.1152/ajprenal.00027.2006. [DOI] [PubMed] [Google Scholar]
- 456.Turner AJ, Murphy LJ. Molecular pharmacology of endothelin converting enzymes. Biochem Pharm. 1996;51:91–102. doi: 10.1016/0006-2952(95)02036-5. [DOI] [PubMed] [Google Scholar]
- 457.Uchida S, Horie M, Yanagisawa M, Matsushita Y, Kurokawa K, Ogata E. Polarized secreton of endothelin-1 and big ET-1 in MDCK cells is inhibited by cell Na+ flux and disrupted by NH4Cl. J Cardiovasc Pharmacol. 1991;17(Suppl 7):S226–S228. doi: 10.1097/00005344-199100177-00065. [DOI] [PubMed] [Google Scholar]
- 458.Uchida S, Takemoto F, Ogata E, Kurokawa K. Detection of endothelin-1 mRNA by RT-PCR in isolated rat renal tubules. Biochem Biophys Res Commun. 1992;188:108–113. doi: 10.1016/0006-291x(92)92356-3. [DOI] [PubMed] [Google Scholar]
- 459.Ujiie K, Terada Y, Nonoguchi H, Shinohara M, Tomita K, Marumo F. Messenger RNA expression and synthesis of endothelin-1 along rat nephron segments. J Clin Invest. 1992;90:1043–1048. doi: 10.1172/JCI115918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Uzuner K, Banks RO. Endothelin-induced natriuresis and diuresis are pressure-dependent events in the rat. Am J Physiol. 1993;265:R90–R96. doi: 10.1152/ajpregu.1993.265.1.R90. [DOI] [PubMed] [Google Scholar]
- 461.Valdenaire O, Lepailleur-Enouf D, Egidy G, Thouard A, Barret A, Vranckx R, Tougard C, Michel JB. A fourth isoform of endothelin-converting enzyme (ECE-1) is generated from an additional promoter molecular cloning and characterization. Eur J Biochem. 1999;264:341–349. doi: 10.1046/j.1432-1327.1999.00602.x. [DOI] [PubMed] [Google Scholar]
- 462.Vassileva I, Mountain C, Pollock DM. Functional role of ETB receptors in the renal medulla. Hypertension. 2003;41:1359–1363. doi: 10.1161/01.HYP.0000070958.39174.7E. [DOI] [PubMed] [Google Scholar]
- 463.Vijayaraghavan J, Scicli AG, Carretero OA, Slaughter C, Moomaw C, Hersh LB. The hydrolysis of endothelins by neutral endopeptidase 24.11 (enkephalinase) J Biol Chem. 1990;265:14150–14155. [PubMed] [Google Scholar]
- 464.Vio CP, Figueroa CD, Caorsi I. Anatomical relationship between kallikrein-containing tubules and the juxtaglomerular apparatus in the human kidney. Am J Hypertens. 1988;1:269–271. doi: 10.1093/ajh/1.3.269. [DOI] [PubMed] [Google Scholar]
- 465.Vogel V, Backer A, Heller J, Kramer HJ. The renal endothelin system in the Prague hypertensive rat, a new model of spontaneous hypertension. Clin Sci. 1999;97:91–98. [PubMed] [Google Scholar]
- 466.Wagner CA, Giebisch G, Lang F, Geibel JP. Angiotensin II stimulates vesicular H+-ATPase in rat proximal tubular cells. Proc Natl Acad Sci U S A. 1998;95:9665–9668. doi: 10.1073/pnas.95.16.9665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Wagner OF, Christ G, Wojita J, Vierhapper H, Parzer S, Nowotny PJ, Schneider B, Waldhäusl W, Binder BR. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267:16066–16068. [PubMed] [Google Scholar]
- 468.Walshe T, Ferguson G, Connell P, O’Brien C, Cahill P. Pulsatile flow increases the expression of eNOS, ET-1, and prostacyclin in a novel in vitro cocultrue model of the retinal vasculature. Invest Opthalmol Vis Sci. 2005;46:375–382. doi: 10.1167/iovs.04-0806. [DOI] [PubMed] [Google Scholar]
- 469.Walter R, Helmle-Kolb C, Forgo J, Binswanger U, Murer H. Stimulation of Na/H exchange activity by endothelin in opossum kidney cells. Pflügers Arch. 1995;430:137–144. doi: 10.1007/BF00373849. [DOI] [PubMed] [Google Scholar]
- 470.Wang H, Sakurai K, Endoh M. Pharmacological analysis by HOE642 and KB-R9032 of the role of Na(+)/H(+) exchange in the endothelin-1-induced Ca(2+) signalling in rabbit ventricular myocytes. Br J Pharmacol. 2000;131:638–644. doi: 10.1038/sj.bjp.0703608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Wang T. Nitric oxide regulates HCO3- and Na+ transport by a cGMP-mediated mechanism in the kidney proximal tubule. Am J Physiol. 1997;272:F242–248. doi: 10.1152/ajprenal.1997.272.2.F242. [DOI] [PubMed] [Google Scholar]
- 472.Wang T, Hropot M, Aronson PS, Giebisch G. Role of NHE isoforms in mediating bicarbonate reabsorption along the nephron. Am J Physiol Renal Physiol. 2001;281:F1117–1122. doi: 10.1152/ajprenal.2001.281.6.F1117. [DOI] [PubMed] [Google Scholar]
- 473.Wang Y, Mishra R, Simonson M. Ca(2+)/Calmodulin-Dependent Protein Kinase II Stimulates c-fos Transcription and DNA Synthesis by a Src-Based Mechanism in Glomerular Mesangial Cells. J Am Soc Nephrol. 2003;14:28–36. doi: 10.1097/01.asn.0000043180.18456.47. [DOI] [PubMed] [Google Scholar]
- 474.Warner TD, Allcock GH, Vane JR. Reversal of established responses to endothelin-1 in vivo and in vitro by the endothelin receptor antagonists, BQ-123 and PD 145065. Br J Pharmacol. 1994;112:207–213. doi: 10.1111/j.1476-5381.1994.tb13053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 475.Webb ML, Dickinson KE, Delaney CL, Liu EC, Serafino R, Cohen RB, Monshizadegan H, Moreland S. The endothelin receptor antagonist, BQ-123, inhibits angiotensin II-induced contractions in rabbit aorta. Biochem Biophys Res Commun. 1992;185:887–892. doi: 10.1016/0006-291x(92)91710-8. [DOI] [PubMed] [Google Scholar]
- 476.Weber MA, Black H, Bakris G, Krum H, Linas S, Weiss R, Linseman JV, Wiens BL, Warren MS, Lindholm LH. A selective endothelin-receptor antagonist to reduce blood pressure in patients with treatment-resistant hypertension: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;374:1423–1431. doi: 10.1016/S0140-6736(09)61500-2. [DOI] [PubMed] [Google Scholar]
- 477.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–134. doi: 10.1161/01.hyp.8.6_pt_2.ii127. [DOI] [PubMed] [Google Scholar]
- 478.Weiner ID, Frank AE, Wingo CS. Apical proton secretion by the inner stripe of the outer medullary collecting duct. Am J Physiol. 1999;276:F606–613. doi: 10.1152/ajprenal.1999.276.4.F606. [DOI] [PubMed] [Google Scholar]
- 479.Weinstein AM. A mathematical model of rat distal convoluted tubule. I. Cotransporter function in early DCT. Am J Physiol Renal Physiol. 2005;289:F699–720. doi: 10.1152/ajprenal.00043.2005. [DOI] [PubMed] [Google Scholar]
- 480.Wellings RP, Corder R, Doherty AM, Vane JR. Antagonism of renal and systemic responses to endothelin-1 infusion with PD 145065. Eur J Pharmacol. 1994;256:201–204. doi: 10.1016/0014-2999(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 481.Wendel M, Knels L, Kummer W, Koch T. Distribution of endothelin receptor subtypes ETA and ETB in the rat kidney. J Histochem Cytochem. 2006;54:1193–1203. doi: 10.1369/jhc.5A6888.2006. [DOI] [PubMed] [Google Scholar]
- 482.Wenzel RR, Ruthemann J, Bruck H, Schafers RF, Michel MC, Philipp T. Endothelin-A receptor antagonist inhibits angiotensin II and noradrenaline in man. Br J Clin Pharmacol. 2001;52:151–157. doi: 10.1046/j.0306-5251.2001.01422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 483.Wesson D. Endogenous endothelins mediate increased distal tubule acidification induced by dietray acid in rats. J Clin Invest. 1997;99:2203–2211. doi: 10.1172/JCI119393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Wesson D, Dolson G. Endothelin-1 increases rat distal tubule acidification in vivo. Am J Physiol. 1997;273:F586–F594. doi: 10.1152/ajprenal.1997.273.4.F586. [DOI] [PubMed] [Google Scholar]
- 485.Wesson DE. Augmented bicarbonate reabsorption by both the proximal and distal nephron maintains chloride-deplete metabolic alkalosis in rats. J Clin Invest. 1989;84:1460–1469. doi: 10.1172/JCI114321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Wesson DE. Combined K+ and Cl- repletion corrects augmented H+ secretion by distal tubules in chronic alkalosis. Am J Physiol. 1994;266:F592–603. doi: 10.1152/ajprenal.1994.266.4.F592. [DOI] [PubMed] [Google Scholar]
- 487.Wesson DE. Dietary acid increases blood and renal cortical acid content in rats. Am J Physiol. 1998;274:F97–103. doi: 10.1152/ajprenal.1998.274.1.F97. [DOI] [PubMed] [Google Scholar]
- 488.Wesson DE. Endogenous endothelins mediate increased acidification in remnant kidneys. J Am Soc Nephrol. 2001;12:1826–1835. doi: 10.1681/ASN.V1291826. [DOI] [PubMed] [Google Scholar]
- 489.Wesson DE. Na/H exchange and H-K ATPase increase distal tubule acidification in chronic alkalosis. Kidney Int. 1998;53:945–951. doi: 10.1111/j.1523-1755.1998.00838.x. [DOI] [PubMed] [Google Scholar]
- 490.Wesson DE. Reduced bicarbonate secretion mediates increased distal tubule acidification induced by dietary acid. Am J Physiol. 1996;271:F670–678. doi: 10.1152/ajprenal.1996.271.3.F670. [DOI] [PubMed] [Google Scholar]
- 491.Wesson DE, Dolson GM. Augmented bidirectional HCO3 transport by rat distal tubules in chronic alkalosis. Am J Physiol. 1991;261:F308–317. doi: 10.1152/ajprenal.1991.261.2.F308. [DOI] [PubMed] [Google Scholar]
- 492.Wesson DE, Nathan T, Rose T, Simoni J, Tran RM. Dietary protein induces endothelin-mediated kidney injury through enhanced intrinsic acid production. Kidney Int. 2007;71:210–217. doi: 10.1038/sj.ki.5002036. [DOI] [PubMed] [Google Scholar]
- 493.Wesson DE, Simoni J. Increased tissue acid mediates a progressive decline in the glomerular filtration rate of animals with reduced nephron mass. Kidney Int. 2009;75:929–935. doi: 10.1038/ki.2009.6. [DOI] [PubMed] [Google Scholar]
- 494.Wesson DE, Simoni J, Prabhakar S. Endothelin-induced increased nitric oxide mediates augmented distal nephron acidification as a result of dietary protein. J Am Soc Nephrol. 2006;17:406–413. doi: 10.1681/ASN.2005070775. [DOI] [PubMed] [Google Scholar]
- 495.Wilkes BM, Susin M, Mento PF, Macica CM, Giraldi EP, Boss E, Nord EP. Localization of endothelin-like immunoreactivity in rat kidneys. Am J Physiol. 1991;260:F913–F920. doi: 10.1152/ajprenal.1991.260.6.F913. [DOI] [PubMed] [Google Scholar]
- 496.Williams JM, Pollock DM. Contribution of prostanoid tp receptors to the pressor and intrarenal haemodynamic response to endothelin. Clin Exp Pharmacol Physiol. 2006;33:253–257. doi: 10.1111/j.1440-1681.2006.04354.x. [DOI] [PubMed] [Google Scholar]
- 497.Wong NL, Tsui JK. Angiotensin regulates endothelin-B receptor in rat inner medullary collecting duct. Metabolism. 2001;50:661–666. doi: 10.1053/meta.2001.23293. [DOI] [PubMed] [Google Scholar]
- 498.Wong NL, Wong BP, Tsui JK. Vasopressin regulates endothelin-B receptor in rat inner medullary collecting duct. Am J Physiol Renal Physiol. 2000;278:F369–374. doi: 10.1152/ajprenal.2000.278.3.F369. [DOI] [PubMed] [Google Scholar]
- 499.Wong S, Brennan FE, Young MJ, Fuller PJ, Cole TJ. A direct effect of aldosterone on endothelin-1 gene expression in vivo. Endocrinology. 2007;148:1511–1517. doi: 10.1210/en.2006-0965. [DOI] [PubMed] [Google Scholar]
- 500.Wong WH, Wong BP, Wong EF, Huang MH, Wong NL. Downregulation of endothelin B receptors in cardiomyopathic hamsters. Cardiology. 1998;89:195–201. doi: 10.1159/000006787. [DOI] [PubMed] [Google Scholar]
- 501.Woodcock E, Land S. Endothelin receptors in rat renal papilla with a high affinity for endothelin-3. Eur J Pharmacol. 1991;208:255–260. doi: 10.1016/0922-4106(91)90103-o. [DOI] [PubMed] [Google Scholar]
- 502.Woodcock EA, Land SL. Functional endothelin ETB receptors on renal papillary tubules. Eur J Pharmacol. 1993;247:93–95. doi: 10.1016/0922-4106(93)90142-v. [DOI] [PubMed] [Google Scholar]
- 503.Worgall S, Manz F, Kleschin K, Feth F, Rascher W. Elevated urinary excretion of endothelin-like immunoreactivity in children with renal disease is related to urine flow rate. Clin Nephrol. 1994;41:331–337. [PubMed] [Google Scholar]
- 504.Wu L, Gao X, Brown RC, Heller S, O’Neil RG. Dual role of the TRPV4 channel as a sensor of flow and osmolality in renal epithelial cells. Am J Physiol Renal Physiol. 2007;293:F1699–1713. doi: 10.1152/ajprenal.00462.2006. [DOI] [PubMed] [Google Scholar]
- 505.Wu-Wong JR, Dayton BD, Opgenorth TJ. Endothelin-1-evoked arachidonic acid release: a Ca(2+)-dependent pathway. Am J Physiol. 1996;271:C869–877. doi: 10.1152/ajpcell.1996.271.3.C869. [DOI] [PubMed] [Google Scholar]
- 506.Xu D, Emoto N, Giaid A, Slaughter C, Kaw S, deWit D, Yanagisawa M. ECE-1: A membrane-bound metalloprotease that catalyzes the proteolytic activation of big endothelin-1. Cell. 1994;78:473–485. doi: 10.1016/0092-8674(94)90425-1. [DOI] [PubMed] [Google Scholar]
- 507.Yamamoto T, Hirohama T, Uemura H. Endothelin B receptor-like immunoreactivity in podocytes of the rat kidney. Arch Histol Cytol. 2002;65:245–250. doi: 10.1679/aohc.65.245. [DOI] [PubMed] [Google Scholar]
- 508.Yamamoto T, Suzuki H, Kubo Y, Matsumoto A, Uemura H. Endothelin A receptor-like immunoreactivity on the basal infoldings of rat renal tubules and collecting ducts. Arch Histol Cytol. 2008;71:77–87. doi: 10.1679/aohc.71.77. [DOI] [PubMed] [Google Scholar]
- 509.Yamamoto T, Uemura H. Distribution of endothelin-B receptor-like immunoreactivity in rat brain, kidney, and pancreas. J Cardiovasc Pharmacol. 1998;31(1):S207–211. doi: 10.1097/00005344-199800001-00058. [DOI] [PubMed] [Google Scholar]
- 510.Yamashita K, Discher D, Hu J, Bishopric N, Webster K. Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J Biol Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- 511.Yanagisawa H, Hammer RE, Richardson JA, Emoto N, Williams SC, Takeda S-i, Clouthier DE, Yanagisawa M. Disruption of ECE-1 and ECE-2 reveals a role for endothelin-converting enzyme-2 in murine cardiac development. J Clin Invest. 2000;105:925–933. doi: 10.1172/JCI7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 513.Yang T, Terada Y, Nonoguchi H, Ujiie K, Tomita K, Marumo F. Effect of hyperosmolality on production and mRNA expression of endothelin-1 in inner medullary collecting duct. Am J Physiol. 1993;264:F684–F689. doi: 10.1152/ajprenal.1993.264.4.F684. [DOI] [PubMed] [Google Scholar]
- 514.Yard BA, Chorianopoulos E, Herr D, van der Woude FJ. Regulation of endothelin-1 and transforming growth factor-beta1 production in cultured proximal tubular cells by albumin and heparan sulphate glycosaminoglycans. Nephrol Dial Transplant. 2001;16:1769–1775. doi: 10.1093/ndt/16.9.1769. [DOI] [PubMed] [Google Scholar]
- 515.Ye Q, Chen S, Gardner DG. Endothelin inhibits NPR-A and stimulates eNOS gene expression in rat IMCD cells. Hypertension. 2003;41:675–681. doi: 10.1161/01.HYP.0000047204.72286.34. [DOI] [PubMed] [Google Scholar]
- 516.Yokokawa K, Kohno M, Johchi M, Horio T, Murakawa K, Yasunari K, Yanagisawa M, Takeda T. Effect of endothelin receptor antagonist, BQ-123, on Ca2+ signaling in cultured rat mesangial cells. Life Sci. 1993;53:1631–1641. doi: 10.1016/0024-3205(93)90187-8. [DOI] [PubMed] [Google Scholar]
- 517.Yu C, Yang Z, Ren H, Zhang Y, Han Y, He D, Lu Q, Wang X, Yang C, Asico LD, Hopfer U, Eisner GM, Jose PA, Zeng C. D(3) Dopamine Receptor Regulation of ETB Receptors in Renal Proximal Tubule Cells From WKY and SHRs. Am J Hypertens. 2009 doi: 10.1038/ajh.2009.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 518.Yukimura T, Notoya M, Mizojiri K, Mizuhira V, Matsuura T, Ebara T, Miura K, Kim S, Iwao H, Song K. High resolution localization of endothelin receptors in rat renal medulla. Kidney Int. 1996;50:135–147. doi: 10.1038/ki.1996.296. [DOI] [PubMed] [Google Scholar]
- 519.Yukimura T, Yamashita Y, Miura K, Kim S, I H, Takai M, Okada T. Renal vasodilating and diuretic actions of a selective endothelin ETB receptor agonist, IRL1620. Eur J Pharmacol. 1994;264:399–405. doi: 10.1016/0014-2999(94)00501-x. [DOI] [PubMed] [Google Scholar]
- 520.Zeidel ML, Brady HR, Kone BC, Gullans SR, Brenner BM. Endothelin, a peptide inhibitor of Na+-K+-ATPase in intact tubular epithelial cells. Am J Physiol. 1989;257:C1101–C1107. doi: 10.1152/ajpcell.1989.257.6.C1101. [DOI] [PubMed] [Google Scholar]
- 521.Zeiler M, Löffler B-M, Bock HA, Thiel G, Basel K. Water diuresis increases endothelin-1 excretion in humans. J Am Soc Nephrol. 1995;6:751. [Google Scholar]
- 522.Zeng C, Asico LD, Yu C, Villar VA, Shi W, Luo Y, Wang Z, He D, Liu Y, Huang L, Yang C, Wang X, Hopfer U, Eisner GM, Jose PA. Renal D3 dopamine receptor stimulation induces natriuresis by endothelin B receptor interactions. Kidney Int. 2008;74:750–759. doi: 10.1038/ki.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 523.Zeng C, Hopfer U, Asico LD, Eisner GM, Felder RA, Jose PA. Altered AT1 receptor regulation of ETB receptors in renal proximal tubule cells of spontaneously hypertensive rats. Hypertension. 2005;46:926–931. doi: 10.1161/01.HYP.0000174595.41637.13. [DOI] [PubMed] [Google Scholar]
- 524.Zeng C, Wang Z, Asico LD, Hopfer U, Eisner GM, Felder RA, Jose PA. Aberrant ETB receptor regulation of AT receptors in immortalized renal proximal tubule cells of spontaneously hypertensive rats. Kidney Int. 2005;68:623–631. doi: 10.1111/j.1523-1755.2005.00440.x. [DOI] [PubMed] [Google Scholar]
- 525.Zhang W, Xia X, Reisenauer MR, Rieg T, Lang F, Kuhl D, Vallon V, Kone BC. Aldosterone-induced Sgk1 relieves Dot1a-Af9-mediated transcriptional repression of epithelial Na+ channel alpha. J Clin Invest. 2007;117:773–783. doi: 10.1172/JCI29850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 526.Zhao X, Imig JD. Kidney CYP450 enzymes: biological actions beyond drug metabolism. Curr Drug Metab. 2003;4:73–84. doi: 10.2174/1389200033336892. [DOI] [PubMed] [Google Scholar]
- 527.Zoccali C, Leonardis D, Parlongo S, Mallamaci F, Postorino M. Urinary and plasma endothelin 1 in essential hypertension and in hypertension secondary to renoparenchymal disease. Nephrol Dial Transplant. 1995;10:1320–1323. [PubMed] [Google Scholar]
- 528.Zoja C, Morigi M, Figliuzzi M, Bruzzi I, Oldroyd S, Benigni A, Ronco P, Remuzzi G. Proximal tubular cell synthesis and secretion of endothelin-1 on challenge with albumin and other proteins. Am J Kid Dis. 1995;26:934–941. doi: 10.1016/0272-6386(95)90058-6. [DOI] [PubMed] [Google Scholar]
- 529.Zoja C, Orisio S, Perico N, Benigni A, Morigi M, Benatti L, Rambaldi A, Remuzzi G. Constitutive expression of endothelin gene in cultured human mesangial cells and its modulation by transforming growth factor-ß, thrombin, and a thromboxane A2 analogue. Lab Invest. 1991;64:16–20. [PubMed] [Google Scholar]


