Abstract
Infection is a risk factor for adverse neurodevelopmental outcome in preterm newborns. Our objective was to characterize the association of postnatal infection with adverse microstructural and metabolic brain development in premature newborns. One hundred seventeen preterm newborns (24–32 weeks gestation) were studied prospectively at a median of 32.0 and 40.3 weeks postmenstrual age: MRI (white matter injury, hemorrhage), MR (magnetic resonance) spectroscopy (metabolism) and diffusion tensor imaging (microstructure). Newborns were categorized as having “no infection”, “clinical infection”, or “positive-culture infection.” We compared brain injuries, as well as metabolic and microstructural development across these infection groups. In 34 newborns, clinical signs were accompanied by positive cultures while 17 had clinical signs of sepsis alone. White matter injury was identified in 34 newborns. In multivariate regression models infected newborns had brain imaging measures indicative of delayed brain development: lower N-acetylaspartate/choline, elevated average diffusivity (DAV) and decreased white matter fractional anisotropy. These widespread brain abnormalities were found in both newborns with positive-culture infection and in those with clinical infection. These findings suggest that postnatal infection, even without a positive culture, is an important risk factor for widespread abnormalities in brain. These abnormalities extend beyond brain injuries apparent with conventional MRI.
INTRODUCTION
White matter injury (WMI) is the most common pattern of brain injury identified in the premature newborn (1, 2). Postnatal infection is now recognized as an important risk factor for WMI in this population (1–4). Of particular relevance to neonatal care, almost half of premature newborns with postnatal infection have neurodevelopmental impairments on follow-up, even when the infection is only evident clinically without positive cultures (5). It is unknown whether these “clinical” infections also increase the risk of brain injury in the premature newborn. Furthermore, while the neurodevelopmental effects of postnatal infection are mediated by WMI in some studies (3) they are not in others (1). Recent evidence using diffusion tensor tractography in premature newborns suggests that postnatal infections are associated with abnormal development of the corticospinal tract as the newborns mature to “term-equivalent” age (6). It is thus imperative to determine whether postnatal infections, even in the absence of positive cultures, are associated with more widespread abnormalities in early brain development.
Advanced MR techniques such as spectroscopic imaging (MRSI) and diffusion tensor imaging (DTI) now enable serial in vivo assessments of brain metabolic and microstructural development in premature newborns (7). Using state of the art brain imaging and detailed characterization of infection in a prospective cohort of premature newborns, we addressed the hypothesis that postnatal infection precedes early widespread abnormalities of brain development.
MATERIAL AND METHODS
Study Population
This study was approved by the University of British Columbia Clinical Research Ethics Board. Newborns were recruited prospectively, with informed consent, at the British Columbia’s Women’s Hospital, the major provincial tertiary-level neonatal referral center, from April 2006 to May 2009. Earlier stages of this cohort were described previously to address the relationship of chorioamnionitis with brain injury and to characterize corticospinal tract development (6, 7). Newborns were eligible if they were delivered between 24–32 weeks gestation, but were excluded if they had: 1) clinical evidence of a congenital malformation or syndrome, 2) antenatal congenital infections, or 3) ultrasound evidence of a large parenchymal hemorrhagic infarction (>2 cm) (8). Of the parents of eligible newborns approached, 117 (54%) consented to participate in this study. Enrolled patients were slightly younger (median: 27.0 versus 28.9 weeks; P=0.003) and smaller (median: 1020 versus 1125 grams; P=0.01) at birth, compared to those not participating.
Magnetic resonance imaging studies
One hundred-seventeen newborns delivered at a median gestational age (GA) of 27.6 weeks (interquartile range (IQR): 26.0–29.6 weeks) were scanned using an MR-compatible isolette (Lammers Medical Technology, Luebeck, Germany) and specialized neonatal head coil (Advanced Imaging Research, Cleveland, USA). Newborns were scanned as soon as they were clinically stable, at a median postmenstrual age of 32.0 weeks (IQR: 30.3–33.6 weeks), and 97 were scanned again at term-equivalent age (median of 40.3 weeks (IQR: 38.7–42.7 weeks)). Detailed imaging methods applied in this cohort have been described previously (7). MRI studies were carried out without pharmacological sedation on a Siemens 1.5 Tesla Avanto scanner with 3D coronal volumetric T1-weighted images and axial fast spin echo T2-weighted images (7). An experienced neuroradiologist, blinded to the newborn’s medical history, reviewed the images. The severity of WMI, intraventricular hemorrhage, and cerebellar hemorrhage, were recorded as described previously (7). MRSI was used to assess brain metabolism by measuring metabolite ratios in eight anatomical regions (Figure 1) (7). N-acetylaspartate (NAA)/choline reflects neuronal integrity and metabolism, and increases with brain maturation (9). The lactate/choline reflects oxidative metabolism and is usually undetectable by term-equivalent age (9). As absolute metabolite quantification is not possible with our MRSI technique, metabolite ratios where used to account for potential changes in intrinsic magnetic resonance properties (in particular T2) of NAA and lactate with hypoxia-ischemia. DTI measures reflect microstructural brain development (10). As the brain matures, average diffusivity decreases due to developing neuronal and glial cell membranes that hinder water diffusion (11). With white matter development, fractional anisotropy (FA) increases with maturation of the oligodendrocyte lineage and early myelination (11). These parameters were measured from 7 white matter and 5 gray matter regions (Figure 2) (7). (Supplemental methods, online).
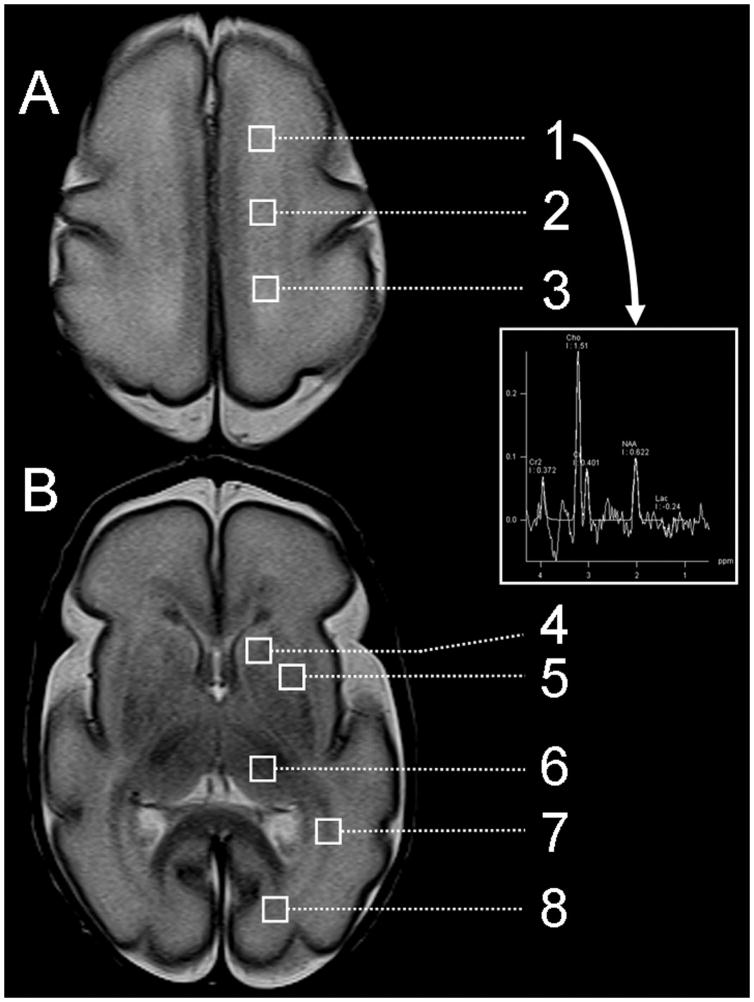
Figure 1. Proton magnetic resonance imaging and regions of interest.
As shown in Figure 1, brain metabolic development was assessed using magnetic resonance spectroscopic imaging in 8 regions of interest at the level of (A) the high centrum semi-ovale and (B) the basal ganglia: high white matter ((1) anterior, (2) central, and (3) posterior), (4) caudate, (5) lentiform nuclei, (6) thalamus, (7) optic radiations, (8) calcarine region. The values of each region were averaged bilaterally. The arrow shows the spectrum of the left frontal white matter. This example is taken from a premature newborn with a normal MR imaging and born at 27 weeks gestation and scanned at 28 weeks postmenstrual age. Legend: Cho=Choline; Cr=Creatine; NAA=N-acetylaspartate; Lac=Lactate.
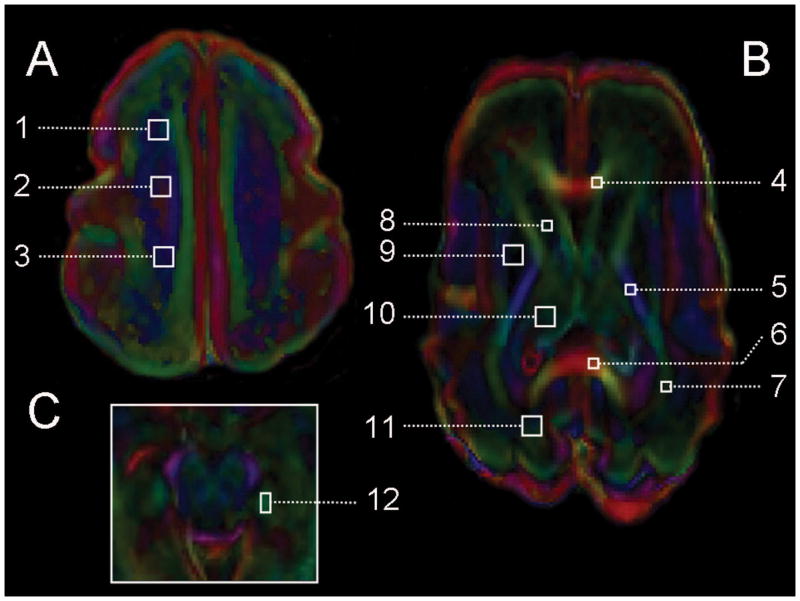
Figure 2. Diffusion tensor imaging and regions of interest.
As shown in Figure 2, brain microstructural development was assessed using diffusion tensor imaging in 12 regions of interest at the level of (A) the high centrum semi-ovale, (B) the basal ganglia, and (C) the midbrain, and include these: high white matter ((1) anterior, (2) central, and (3) posterior), (4) genu of the corpus callosum, (5) posterior limb of the internal capsule, (6) splenium of the corpus callosum, (7) optic radiation, (8) caudate, (9) lentiform nuclei, (10) thalamus, (11) calcarine region, and (12) hippocampus. The values of each region were averaged bilaterally. This example is taken from a premature newborn with a normal MR imaging and born at 27 weeks gestation and scanned at 28 weeks postmenstrual age. The color convention used to display the predominant diffusion direction has red representing right–left, green representing anterior-posterior, and blue representing superior-inferior anatomical directions.
Postnatal infection
Postnatal infection (occurring >3 days of life) was recorded using the categories proposed by Stoll et al: 1) absence of infection, 2) clinical infection alone, 3) positive-culture infection, 4) combination of necrotizing enterocolitis (NEC) and positive-culture sepsis, and 5) meningitis with or without positive-culture sepsis (5). Culture was “positive” if a pathogen was found in the blood, urine, or cerebrospinal fluid. With clinical respiratory infection, ≥4 white blood cells per field associated with a specific pathogen in the tracheal aspirates was considered as a positive culture. Given the small number of newborns in this cohort, categories 3, 4, and 5 were grouped as “positive-culture infection”. As not all newborns had routine lumbar punctures, we did not exclude newborns with meningitis; rather, we examined whether the final statistical models differed when newborns diagnosed with meningitis were removed. Other clinical data were collected systematically by chart review (7).
Data Analysis
We hypothesized that postnatal infection, even in the absence of positive culture, is associated with widespread abnormalities of early brain development. Statistical analysis was performed using Stata 9.2 (Stata Corporation, College Station, Texas) and R version 2.11 (12, 13). Clinical characteristics of the newborns were compared using Fisher’s exact test and Kruskall Wallis test for categorical and continuous data respectively. The association of postnatal infection and other clinical variables with WMI was tested with logistic regression. The mean values of NAA/choline and lactate/choline ratios, and DAV and FA were compared between the newborns without infection to those with clinical infection and those with positive-culture infection, in a generalized least squares (GLS) regression model for repeated measures, adjusting for postmenstrual age at MRI scan, multiple regions of interest, and presence of WMI. A log-transformed outcome variable was used to determine the percent differences of the MR measures. Interaction terms were examined (i) to determine whether postnatal infection modified the change of these MR parameters over time and (ii) to explore whether the effect of infection varied across regions. Interaction terms which improved predictive performance as assessed by the Akaike Information Criterion (AIC) were incorporated in reported estimates (14). To explore the impact of potential clinical confounding variables on the association of postnatal infection with measures of brain development, we examined an expanded model that included terms for GA at birth, birth weight, patent ductus arteriosus, necrotizing enterocolitis, hypotension, and days of mechanical ventilation.
RESULTS
Postnatal infection and other risk factors for brain injury
Fifty-one newborns (44%) were infected before the first scan: 17 had clinical infection while 34 had positive-culture infection. Of those with positive-culture infection, 13 newborns had sepsis with NEC and 4 with meningitis. Some patients had multi-system infections or were infected with more than one organism. Of all “positive culture infections” up to term age, most were sepsis (36 cases). Urinary tract infection, pneumonia and meningitis were found in 9, 6 and 4 newborns respectively. The most common pathogen was Staphyloccocus species (30 cases), followed by E. coli (6), C. albicans (6), Enteroccocus species (6), Klebsiella species (3) and others (4). Twenty-four newborns had multiple infections during their neonatal intensive care unit (NICU) stay.
Compared to non-infected newborns, those with postnatal infection were younger and smaller at birth, with more systemic illness (Table 1). Among 44 newborns with hypotension, 11 experienced it during an infection episode, 5 during a NEC episode, and during the first week of life in the remainder.
Table 1.
Clinical characteristics of the newborns with and without postnatal infection (prior to the first MR scan)
| Median (IQR) or Number (%) | No infection | Clinical infection | Positive-culture infection | P-value |
|---|---|---|---|---|
| Number | 66 | 17 | 34 | |
| Prenatal clinical features | ||||
| Male | 30 (45%) | 8 (47%) | 19 (56%) | 0.6 |
| Gestational age at birth (wks) | 29.0 (27.0–30.4) | 27.3 (26.3–27.7) | 25.9 (25.7–27.9) | 0.0001 |
| Birth weight (g) | 1155 (925–1380) | 945 (805–1120) | 844 (731–955) | 0.0001 |
| Head circumference (cm) | 26.5 (24.2–28.2) | 25.0 (22.5–25.5) | 24 (22.5–25.0) | 0.0002 |
| Histopathological chorioamnionitis | 17 (26%) | 10 (59%) | 11 (32%) | 0.05 |
| Early postnatal clinical features | ||||
| Cesarean delivery | 34 (52%) | 14 (82%) | 21 (62%) | 0.06 |
| APGAR score at 5 minutes | 8 (6–9) | 7 (7–8) | 7 (6–8) | 0.4 |
| Hyaline membrane disease | 47 (71%) | 16 (94%) | 33 (97%) | 0.002 |
| Late postnatal clinical features | ||||
| Necrotizing enterocolitis | 5 (8%) | 6 (35%) | 13 (38%) | <0.001 |
| Patent ductus arteriosus | 21 (32%) | 11 (65%) | 24 (71%) | <0.001 |
| Hypotension | 15 (23%) | 11 (65%) | 18 (53%) | 0.001 |
| Intubation (days) | 1 (0–9) | 11 (3–36) | 35.5 (6–62) | 0.0001 |
| Brain imaging | ||||
| Postmenstrual age at 1st scan (wks) | 31.9 (30.3–33.0) | 30.6 (29.6–33.6) | 32.4 (30.9–34.6) | 0.04 |
| White Matter Injury | 16 (24%) | 5 (29%) | 13 (38%) | 0.3 |
| Intraventricular Hemorrhage (any grade) | 28 (42%) | 9 (53%) | 18 (53%) | 0.4 |
| Cerebellar Hemorrhage | 2 (3%) | 4 (24%) | 8 (24%) | 0.003 |
| Neurological outcome at term- equivalent age | ||||
| Chronic lung disease | 8 (12%) | 8 (47%) | 21 (62%) | <0.001 |
| Abnormal neuromotor score | 32 (56%) | 13 (93%) | 26 (87%) | 0.001 |
| G-tube feed | 11 (18%) | 10 (67%) | 23 (72%) | <0.001 |
| Respiratory support* | 1 (2%) | 1 (7%) | 5 (16%) | 0.02 |
Respiratory support: oxygen therapy, continuous positive airway pressure/synchronized intermittent positive airway pressure, or mechanical ventilation
Postnatal infection and brain injury
WMI was found in 34 (29%) newborns: 10 mild, 14 moderate, and 10 severe. Cystic periventricular leukomalacia was seen on clinical head ultrasound in only 4 newborns. On univariable analysis, the presence of postnatal infection prior to the first scan is associated with an increased risk of cerebellar hemorrhage (Table 1). In a multivariable model adjusting for GA at birth and birth weight, the risk of WMI on the first scan increased with positive-culture infection (Odds Ratio=3.1; 95% Confidence Interval: 1.04 to 9.4; P=0.04), but not clinical infection alone (OR=2.1; 95% CI: 0.6 to 7.7; P=0.3). We then examined the relationship of positive culture infections with WMI on the first scan adjusting for GA at birth, birth weight and patent ductus arteriosus, necrotizing enterocolitis, hypotension and days of mechanical ventilation: positive culture infections remained a significant risk for WMI (OR=3.4; CI: 1.06 to 10.8; P=0.04). Of the 4 newborns with meningitis, 2 had recurrent episodes of sepsis, and all had WMI (1 mild, 2 moderate, 1 severe). The risk of cerebellar hemorrhage increased with positive-culture infection (OR=8.9; 95% CI: 1.4 to 55.9; P=0.02), as well as with clinical infection alone (OR=15.7; 95% CI: 1.8 to 133.4.; P=0.01) when adjusting for GA at birth, birth weight, and IVH severity.
Postnatal infection: association with abnormal brain development
When examining brain metabolic development from the first to the term-equivalent MR scans with adjustment for age at scan, regions of interest and presence of WMI, postnatal infection was found to be associated with significantly lower NAA/choline; this effect was especially pronounced on the second scan. Newborns with postnatal infection had 5.2% lower NAA/choline on the first scan (95% CI: −9.1% to −1.2%; P=0.01) and 12.7% lower NAA/choline on the second scan (95% CI: −16.5% to −8.9%; P<0.0001). In longitudinal models, newborns with postnatal infections had a slower rate of increase in NAA/choline than newborns without a postnatal infection: NAA/choline was a further 7.5% lower in newborns with infection on the second scan relative to the first (95% CI: −11.2% to −3.8%; P<0.0001). These effects were similar when newborns with meningitis were excluded from the model. The alterations in NAA/choline did not differ significantly in newborns with clinical infection relative to those with positive culture infections (P=0.59). Furthermore, the association of postnatal infections with lower NAA/choline did not differ meaningfully across brain regions. In an expanded model that included potential clinical confounding variables (GA at birth, birth weight, patent ductus arteriosus, necrotizing enterocolitis, hypotension, and days of mechanical ventilation), postnatal infections remained significant associated with lower NAA/choline: 4.6% % lower NAA/choline on the first scan (P=0.019) and 7.0% lower NAA/choline on the second scan (P=0.001).
Lactate/choline did not differ significantly in newborns with and without postnatal infection with adjustment for GA at scan and presence of WMI (P=0.28).
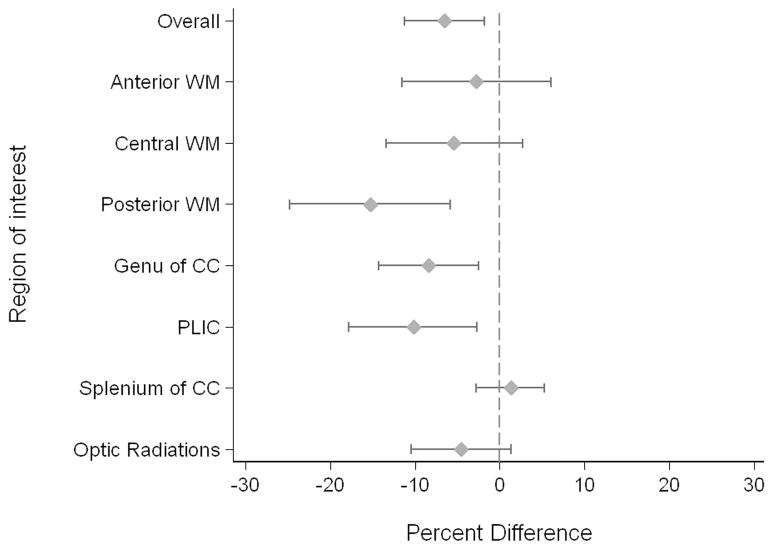
When examining measures of white matter microstructural development from the first to the term-equivalent MR scan with adjustment for GA at scan and presence of WMI, FA was lower in newborns with infection relative to newborns without infection (overall: − 6.5%; 95% CI: −11.2% to −1.8%; P=0.006). There was a significant interaction of infection and white matter regions, with the most pronounced difference observed in the posterior white matter (Figure 3). The effect of infection on FA did not differ significantly in newborns with clinical infection alone and those with positive culture infection (P=0.63). Yet, when the 4 newborns with meningitis were excluded from the model, the association of infection and FA was attenuated: 4.5% lower in newborns with infection (95% CI: −9.4% to 0.01%; P=0.06). In the expanded model that included potential clinical confounding variables, postnatal infections remained significant associated with lower overall FA (−8.4%; P<0.0001). The reduced FA observed in infected newborns resulted from an increase in λ2/λ3 relative to λ1in infected newborns.
Figure 3. Fractional Anisotropy: Infected Newborns Relative to Non-Infected Newborns.
The mean difference, with 95% confidence intervals, is plotted for the overall effect and each region of interest in the white matter.
Abbrevations: WM=white matter, CC=corpus callosum, PLIC=posterior limb of the internal capsule.
In a similar model, brain DAV in white matter regions was 5.1% higher in the newborns with postnatal infection on the second scan (95% CI: 3.2% to 7.0%; P<0.0001). There was no significant interaction of infection with GA at scan on DAV, nor did the association of postnatal infections with higher DAV differ meaningfully across white matter regions. Average diffusivity in gray matter regions on the second scan did not differ in newborns with postnatal infection (P=0.44). The association of infection with DAV was similar when newborns with meningitis were excluded from the model. In the expanded model that included potential clinical confounding variables, postnatal infections remained significant associated with higher white matter DAV on the second scan (5.4%; 95% CI: 2.1% to 8.7% P=0.001).
Postnatal infections and early neurological outcomes
All newborns in this cohort except one survived to term-equivalent age with clinical outcomes available in 109 (93%). Compared to non-infected newborns at term-equivalent age, the newborns who were exposed to postnatal infection were more likely to have chronic lung disease, have higher neuromotor scores (i.e. were neurologically more impaired), and need respiratory support and need G-tube feeding (Table 1)
DISCUSSION
The observation that infections affecting premature newborns in the NICU precede widespread abnormalities of brain development, in addition to brain injury on MRI (e.g. WMI and cerebellar hemorrhage), have a number of important implications for the care of these newborns. Widespread abnormalities of brain development are seen in newborns with postnatal infection and are independent of extreme prematurity and common neonatal co-morbidities. These brain abnormalities are seen even when the infection is only clinical without positive cultures. These findings are consistent with the significant increase in risk for adverse neurodevelopmental outcomes observed in premature newborns; deficits in motor and cognitive function are most apparent in premature newborns with postnatal infections, even when they are only evident clinically. Importantly, the full spectrum of brain abnormalities related to infection was not apparent on conventional MRI nor were they fully apparent on the earliest MR evaluation. With serial quantitative brain imaging, the impact of postnatal infection was most readily detected at term-equivalent age, suggesting an impairment of ongoing brain development. Thus, rigorous neurodevelopmental follow-up of these high-risk newborns is indicated, even in the absence of positive bacteriologic cultures or WMI on MRI. These data also provide novel insights into how infection impacts the brain and points to the critical need not only to prevent WMI but also to optimize brain development following a postnatal infection.
Early brain development: the effect of infection
The in vivo demonstration that postnatal infection is associated with widespread abnormalities of brain development that become more apparent over time builds on the current concept that infection and inflammation constitute one of the major initiating mechanisms in the pathogenesis of injury to the immature brain. Markers of intrauterine T-cell activation and increased pro-inflammatory cytokine concentrations in the serum and cerebrospinal fluid are seen in preterm newborns with WMI on MRI (15). Some of these cytokines appear to be toxic to the developmentally regulated cell populations prevalent in the immature white matter (16). Their presence may also induce a microglial response with generation of free radicals and glutamate-mediated excitoxicity (17). Other experimental and clinical studies have demonstrated that “endotoxin”, exogenous polysaccharide, by binding and interacting with Toll-like receptors activates a cascade of immune reactions that are highly deleterious to the survival of pre-oligodendrocytes (18, 19). The widespread abnormalities of brain metabolic and microstructural development detected in our study are a plausible consequence of this inflammatory insult. The widespread abnormalities in brain development observed in this cohort following infection are consistent with the changes in corticospinal tract development observed in a smaller subset of these newborns studied serially with diffusion tensor tractography (6). Together, these findings may explain the observation of “progressive” WMI following postnatal infection (4), given that the vulnerable cell population (the oligodendroglia progenitor) may have failed to mature to the “resistant” myelin-forming oligodendrocyte.
The association of postnatal infection on NAA/choline and DAV persisted even when the four newborns with meningitis were excluded. In contrast, the association of postnatal infection with white matter FA values was attenuated when the four newborns with CSF confirmed meningitis were excluded. This suggests that meningitis impacts white matter microstructure beyond that visualized on conventional MRI as WMI.
The recent finding that chorioamnionitis (i.e. antenatal infection) was not a significant risk factor for abnormal brain development (7, 20) contrasts with the association of postnatal infection with impaired brain development. The germs involved in chorioamnionitis and postnatal infections may differ (2, 3), although placental culture was not always available in previous studies. Outside the intra-uterine environment, the newborn may be more vulnerable to the consequences of infection. This may relate to an increased susceptibility to other mediating factors such as more intensive need for mechanical ventilation, limited nutrition, and hypotension (21).
The mechanism for white matter abnormalities in culture-negative, clinically-infected infants remains poorly understood. In addition to inflammation, a second upstream mechanism in the pathogenesis of WMI is hypoxia-ischemia (17). In our cohort, hypotension was seen with similar frequency in newborns with clincial sepsis and in those with positive cultures. Growing evidence suggests that pressure-passive cerebral circulation is common in premature newborns (22). Cerebrovascular autoregulation in the developing brain seems vulnerable to exogenous factors, and the presence of systemic cytokines, triggered by an episode of sepsis or NEC, may lead to its disturbance (23). In experimental models, it is recognized that infection and inflammation potentiate the effects of hypoxia-ischemia (24). Thus, white matter abnormalities related to infection, with or without positive cultures, may relate to inflammation or disturbed cerebral blood flow, or some combination of the two.
Together, our findings stress the need to prevent and treat postnatal infection in premature newborns given its potential devastating impact on neurological outcomes (5). As suggested by a cluster randomized control trial, continuous quality improvement method can reduce nosocomial infection rates in the NICU (3). Our data suggest that even clinical sepsis with negative cultures significantly affects brain development. This finding is in keeping with a recent data showing that presumed and definite bacteremia in extremely low gestational age infants are associated with similar postnatal comorbidities, and highlights the importance of this common condition (25). Recent data from experimental models suggest the potential for novel therapeutic approaches to prevent the white matter abnormalities associated with sepsis (26).
Limitations
The clinical characteristics of our cohort, including the rate of WMI (1, 27, 28) and postnatal infection (3, 29), are similar to that of other groups and suggest that our findings are applicable to other preterm populations even though the study was carried out in a single center. Due to their medical condition, newborns with positive culture infections were generally scanned at a later chronological age. While all analyses were adjusted for postmenstrual age at scan, if there were residual confounding, the age difference would have nonetheless biased the newborns with infection to appear more mature, suggesting that differences might have been underestimated. The impact of these striking differences in the MRI outcomes between the newborns with and without postnatal infection on the character and severity of adverse long term neurodevelopmental outcomes will be examined with long-term follow-up of this cohort.
Conclusions
Postnatal infection, even without a positive culture, is an important risk factor for widespread abnormalities in brain metabolic and microstructural brain development. These abnormalities extend beyond brain injuries apparent with conventional MRI, such as white matter injury. The finding that postnatal infection is an important risk factor for altered brain development has direct and critical clinical implications due to the treatable and preventable nature of this condition.
Acknowledgments
We thank the children and their parents who generously participated in this study.
Statement of financial support: This work is supported by a Canadian Institutes for Health Research (CIHR) operating grant (CHI 151135). S.P.M. is supported by a Canada Research Chair in Neonatal Neuroscience, and by CIHR Clinician-Scientist and Michael Smith Foundation for Health Research Scholar awards. E.W.Y.T. is a Cerebral Palsy International Research Foundation Ethel & Jack Hausman Clinical Research Scholar.
Footnotes
The authors have no conflict of interest or potential financial interests to disclose.
References
- 1.Miller SP, Ferriero DM, Leonard C, Piecuch R, Glidden DV, Partridge JC, Perez M, Mukherjee P, Vigneron DB, Barkovich AJ. Early brain injury in premature newborns detected with magnetic resonance imaging is associated with adverse early neurodevelopmental outcome. J Pediatr. 2005;147:609–616. doi: 10.1016/j.jpeds.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Leviton A, Allred EN, Kuban KC, Hecht JL, Onderdonk AB, O’Shea TM, Paneth N. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatr Res. 2010;67:95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah DK, Doyle LW, Anderson PJ, Bear M, Daley AJ, Hunt RW, Inder TE. Adverse neurodevelopment in preterm infants with postnatal sepsis or necrotizing enterocolitis is mediated by white matter abnormalities on magnetic resonance imaging at term. J Pediatr. 2008;153:170–175. doi: 10.1016/j.jpeds.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 4.Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, Ferriero DM, Miller SP. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. Pediatrics. 2008;122:299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004;292:2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 6.Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP. Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr. 2010;156:882–888. doi: 10.1016/j.jpeds.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chau V, Poskitt KJ, McFadden DE, Bowen-Roberts T, Synnes A, Brant R, Sargent MA, Soulikias W, Miller SP. Effect of chorioamnionitis on brain development and injury in premature newborns. Ann Neurol. 2009;66:155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 8.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 9.Kreis R, Hofmann L, Kuhlmann B, Boesch C, Bossi E, Huppi PS. Brain metabolite composition during early human brain development as measured by quantitative in vivo 1H magnetic resonance spectroscopy. Magn Reson Med. 2002;48:949–958. doi: 10.1002/mrm.10304. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC. Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol. 2002;23:1445–1456. [PMC free article] [PubMed] [Google Scholar]
- 11.Drobyshevsky A, Song SK, Gamkrelidze G, Wyrwicz AM, Derrick M, Meng F, Li L, Ji X, Trommer B, Beardsley DJ, Luo NL, Back SA, Tan S. Developmental changes in diffusion anisotropy coincide with immature oligodendrocyte progression and maturation of compound action potential. J Neurosci. 2005;25:5988–5997. doi: 10.1523/JNEUROSCI.4983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Team RDC. R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna: 2007. [Google Scholar]
- 13.R: Foundation for statistical computing. Vienna: 2010. http://www.R-Project.org. [Google Scholar]
- 14.Burnham KP, Anderson DR. Understanding AIC and BIC in model selection. Sociol Methods Res. 2004;33:261. [Google Scholar]
- 15.Ellison VJ, Mocatta TJ, Winterbourn CC, Darlow BA, Volpe JJ, Inder TE. The relationship of CSF and plasma cytokine levels to cerebral white matter injury in the premature newborn. Pediatr Res. 2005;57:282–286. doi: 10.1203/01.PDR.0000148286.53572.95. [DOI] [PubMed] [Google Scholar]
- 16.Back SA. Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms. Ment Retard Dev Disabil Res Rev. 2006;12:129–140. doi: 10.1002/mrdd.20107. [DOI] [PubMed] [Google Scholar]
- 17.Volpe JJ. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol. 2009;8:110–124. doi: 10.1016/S1474-4422(08)70294-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lehnardt S, Lachance C, Patrizi S, Lefebvre S, Follett PL, Jensen FE, Rosenberg PA, Volpe JJ, Vartanian T. The toll-like receptor TLR4 is necessary for lipopolysaccharide-induced oligodendrocyte injury in the CNS. J Neurosci. 2002;22:2478–2486. doi: 10.1523/JNEUROSCI.22-07-02478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann O, Braun JS, Becker D, Halle A, Freyer D, Dagand E, Lehnardt S, Weber JR. TLR2 mediates neuroinflammation and neuronal damage. J Immunol. 2007;178:6476–6481. doi: 10.4049/jimmunol.178.10.6476. [DOI] [PubMed] [Google Scholar]
- 20.Reiman M, Kujari H, Maunu J, Parkkola R, Rikalainen H, Lapinleimu H, Lehtonen L, Haataja L. Does placental inflammation relate to brain lesions and volume in preterm infants? J Pediatr. 2008;152:642–647. doi: 10.1016/j.jpeds.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 21.Lee SK, McMillan DD, Ohlsson A, Pendray M, Synnes A, Whyte R, Chien LY, Sale J. Variations in practice and outcomes in the Canadian NICU network: 1996–1997. Pediatrics. 2000;106:1070–1079. doi: 10.1542/peds.106.5.1070. [DOI] [PubMed] [Google Scholar]
- 22.Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, du Plessis AJ. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatr Res. 2007;61:467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- 23.Yanowitz TD, Potter DM, Bowen A, Baker RW, Roberts JM. Variability in cerebral oxygen delivery is reduced in premature neonates exposed to chorioamnionitis. Pediatr Res. 2006;59:299–304. doi: 10.1203/01.pdr.0000196738.03171.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larouche A, Roy M, Kadhim H, Tsanaclis AM, Fortin D, Sebire G. Neuronal injuries induced by perinatal hypoxic-ischemic insults are potentiated by prenatal exposure to lipopolysaccharide: animal model for perinatally acquired encephalopathy. Dev Neurosci. 2005;27:134–142. doi: 10.1159/000085985. [DOI] [PubMed] [Google Scholar]
- 25.Patel S, Dammann O, Martin CR, Allred EN, Leviton A. Presumed and definite bacteremia in extremely low gestational age newborns. Acta Paediatr. 2010;100:36–41. doi: 10.1111/j.1651-2227.2010.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loron G, Olivier P, See H, Le Sache N, Angulo L, Biran V, Brunelle N, Besson-Lescure B, Kitzis MD, Pansiot J, Bingen E, Gressens P, Bonacorsi S, Baud O. Ciprofloxacin prevents myelination delay in neonatal rats subjected to E. coli sepsis. Ann Neurol. 2011;69:341–351. doi: 10.1002/ana.22190. [DOI] [PubMed] [Google Scholar]
- 27.Cornette LG, Tanner SF, Ramenghi LA, Miall LS, Childs AM, Arthur RJ, Martinez D, Levene MI. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Arch Dis Child Fetal Neonatal Ed. 2002;86:F171–177. doi: 10.1136/fn.86.3.F171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dyet LE, Kennea N, Counsell SJ, Maalouf EF, Ajayi-Obe M, Duggan PJ, Harrison M, Allsop JM, Hajnal J, Herlihy AH, Edwards B, Laroche S, Cowan FM, Rutherford MA, Edwards AD. Natural history of brain lesions in extremely preterm infants studied with serial magnetic resonance imaging from birth and neurodevelopmental assessment. Pediatrics. 2006;118:536–548. doi: 10.1542/peds.2005-1866. [DOI] [PubMed] [Google Scholar]
- 29.Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, Lemons JA, Donovan EF, Stark AR, Tyson JE, Oh W, Bauer CR, Korones SB, Shankaran S, Laptook AR, Stevenson DK, Papile LA, Poole WK. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]