Abstract
Bronchopulmonary dysplasia (BPD), the chronic lung disease of prematurity, is the most common complication in extreme premature infants (born before 28 weeks gestation). Despite advances in perinatal care, modern clinical management remains devoid of therapies specifically promoting lung repair and lung growth. Recent progress in stem cell biology has uncovered the promise of stem/progenitor cells to repair damaged organs. Contrary to the original theory that stem cells engraft and repopulate the damaged organ, evidence suggests that stem cells act via a paracrine mechanism. This review highlights the pre-clinical evidence for the therapeutic potential of cell-based therapies in animal models of neonatal chronic lung injury and the multiple therapeutic avenues offered by soluble stem cell-derived factors.
INTRODUCTION
The incidence of premature delivery in North America is at 12.5% and rising (1). Prematurity is the leading cause of perinatal mortality and morbidity, placing these neonates at high-risk for long-term medical impairments such as bronchopulmonary dysplasia (BPD). First documented in 1967 by Northway et al., BPD was described as a chronic lung disease following mechanical ventilation and oxygen therapy for acute respiratory failure at birth (2). Since then, antenatal steroids and postnatal surfactant have aided in overcoming the biochemical immaturity of the lung. These advances in perinatal care together with more incremental improvements enabled neonatologists to push back the limits of viability from then 34 weeks gestation to today around 24 weeks gestation. Injury to more immature lungs changed the pathology of BPD (3, 4). Today, BPD is characterized by impaired alveolar development and dysmorphic pulmonary microvascular growth, along with a lesser degree of inflammation and fibrosis compared to the original BPD (5). Injury at these earlier stages may be more challenging to prevent and increases the risk of long-term consequences including pulmonary hypertension and early onset emphysema (6), which add to the burden of health care (7, 8). Thus, therapies that promote both lung repair and lung growth are desirable.
Recent insights into stem cell biology promise the regeneration of damaged organs. Stem cells are capable of self-renewal and differentiation into specialized cell types and thus have the potential to promote organogenesis, tissue regeneration, maintenance and repair (9). Mesenchymal stromal cells (MSC) attracted particular interest because of their ease of isolation, characterization, apparent multipotency and pleiotropic effects. Adult bone marrow-derived MSCs (BMSCs) apparently differentiate into cells of various non-hematopoietic tissues. BMSC studies in various disease models, including cardiovascular and neurodegenerative disorders, demonstrated their efficacy in attenuating organ injury (10–13). The demonstration that a bone marrow derived stem cell could differentiate into alveolar epithelial cells ignited stem cell research in the lung (14). Accordingly, pre-clinical studies suggested that bone marrow derived stem/progenitor cells were capable of migrating to the injured lung to promote repair (15) and administration of exogenous BMSCs prevented lung injury in various adult lung disease models (reviewed in Weiss et al. (9)). These studies offered substantial promise to mitigate the impaired alveolar growth in experimental models mimicking BPD. The multipotency and self-renewal of stem cells make cell-based therapies appealing for providing both lung injury prevention and lung growth.
Cell-based therapies to prevent experimental chronic neonatal lung injury - Proof of concept
In 2007, Tian et al. showed that intravenous injection of rat BMSCs could ameliorate neonatal lung injury (16). Shortly after, two simultaneously published papers confirmed the therapeutic potential of BMSCs. Intravenously-delivered BMSCs reduced alveolar loss and lung inflammation, and prevented pulmonary hypertension in hyperoxia-induced mice (17). Likewise, intratracheal delivery of BMSCs increased survival and exercise capacity of hyperoxia-exposed rats while attenuating alveolar and vascular injury and pulmonary hypertension (18). Subsequent studies also showed benefits in weight gain (19) and decreased fibrosis (20).
A clinically relevant source of stem cells, especially for the treatment of neonatal diseases, is offered by umbilical cord blood (UCB) (21). UCB contains stem cells that are easily accessible at birth and also capable of differentiating into various cell types (22–24), including alveolar epithelial cells (25). Intratracheal and intraperitoneal administration of human UCB-derived MSCs also improved alveolar growth through various mechanisms (26) and in a dose-dependent manner: 5×103 cells failed to attenuate both hyperoxia-induced lung injury and inflammation, while 5×104 and 5×105 cells attenuated both hyperoxia-induced injuries and inflammatory responses, but the latter dose was more effective (27). Human cord-derived pericytes and UCB-derived MSCs not only prevented but could also repair lung injury in neonatal rats when administered two weeks after established hyperoxia-induced lung injury (28). Long term assessment at six months showed persistent improvement in lung architecture and exercise capacity and no adverse effects were observed (28).
While MSCs are affirming their promise in regenerative medicine, other stem and progenitor cells are emerging. Amnion epithelial cells prevent antenatal lipopolysaccharide (29)-and ventilation (30)-induced lung injury in fetal sheep. Multipotent amniotic fluid-derived stem cells are capable of differentiating into lineages of all three embryonic germ layers and promote alveolar epithelial cell wound healing and lung growth (31, 32). Consistent with the importance of angiogenesis during lung growth, injury and repair (33), bone marrow-derived angiogenic cells - a novel population of bone marrow myeloid progenitor cells that express angiogenic markers - demonstrated the capacity to restore impaired alveolar and vascular lung growth in hyperoxia-exposed newborn mice (34).
Overall, these observations (summarized in Table 1) provide evidence for the therapeutic benefit of bone marrow and cord blood-derived MSCs in chronic oxygen-induced lung injury in rodents. A recurrent finding, however, is the paucity of cell engraftment, suggesting that stem cell properties such as self-renewal and differentiation are not required for their therapeutic action (35). This finding has led to the hypothesis that MSCs act through a paracrine effect (36), rather than through cell replacement. This realization has expanded the therapeutic options of cell-based therapies.
Table 1.
Stem/progenitor cell pre-clinical trials in experimental neonatal lung diseases
| Cell Type | Source/Route/Control cell | Animal Model | Age of Animals | Outcomes | Reference |
|---|---|---|---|---|---|
| MSC | BM/IV/no | 95% hyperoxia (rat) | P-10 | Reduced levels of TNF-alpha and TGF-beta1, increased radial alveolar count. | (16) |
| MSC MSC-CM |
BM/IT/PASMC | 95% hyperoxia (rat) | P-14 |
in vitro: preferential MSC migration toward O2-damaged lungs. MSC-CM prevented O2-induced AEC2 apoptosis, accelerated AEC2 wound healing and enhanced endothelial cord formation. in vivo: attenuated alveolar and vascular injury, and PH. |
(18) |
| MSC | BM/IV/no | 95% hyperoxia (rat) | P-3, P-7, P-14 | Improved weight gain, prevented alveolar growth arrest and suppressed lung inflammation. | (19) |
| MSC-CM | BM/IP/lung fibroblasts | 95% hyperoxia (rat) | P-14 | Preserved alveolar growth. CM from O2-exposed, preconditioned BMSCs exerted more potent therapeutic potential and prevented PH. | (39) |
| MSC MSC-CM |
BM/IV/PASMC for CM | 75% hyperoxia (mouse) | P-14 | MSCs reduced alveolar loss and lung inflammation, prevented PH. MSC-CM had a more pronounced effect, prevented alveolar and lung vascular injury. | (17) |
| MSC | BM/IP/no | 60% hyperoxia (mouse) | P-45 |
in vitro: co-culturing of injured lung tissue increased migration potential of BMSC and SP-C expression. in vivo: BMSC home to injured lung, express SP-C, improve pulmonary architecture, attenuate pulmonary fibrosis and increase survival rate. |
(20) |
| MSC-CM | BM/IV/lung fibroblasts | 75% hyperoxia (mouse) | P-14 | Reversed parenchymal fibrosis and peripheral PA devascularisation, partially reversed alveolar injury, normalized lung function, reversed PH and RVH and attenuated peripheral PA muscularization. | (37) |
| MSC MSC-CM |
BM/IV/PASMC | 75% hyperoxia (mouse) | P-10 |
in vitro: MSC-CM increased BASCs growth. in vivo: MSCs & MSC-CM increased BASCs in lungs. |
(41) |
| MSC MSC-CM |
BM/IT/no | 90% hyperoxia (rat) | P-16, P-33, P-100 | Improved alveolarization and lung vascular growth with MSC and MSC-CM up to 3 months post-treatment. Decreased inflammation and up-regulation of angiogenic factors. | (38) |
| MSC | UCB/IT & IP/no | 95% hyperoxia (rat) | P-14 | IT & IP: attenuated the increase in TUNEL-positive cells, myeloperoxidase activity, and IL-6 mRNA level. IT: improved alveolarization and decreased lung collagen, TNF-alpha and TGF-beta mRNA, alpha-SMA protein. |
(26) |
| MSC | UCB/IT/no | 95% hyperoxia (rat) | P-14 | Improved alveolarization, decreased lung collagen, and attenuated lung inflammation (decreased myeloperoxidase activity, TNF-alpha, IL-1beta, IL-6, TGF-beta mRNA, up- regulation of cytosolic and membrane p4phox) in a dose dependent manner. | (27) |
| MSC & Pericytes | Umbilical cord & UCB/IT/Human neonatal dermal fibroblasts | 95% hyperoxia (rat) | P-22, P-35, 6 months | Improved alveolarization and lung vascular growth with whole cell and cell-free CM. Prevention and rescue. Efficacy and safety up to 6 months post-treatment. | (28) |
| Myeloid progenitor | BM/IV/embryonic EPC, mouse embryonic fibroblasts | 80% hyperoxia (mice) | P-21 | Restored lung structure. | (34) |
| Epithelial | Amnion/IV/no | LPS (sheep) | Improved lung function and structure (lung volume, tissue-to- airspace ratio, and septal crest density), reduced inflammatory cytokines (TNF-alpha, IL-1beta, IL-6). | (29) | |
| Epithelial | Amnion/IV/no | Ventilation (sheep) | Attenuated lung fibrosis and normalized secondary septal crests. Differentiated into AEC1 and AEC2 in the injured lung. | (30) |
Abbreviations: AEC1/2: alveolar epithelial type I/II cell, BASC: bronchioalveolar stem cells, BM: bone marrow, CM: conditioned media, EPC: endothelial progenitor cell, IV: intravenous, IT: intratracheal, IP: intraperitoneal, LPS: lipopolysaccharide, P-(n): postnatal day-(n), PA: pulmonary artery, PASMC: PA smooth muscle cells, PH: pulmonary hypertension, RVH: right ventricular hypertrophy, SMA: smooth muscle actin, SP-C: surfactant protein C, TNF: tumor necrosis factor, TUNEL: terminal deoxynucleotidyl transferase dUTP nick end labeling, UCB: umbilical cord blood.
MSCs prevent lung injury via a paracrine mechanism - it’s in the juice
Several lines of evidence suggest that MSCs act via a paracrine mechanism to protect the developing lung from injury. In vitro cell-free BMSC conditioned media prevented hyperoxia-induced alveolar epithelial cell apoptosis, accelerated alveolar epithelial cell wound healing and preserved endothelial cord formation on matrigel during hyperoxia (18).
In vivo, the paracrine effect could also be inferred from the efficacy of intraperitoneal administration of MSCs in preventing oxygen-induced neonatal lung injury (20, 26). Accordingly, Aslam et al. provided direct in vivo evidence showing that a single injection of cell-free BMSC-derived conditioned media had a more pronounced effect on alveolar injury and fibrosis prevention than BMSCs themselves (17). In a follow-up study, a single intravenous dose of BMSC-derived conditioned media normalized lung function, reversed alveolar injury and pulmonary hypertension (37). A single intratracheal injection of BMSC or BMSC-free conditioned media protected from oxygen-induced alveolar and vascular injury with a persistent benefit followed up to 3 months (38). Likewise, cell-free conditioned media derived from human UCB-MSCs and pericytes prevented and reversed arrested alveolar growth and lung function in hyperoxic-exposed rats with persistent benefits at 6 months of age and without adverse effect on lung structure or tumour formation (28). Dose-response studies have not yet been performed.
Although the therapeutic benefit of the conditioned media is undeniable, a potential caveat of this strategy is the lack of cell adaptation to the local injurious environment. In an attempt to overcome this potential limitation, Waszak et al. exposed BMSCs to a “deleterious BPD environment” by priming them ex vivo in hyperoxia for 24 hours (39). Conditioned media collected from these preconditioned cells and injected into hyperoxic rats exerted a more potent therapeutic effect in vivo on lung architecture compared to non-preconditioned media.
Thus, rather than replacing injured cells and differentiating into lung cells, MSCs may release factors that protect resident lung cells from injury or modulate the function of inflammatory cells. Tropea and colleagues recently provided evidence for such a scenario. Bronchio-alveolar stem cells (BASCs) are putative epithelial lung stem/progenitor cells at the bronchio-alveolar junction, capable of self-renewal and differentiation in culture, and also proliferate in response to alveolar injury (40). Both BMSC and BMSC-derived conditioned media increase the number of BASCs in neonatal mice exposed to hyperoxia (41). This study also offers new therapeutic perspectives, i.e. the protection of resident lung progenitor cells rather than exogenous supplementation of stem cells. In addition, there is increasing evidence that MSCs interact with inflammatory cells to modulate their response to injury. MSCs can direct macrophages from a M1 (pro-inflammatory) to a M2 (healer) phenotype in various disease conditions (42, 43). Overall, these observations suggest that cell-free conditioned media exerts similar therapeutic benefit to the cell itself. The exciting challenge is how to harness the multiple healing properties of stem cells.
MSCs prevent lung injury via a paracrine mechanism - what’s in the Juice?
Indeed, the identification of soluble factors in the conditioned media may allow the discovery of novel healing molecules that each by itself or in combination could yield new therapeutic options (44). Besides factors already known to be lung protective including keratinocyte growth factor (45), vascular endothelial growth factor (46) or adiponectin (47), novel molecules secreted by MSCs have already been identified and shown therapeutic benefit in various disease models, such as staniocalcin-1 (48) – a potent anti-oxidant – or tumor necrosis factor-alpha-stimulated gene/protein 6 (TSG-6) (49) – a potent anti-inflammatory protein.
From a clinical perspective, a relevant question for the design of clinical trials is to determine the most efficacious and safest stem cell-based approach: whole cell therapy vs. cell free-derived conditioned media vs. identification of single bioactive molecules vs. identification and determination of the most efficacious combination of molecules. This daunting task could be circumvented by the recent recognition that MSCs release membrane vesicles, exosomes in particular, that act as nano-packages containing a combination of bioactive molecules and microRNA (miRNA) (50). miRNA are small non-coding RNA molecules involved in transcriptional regulation of gene expression. In particular, miRNA could become interesting therapeutic targets in the prevention of BPD (51) by silencing specific genes with deleterious effects during lung injury. Exosomes are 40–100 nm in size and represent a specific subtype of secreted membrane vesicles formed through the fusion of multivesicular endosomes with the plasma membrane. Although known for several decades, membrane vesicles have long been thought of as mere cell debris. Recent evidence, however, suggests that MSC-derived exosomes play important roles in cell communication and mediate the therapeutic benefit of MSCs. For example, MSC-derived exosomes attenuate lung macrophage influx, decrease pro-inflammatory cytokine levels in the bronchoalveolar lavage and prevent pulmonary vascular remodeling and hypoxic-induced pulmonary hypertension in mice (52). With the exosomes removed, the conditioned media showed no therapeutic effect in this model. Similar therapeutic benefits of MSC-derived exosomes are reported in kidney (53) and cardiac (54) injury. A limitation for the exploitation of exosomes as therapeutic tools remains the process of isolation, characterization, quality control, and large-scale manufacturing. Novel findings continue to uncover the mechanisms by which MSCs protect resident lung cells including the transfer of mitochondria via nanotubes (55). These pleiotropic effects (Figure 1) open exciting avenues in particular for multifactorial diseases such as BPD and provide traction for the discoveries of cell-free products.
Figure 1.
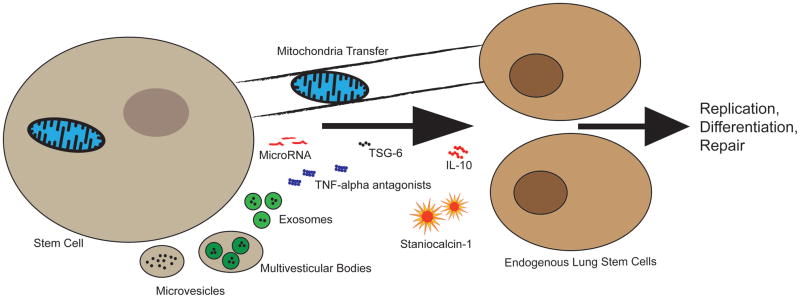
Schematic representation of possible repair mechanisms associated with stem cells. Many therapeutic mechanisms for stem cells have recently emerged. These include microparticle carriers such as microvesicles, exosomes or multivesicular bodies which are speculated to be released by stem cells and elicit a therapeutic response. MicroRNA packaged in these vesicles or as a sole effector may also play a therapeutic role. The role of secreted soluble proteins/peptides in neonatal and adult lung injury has been extensively studied. This has lead to the discovery of promising bioactive molecules such as the anti-inflammatory IL-10, Staniocalcin-1, TSG-6 and TNF-alpha antagonists, the combination of which may contribute to the pleiotropic effects promoting repair. Recent evidence also unveiled therapeutic mitochondria transfer via nanotubes. These mechanisms can signal endogenous stem cells to amplify or transduce similar repair actions.
Considerations for Clinical Trials
A clinical trial testing the safety and efficacy of MSCs in adult patients with COPD has been completed (56). Although this clinical trial was predominantly for safety, no substantial evidence of efficacy of the MSCs was recorded. More recently, a phase I clinical trial testing the safety of human umbilical cord blood derived MSCs in nine premature infants at risk of developing BPD has been completed (ClinicalTrials.gov: NCT01297205). Although this study upholds the safety of MSC therapy, long-term follow-up is warranted. Further clinical trials are already planned (ClinicalTrials.gov: NCT01207869, NCT01828957).
Pre-clinical studies have generated proof of concept evidence that cell-based therapies can prevent and restore experimental neonatal lung injury in rodent and sheep. Rather than cell replacement, the therapeutic benefit of stem cells is mediated through a paracrine effect. It is likely that the combination of bioactive molecules contained in the conditioned media provide the compounding pleiotropic effects attributed to MSCs. Administration of the entire cocktail containing unidentified products may conjure unforeseen side effects and some components may be more beneficial in repair. Thus, further specification of which molecule(s) have reparative properties and/or the isolation of specific micro/nano carriers such as exosomes may lead to pharmacological therapies for BPD.
The cell most suitable for clinical trials appears to be MSCs, likely because of their ease of isolation, characterization and pleiotropic effects. However, endothelial progenitor cells and other stem/progenitor cells have also proven to be effective in pre-clinical BPD models. These various cells differ in their roles and respective factors, therefore possibly producing a more pronounced effect when administered in concert (57), although this remains to be proven in the lung.
Likewise, the source of cells is an important consideration. Umbilical cord and cord blood are easily accessible at birth and may have more potent repair capabilities than adult BMSCs. Autologous UCB-derived cell therapies may avoid immunological risks and allows the use of minimally manipulated cells. However, given the immunological properties of MSCs, allogeneic cell therapy is feasible and may facilitate the logistics of cell-based therapies.
The timing of the treatment is another factor to be resolved. Recent pre-clinical evidence showed UCB-derived MSCs time-dependently attenuated hyperoxia-induced injury, eliciting significant protection in the early, but not the late phase of inflammation (58). In the clinic, the early identification of infants at the most risk of developing BPD through the use of estimators and models may allow for the selection of an appropriate patient population. Patients with early and persistent pulmonary dysfunction have a close to 70% risk of developing BPD, as defined by Laughon et al. (59), and may represent an at-risk population of choice for cell-based therapies.
Finally, the safety of each of these cell-based therapies must be investigated thoroughly in well-designed pre-clinical trials including large animal models.
In summary, as the incidence of prematurity and chronic neonatal lung disease rises (60), novel therapies are required. Pre-clinical studies have brought substantial promise in developing an effective clinical therapy that could fulfill the dual role of preventing injury and promoting lung growth. The paracrine effect of cell-based therapies has opened unexpected therapeutic options through the identification of individual molecules or mechanisms including microRNA, mitochondrial transfer and microparticles. The promise may not lie in the stem cell itself, but rather in its vast array of bioactive mediators - ‘it’s in the juice’.
Acknowledgments
STATEMENT OF FINANCIAL SUPPORT: B.T. is supported by the Canadian Institute of Health Research (CIHR), the Ottawa Hospital Research Institute (OHRI), and the Children’s Hospital of Eastern Ontario Research Institute (CHEORI). M.E.F. was supported by a summer studentship from Alberta Innovates Health Solutions (AIHS).
Footnotes
DISCLOSURE: The authors have nothing to disclose.
References
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Northway WH, Jr, Rosan RC, Porter DY. Pulmonary disease following respirator therapy of hyaline-membrane diseaseBronchopulmonary dysplasia. N Engl J Med. 1967;276:357–368. doi: 10.1056/NEJM196702162760701. [DOI] [PubMed] [Google Scholar]
- 3.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–1729. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 4.Jobe AJ. The new BPD: an arrest of lung development. Pediatr Res. 1999;46:641–643. doi: 10.1203/00006450-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 5.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357:1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 6.Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–269. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- 7.Ireys HT, Anderson GF, Shaffer TJ, Neff JM. Expenditures for care of children with chronic illnesses enrolled in the Washington State Medicaid program, fiscal year 1993. Pediatrics. 1997;100:197–204. doi: 10.1542/peds.100.2.197. [DOI] [PubMed] [Google Scholar]
- 8.Wong PM, Lees AN, Louw J, et al. Emphysema in young adult survivors of moderate-to-severe bronchopulmonary dysplasia. Eur Respir J. 2008;32:321–328. doi: 10.1183/09031936.00127107. [DOI] [PubMed] [Google Scholar]
- 9.Weiss DJ, Bertoncello I, Borok Z, et al. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8:223–272. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pereira RF, O’Hara MD, Laptev AV, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1998;95:1142–1147. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah RV, Mitchell RN. The role of stem cells in the response to myocardial and vascular wall injury. Cardiovasc Pathol. 2005;14:225–231. doi: 10.1016/j.carpath.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11:1255–1265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- 13.Phinney DG, Prockop DJ. Concise review: mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair--current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- 14.Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 15.Griffiths MJ, Bonnet D, Janes SM. Stem cells of the alveolar epithelium. Lancet. 2005;366:249–260. doi: 10.1016/S0140-6736(05)66916-4. [DOI] [PubMed] [Google Scholar]
- 16.Tian ZF, Du J, Wang B, Hong XY, Feng ZC. Intravenous infusion of rat bone marrow-derived mesenchymal stem cells ameliorates hyperoxia-induced lung injury in neonatal rats. Nan Fang Yi Ke Da Xue Xue Bao. 2007;27:1692–1695. [PubMed] [Google Scholar]
- 17.Aslam M, Baveja R, Liang OD, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. Am J Respir Crit Care Med. 2009;180:1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Haaften T, Byrne R, Bonnet S, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. Am J Respir Crit Care Med. 2009;180:1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang H, Fang J, Su H, et al. Bone marrow mesenchymal stem cells attenuate lung inflammation of hyperoxic newborn rats. Pediatr Transplant. 2012;16:589–598. doi: 10.1111/j.1399-3046.2012.01709.x. [DOI] [PubMed] [Google Scholar]
- 20.Zhang X, Wang H, Shi Y, et al. Role of bone marrow-derived mesenchymal stem cells in the prevention of hyperoxia-induced lung injury in newborn mice. Cell Biol Int. 2012;36:589–594. doi: 10.1042/CBI20110447. [DOI] [PubMed] [Google Scholar]
- 21.McGuckin CP, Forraz N. Umbilical cord blood stem cells--an ethical source for regenerative medicine. Med Law. 2008;27:147–165. [PubMed] [Google Scholar]
- 22.Hou L, Cao H, Wang D, et al. Induction of umbilical cord blood mesenchymal stem cells into neuron-like cells in vitro. Int J Hematol. 2003;78:256–261. doi: 10.1007/BF02983804. [DOI] [PubMed] [Google Scholar]
- 23.Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. doi: 10.1182/blood-2003-05-1670. [DOI] [PubMed] [Google Scholar]
- 24.Kogler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berger MJ, Adams SD, Tigges BM, et al. Differentiation of umbilical cord blood-derived multilineage progenitor cells into respiratory epithelial cells. Cytotherapy. 2006;8:480–487. doi: 10.1080/14653240600941549. [DOI] [PubMed] [Google Scholar]
- 26.Chang YS, Oh W, Choi SJ, et al. Human umbilical cord blood-derived mesenchymal stem cells attenuate hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2009;18:869–886. doi: 10.3727/096368909X471189. [DOI] [PubMed] [Google Scholar]
- 27.Chang YS, Choi SJ, Sung DK, et al. Intratracheal transplantation of human umbilical cord blood derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843–1854. doi: 10.3727/096368911X565038. [DOI] [PubMed] [Google Scholar]
- 28.Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax. 2012 doi: 10.1136/thoraxjnl-2012-202323. e-pub ahead of print 15 December 2012. [DOI] [PubMed] [Google Scholar]
- 29.Vosdoganes P, Hodges RJ, Lim R, et al. Human amnion epithelial cells as a treatment for inflammation-induced fetal lung injury in sheep. Am J Obstet Gynecol. 2011;205:156, e26–33. doi: 10.1016/j.ajog.2011.03.054. [DOI] [PubMed] [Google Scholar]
- 30.Hodges RJ, Jenkin G, Hooper SB, et al. Human amnion epithelial cells reduce ventilation-induced preterm lung injury in fetal sheep. Am J Obstet Gynecol. 2012;206:448, e8–15. doi: 10.1016/j.ajog.2012.02.038. [DOI] [PubMed] [Google Scholar]
- 31.Buckley S, Shi W, Carraro G, et al. The milieu of damaged alveolar epithelial type 2 cells stimulates alveolar wound repair by endogenous and exogenous progenitors. Am J Respir Cell Mol Biol. 2011;45:1212–1221. doi: 10.1165/rcmb.2010-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pederiva F, Ghionzoli M, Pierro A, De Coppi P, Tovar JA. Amniotic fluid stem cells rescue both in vitro and in vivo growth, innervation and motility in nitrofen-exposed hypoplastic rat lungs through paracrine effects. Cell Transplant. 2012 doi: 10.3727/096368912X657756. e-pub ahead of print 8 October 2012. [DOI] [PubMed] [Google Scholar]
- 33.Thebaud B, Abman SH. Bronchopulmonary dysplasia: where have all the vessels gone? Roles of angiogenic growth factors in chronic lung disease. Am J Respir Crit Care Med. 2007;175:978–985. doi: 10.1164/rccm.200611-1660PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balasubramaniam V, Ryan SL, Seedorf GJ, et al. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol Lung Cell Mol Physiol. 2010;298:L315–323. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19:35–42. doi: 10.1038/nm.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JW, Fang X, Krasnodembskaya A, Howard JP, Matthay MA. Concise review: Mesenchymal stem cells for acute lung injury: role of paracrine soluble factors. Stem Cells. 2011;29:913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hansmann G, Fernandez-Gonzalez A, Aslam M, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ. 2012;2:170–181. doi: 10.4103/2045-8932.97603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sutsko RP, Young KC, Ribeiro A, et al. Long-term reparative effects of mesenchymal stem cell therapy following neonatal hyperoxia-induced lung injury. Pediatr Res. 2013;73:46–53. doi: 10.1038/pr.2012.152. [DOI] [PubMed] [Google Scholar]
- 39.Waszak P, Alphonse R, Vadivel A, Ionescu L, Eaton F, Thebaud B. Preconditioning enhances the paracrine effect of mesenchymal stem cells in preventing oxygen-induced neonatal lung injury in rats. Stem Cells Dev. 2012;21:2789–2797. doi: 10.1089/scd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 40.Kim CF, Jackson EL, Woolfenden AE, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 41.Tropea KA, Leder E, Aslam M, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L829–837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ionescu L, Byrne RN, van Haaften T, et al. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JW, Fang X, Gupta N, Serikov V, Matthay MA. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A. 2009;106:16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thebaud B, Ladha F, Michelakis ED, et al. Vascular endothelial growth factor gene therapy increases survival, promotes lung angiogenesis, and prevents alveolar damage in hyperoxia-induced lung injury: evidence that angiogenesis participates in alveolarization. Circulation. 2005;112:2477–2486. doi: 10.1161/CIRCULATIONAHA.105.541524. [DOI] [PubMed] [Google Scholar]
- 47.Ionescu LI, Alphonse RS, Arizmendi N, et al. Airway delivery of soluble factors from plastic-adherent bone marrow cells prevents murine asthma. Am J Respir Cell Mol Biol. 2012;46:207–216. doi: 10.1165/rcmb.2010-0391OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Block GJ, Ohkouchi S, Fung F, et al. Multipotent stromal cells are activated to reduce apoptosis in part by upregulation and secretion of stanniocalcin-1. Stem Cells. 2009;27:670–681. doi: 10.1002/stem.20080742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaput N, Thery C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–440. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 51.Dong J, Carey WA, Abel S, et al. MicroRNA-mRNA interactions in a murine model of hyperoxia-induced bronchopulmonary dysplasia. BMC Genomics. 2012;13:204. doi: 10.1186/1471-2164-13-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee C, Mitsialis SA, Aslam M, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126:2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037–3042. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 54.Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med. 2011;6:481–492. doi: 10.2217/rme.11.35. [DOI] [PubMed] [Google Scholar]
- 55.Islam MN, Das SR, Emin MT, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weiss DJ, Casaburi R, Flannery R, Leroux-Williams M, Tashkin DP. A Placebo-Controlled Randomized Trial of Mesenchymal Stem Cells in Chronic Obstructive Pulmonary Disease. Chest. 2012 doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aguirre A, Planell JA, Engel E. Dynamics of bone marrow-derived endothelial progenitor cell/mesenchymal stem cell interaction in co-culture and its implications in angiogenesis. Biochem Biophys Res Commun. 2010;400:284–291. doi: 10.1016/j.bbrc.2010.08.073. [DOI] [PubMed] [Google Scholar]
- 58.Chang YS, Choi SJ, Ahn SY, et al. Timing of umbilical cord blood derived mesenchymal stem cells transplantation determines therapeutic efficacy in the neonatal hyperoxic lung injury. PLoS One. 2013;8:e52419. doi: 10.1371/journal.pone.0052419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laughon M, Allred EN, Bose C, et al. Patterns of respiratory disease during the first 2 postnatal weeks in extremely premature infants. Pediatrics. 2009;123:1124–1131. doi: 10.1542/peds.2008-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah PS, Sankaran K, Aziz K, et al. Outcomes of preterm infants <29 weeks gestation over 10-year period in Canada: a cause for concern? J Perinatol. 2012;32:132–138. doi: 10.1038/jp.2011.68. [DOI] [PubMed] [Google Scholar]


