Abstract
Nearly 25 years ago, the shared interests of psychologists and biologists in understanding the neural basis of social behavior led to the inception of social neuroscience. In the past decade, this field has exploded, in large part due to the infusion of studies that use fMRI. At the same time, tensions have arisen about how to prioritize a diverse range of questions and about the authority of neurobiological data in answering them. The field is now poised to tackle some of the most interesting and important questions about human and animal behavior but at the same time faces uncertainty about how to achieve focus in its research and cohesion among the scientists who tackle it. The next 25 years offer the opportunity to alleviate some of these growing pains, as well as the challenge of answering large questions that encompass the nature and bounds of diverse social interactions (in humans, including interactions through the internet); how to characterize, and treat, social dysfunction in psychiatric illness; and how to compare social cognition in humans with that in other animals.
I. What Is Social Neuroscience?
We live in a world that is largely socially constructed, our lives are replete with social interactions every day, and it has been suggested that an understanding of our social behavior could answer questions about who we are, how we differ from other animals, and what defines the nature of our conscious experience. Moreover, the importance of social encounters is ubiquitous across all animal species. These facts together with our intense personal interest in the behaviors and minds of other people have spawned a rich and long history of investigation in the social sciences. Recently, these investigations incorporated neurobiological tools, giving birth to the field of social neuroscience.
But what exactly is social neuroscience? It encompasses all levels of biological analysis (genetic polymorphisms, neurotransmitters, circuits and systems, as well as collective behavior in groups) and stages of processing (sensory systems, perception, judgment, regulation, decision-making, action), a diversity often emphasized in overviews of the field (Adolphs, 2010; Cacioppo et al., 2001). A principled definition of social neuroscience thus begins by saying that it is the study of the neural basis of social behavior and then elaborates from there. However, this elaboration leaves open a wide range of methods to be employed, species to be studied, and theoretical frameworks to anchor the findings, with disagreements about the relative merits of all of these components. These disagreements are reflected in the priorities of faculty searches, funding agencies, and journal publications.
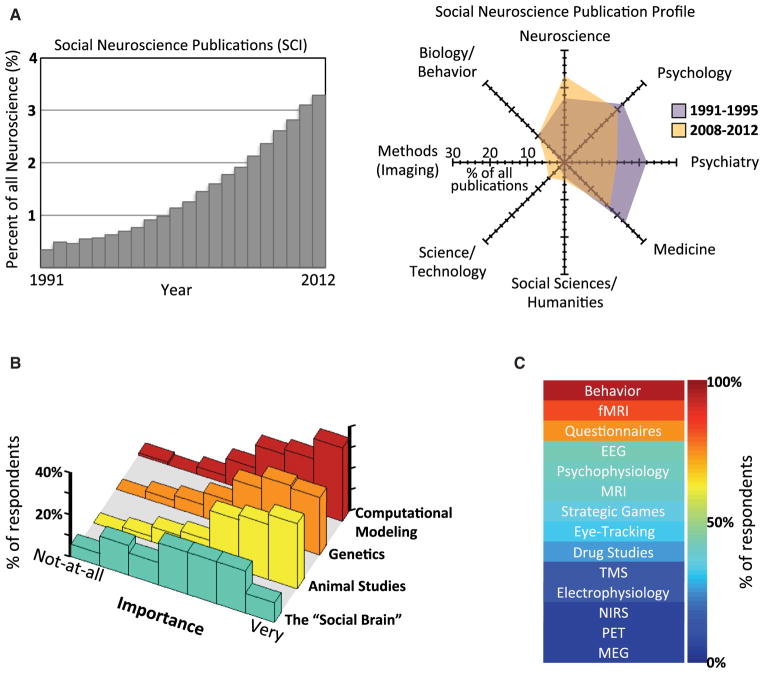
The term “social neuroscience” was first coined in the early 1990s (Cacioppo and Berntson, 1992; Cacioppo et al., 2001) in reference to a fledgling movement that emphasized a broad and multilevel approach to the study of the neural basis of social behavior (see Lieberman, 2012 and Singer, 2012 for historical overviews from both American and European perspectives). This gestation was accompanied by a proposal that social processing in primates was subserved by a specific brain system (Brothers, 1990), as well as by initial neuroimaging studies of social cognition in humans using PET (Fletcher et al., 1995; Happé et al., 1996; Morris et al., 1996), but the tools available at the time were limited. This is likely one reason why the field at the outset emphasized animal studies, where invasive experimental approaches were already well established. Social neuroscience underwent a major transformation in the late 1990s with the advent of fMRI, which led to the emergence of “social cognitive neuroscience” (Ochsner and Lieberman, 2001), a subdiscipline that has now grown to constitute a large component of the field. The two main societies for social neuroscience, the Society for Social Neuroscience (S4SN) and the Social and Affective Neuroscience Society (SANS), emphasize these dual origins, respectively. However, the field is still very much in its infancy: SANS was established in 2008, S4SN was only established in 2010 (each has about 300 members), and a European society is just emerging (ESAN). These societies are comparable in size to organizations such as the Society for Neuroeconomics (which is slightly older and larger) but are far smaller than the Cognitive Neuroscience Society (founded in 1994; membership > 2,000) or the Society for Neuroscience (founded in 1969; membership > 40,000). The two flagship journals of social neuroscience, Social Cognitive and Affective Neuroscience (“SCAN,” publisher: Oxford Press) and Social Neuroscience (publisher: Taylor and Francis), predate the societies only slightly (both were founded in 2006). SANS and S4SN each have about one-third international members, including growing constituencies in South America and Asia (two venues for S4SN’s annual meetings) and a strong student representation, reflecting a young, vibrant, and rapidly growing community. Currently amounting to just over 3%, extrapolation suggests that by the early 2020s, social neuroscience publications could constitute 10% of all neuroscience publications (Figure 1A).
Figure 1. What Is Social Neuroscience?
(A) Metrics of publications over the years. Left: The graph plots the proportion of publications in social neuroscience relative to those in all of neuroscience, using Web of Science and methodology described in Matusall et al. (2011) (updated). Right: Past and current emphases in social neuroscience, obtained by mapping publications in social neuroscience onto the topics shown (see Matusall et al., 2011 for details).
(B) How important to social neuroscience are four major themes (differently colored rows)? The figure shows histograms of the distribution of online responses obtained from ca. 85 members of the Society for Social and Affective Neuroscience (SANS) and the Society for Social Neuroscience (S4SN).
(C) The methods (in rank order) used by social neuroscientists; data from the same respondents as in (B). Abbreviations are as follows: functional magnetic resonance imaging (fMRI), electroencephalography (EEG), magnetic resonance imaging (MRI), transcranial magnetic stimulation (TMS), near infrared spectroscopy (NIRS), positron emission tomography (PET), and magnetoencephalography (MEG).
Many programmatic questions are currently debated in the field. How important is it to relate social behavior to microscopic neurobiological and genetic levels? How important is it to study animal species other than humans? How important is translational work in comparison to basic research? To get an initial overview of how people think about some of these questions, we asked a sample of social neuroscientists to weigh in. Their answers illustrate the broad base that constitutes social neuroscience, the acknowledgment of intense interdisciplinary effort, and the sense of an open landscape in the years ahead (see Figures 1B and 1C; Table 3). Although social neuroscience needs to be broad, it also needs a focus for nucleation, otherwise it threatens simply to merge with cognitive neuroscience or splinter into an array of otherwise unrelated projects. And of course, there is a focus: it is the word “social” that is raising questions about how best to circumscribe this term.
Table 3.
The Future of Social Neuroscience
| What Do Social Neuroscientists Say?
| ||
|---|---|---|
| Current Research Interests | Social Neuroscience Is Currently Lacking | Future of Social Neuroscience |
| Emotion | Statistical/Methodological Rigor | Applied Science |
| Clinical Disorders | Ecological Validity | Computational Approaches |
| Self-Regulation | Interdisciplinary Integration | Networks in the Brain |
| Development | Computational Approaches | Real-World Behaviors |
| Decision-Making | Theory | Social Interaction |
What are the open questions? The table summarizes an inventory of what is currently being studied, what is thought to be missing, and what the future may hold, obtained from the same respondents as in Figure 1B. Respondents were asked to provide 3–5 keywords that best described the following: (1) [their] current research interests; (2) areas in which social neuroscience is lacking; and (3) the future of social neuroscience. The resulting sets of keywords were sorted into umbrella categories, and the top five categories for each question were identified. The results are displayed for each question in rank order. The gray level of the background indicates the rank (i.e., categories with the same color had identical rank).
In studying the “social,” social neuroscience is about the neurobiology involved in perceiving, thinking about, and behaving toward other people. But it also encompasses conspecific interactions between nonhuman animals, the anthropomorphization of stimuli that are not really social at all, and thinking about oneself. The underlying presumption is that these are all intimately related: animals evolved neural mechanisms for interacting with one another and with other species commonly encountered. Conspecifics, predators, and prey thus all require particular repertoires of behavioral interactions, made possible by particular suites of cognitive and neurobiological processes. In humans, these can be applied very widely and flexibly, including cases of anthropomorphization and thinking about ourselves. In addition, they extend beyond typical dyadic interactions to both the larger-scale collective interactions of groups and the indirect and symbolic interactions of individuals through the internet, all hot topics for future study, as we note further below. If all these diverse forms of social behavior were to recruit overlapping processes and activate overlapping brain regions in neuroimaging studies, we would gain confidence that they are sufficiently cohesive to substantiate the field of social neuroscience. Indeed, this is the strong picture that is emerging so far.
All of the features and challenges noted above also make social neuroscience an incredibly exciting field, and one highly attractive to young scientists. There is a plethora of open questions (Tables 2 and 3), a wide range of parent disciplines from which the field can be approached (Figure 1B), and a strong sense of ongoing and impending progress. Whereas previous generations of social neuroscientists were trained in different fields, we are now coming into our first batch of constituents reared in this multidisciplinary environment; whereas several hurdles and critiques were tackled in the recent past, the field has now synthesized initial views of the “social brain” (Figure 2) and generated powerful new approaches to mining and modeling data (Table 1). Next, we briefly take stock of the major current themes, before extrapolating into the future.
Table 2.
What Is Known and Not Known in Social Neuroscience
| What We Knew all along (but Sometimes Forgot) | What We Have Learned | What We Still Need to Know |
|---|---|---|
|
All animals show social behavior. Thus, we should study not only humans. |
Social processes cannot be localized to one brain region. There are distributed systems. |
Are social processes different from nonsocial processes? If so, why and how? |
|
All behavior depends on the brain. Thus, neurobiology can inform social psychology. |
fMRI results cannot be interpreted easily. You need an expert community for advice. |
How far down can we translate social concepts? What vocabulary can we apply across all levels? |
|
The brain interacts with the body. Thus, body and immune system also matter. |
A single discipline is inadequate to understand social behavior. You need collaboration. |
What is unique about human social cognition? And how is any uniqueness represented at the neural level? |
|
There are individual differences. Thus, we have to study individuals as well as groups. |
Our concepts for social processes need revision. Not all good old theories will survive. |
What are the changes in social cognition across the lifespan? How does it emerge in infancy, childhood, adolescence; how does it change in aging? |
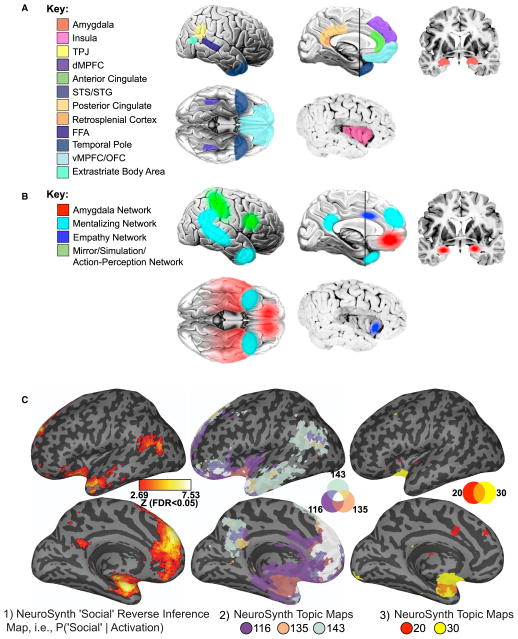
Figure 2. Three Views of the Social Brain.
(A) The original view elaborated a set of brain structures originally proposed by Leslie Brothers (Brothers, 1990).
(B) The current view ties subsets of these structures together into functional networks that subserve particular components of social cognition; both (A) and (B) are from Kennedy and Adolphs (2012).
(C) Hints of a future view in which brain networks are derived by mining large data sets (NeuroSynth; Yarkoni et al., 2011). Left: Lateral (top) and medial (bottom) views of a reverse-inference map (generated using 293 studies) indicate the likelihood that the term “social” was used in a study given the presence of activation, i.e., p(term|activation) (brain activity displayed using NeuroLens; http://www.neurolens.org). We compared this map to that of 200 independently identified Topic maps (Yarkoni et al., 2011; http://neurosynth.org) and identified those that were based on more than 30 studies and that either covered more than 50% of the “social” term map (middle) or were more than 50% covered by the “social” term map (right). Topic 116 was primarily concerned with emotion; Topic 135 with social games and interactions; Topic 143 with mentalizing; Topic 20 with fear and arousal; and Topic 30 with consciousness and awareness. Although these data-mining results should be considered preliminary, they suggest several intriguing patterns: dorsomedial prefrontal cortex appears to subserve a general role, appearing ubiquitously across the networks, whereas regions of the precuneus may be involved more selectively, distinguishing between emotion and social games. It is also interesting to observe that the amygdala is identified in all maps with the exception of Topic 143 (mentalizing). Approaches such as the example we show here should be used in future studies that make an effort to combine and reconcile data-mining results with the results of particular experimental studies.
Table 1.
Three Approaches to Identifying Core Social Processes
| Approach | Examples | Pros | Cons |
|---|---|---|---|
| (A) Social psychology theories |
|
|
|
| (B) Data-driven ontology |
|
|
|
| (C) Computational models |
|
|
|
We outline three very different approaches that each have strengths and weaknesses, together with a few well-known examples from each. All three are currently in use, although (B) and (C) are much more recent than (A). Our own prescription would be to make use of all three and vet them against one another, something almost never undertaken currently but eminently possible. For instance, (A) and (B) could be used to generate models under (C); the results from this could be used to refine (A). Or, (B) could be used to check results from (A) and/or (C) against the large corpus of studies in the literature. We do not believe that we can completely dispense with any of the three, as (A) is essential in giving us theoretical frameworks rich and intuitive enough to let us understand social cognition; (B) is essential in linking our concepts to cumulative data; and (C) is essential in embedding the concepts in the brain’s computations and likely best at translating across different levels.
II. Where Are We Now?
Social neuroscience has made major contributions in many respects. One methodological accomplishment has been to help develop and refine fMRI methods, an advance linked in part to prior critiques we note below. A topical contribution has been the study of individual differences in social behavior. This topic is now often related to genotypic differences (Green et al., 2008) and even to structural brain differences (Kanai and Rees, 2011), with investigation of the effects of culture a hot topic (Rule et al., 2013). There have been major extensions also to understanding psychiatric illness (Cacioppo et al., 2007), as well as the effects of stress and immune function on mood in healthy people (Eisenberger and Cole, 2012). And there has been a recent flurry of attention to real social interactions (as opposed to mere simulations of them), an aspect that has almost spawned its own subdiscipline and is of interest to cognitive scientists more broadly (Schilbach et al., 2013).
A good example of one of the earliest success stories in social neuroscience began in the late 1980s and early 1990s with the discovery of the roles of the neuropeptides oxytocin (OT) and arginine vasopressin (AVP) in social affiliative behaviors. Not only did this work result in a string of elegant papers dissecting the neural circuits and genetic polymorphisms governing affiliative behavior in an animal model (voles; Insel and Young, 2001), but it was also extended to behavioral and neuroimaging studies in humans, including extensions to treatments of psychiatric disorders (Baumgartner et al., 2008; Insel and Young, 2001; Kosfeld et al., 2005; McCall and Singer, 2012). Previously known to play a role in bodily processes related to mammalian child-rearing (OT) and kidney function (AVP), it is now well established that both OT and AVP influence a broad range of social behaviors. In nonhuman mammals, OT has been shown to underlie social bonding behaviors, AVP has been linked to long-term pair bonding and male aggression, and the brain regions in which receptors for these peptides are found have been drawn into a circuit for processing social signals that mediate these behaviors. More than that, genetic polymorphisms in the receptor genes have been linked to species differences in social behavior, providing a story that cuts powerfully across widely different levels of analysis (Insel and Fernald, 2004; Insel and Young, 2001). In the past decade, researchers have begun to explore the influence of OT (which can be delivered intranasally) and, to a lesser extent, AVP on human social behavior: OT can increase social trust (Kosfeld et al., 2005), normal variation in the receptor distribution for OT and AVP in the human population has been linked to measures of altruism and empathy, and OT administration has even been proposed as one component for treating autism (Yamasue et al., 2012). Although it has also become clear that the effect of OT on social behavior is highly dependent on individual differences and context, the topic remains a rich future area of study linking pharmacological, ecological, and psychiatric approaches.
Another major achievement of social neuroscience has been the linking of social and physical health (Eisenberger and Cole, 2012; Eisenberger, 2012). Early work identifying the neural correlates of social pain (e.g., from exclusion or rejection by others) found a remarkable overlap with systems involved in physical pain and linked individual differences in physical and social pain sensitivity. Perhaps even more telling was that experiences that increased social pain also strongly influenced physical pain, and vice versa (Eisenberger, 2012). On the flip side, social support has been shown to reduce both subjective reports and neural responses related to physical pain, while taking Tylenol reduces not only physical pain but also hurt feelings and neural responses to social exclusion (Dewall et al., 2010). Far from simply justifying the shared (though often underappreciated) sense that social pain is as real as physical pain, the establishment of this link between the two has opened up a broad range of new studies, emphasizing the highly interactive nature of social cognition and behavior (a topic to which we will return below).
Perhaps in part as a consequence of the inherent attraction of the questions investigated by social neuroscience, the field has received considerable attention from the media and hence also the general public. This has not always been a good thing. Some overpromotion of early findings in the field resulted in a subsequent backlash against social neuroscience for its failure to deliver on those earlier promises. Particularly acute was a recent episode highlighting the difficulty of supporting many claims drawn from statistical analyses of neuroimaging data (Vul et al., 2009), an issue that pertains to both cognitive neuroscience and social psychology more broadly, but that came to a head at the intersection of these two fields. Social neuroscience, as well as the neuroimaging and psychology fields in general, has been considerably sensitized to these issues, with the overall result that statistical inferences are applied more cautiously by authors and better scrutinized by journal reviewers, publication biases are being exposed in the literature, and increased value has been assigned to replication (Francis, 2012; Green et al., 2008; Kriegeskorte et al., 2010; Poldrack, 2011). However, given the complexity of the phenomena studied by social neuroscience, these issues will continue to demand attention. Their exposure is shaping collective efforts to control for false-positive findings and to construct large databases against which new results can be compared and interpreted (Poldrack, 2011; Yarkoni et al., 2011). With social neuroscience now inoculated with the above critiques, the field is ready to tackle a number of current “hot topics” that we mention only briefly here for the sake of space.
(A) Interactive Neuroscience
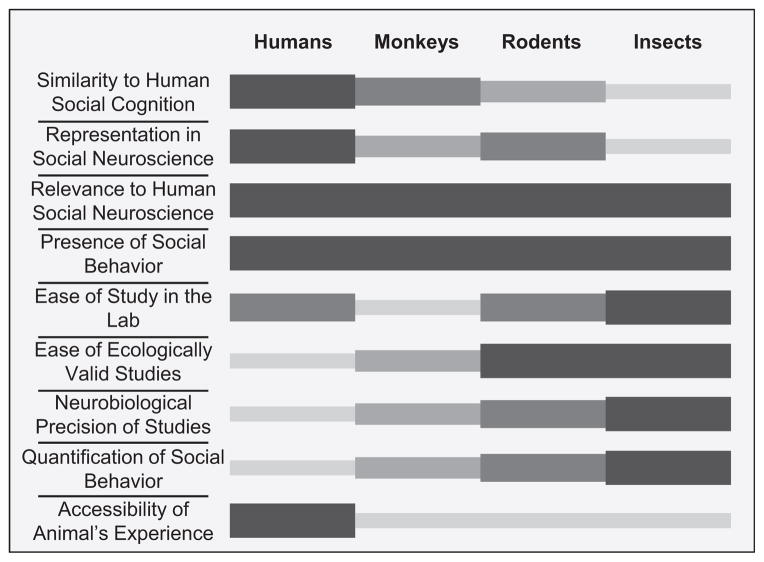
The processes that come into play during real social interactions have been dubbed the “dark matter” of social neuroscience (Schilbach et al., 2013). Studying ecologically valid social interactions in humans is often difficult for two simple reasons: it is ethically tricky (in many cases requiring deception because people otherwise know they are part of an experiment), and it relinquishes some degree of experimental control. It is also an unusually rich and interesting topic, exactly what social psychologists would wish to study and many neurobiologists think is too fuzzy to study. One prescription for the future might be to draw on both of these fields and to study real social interactions—but in well-controlled animal models. Animals usually do not know they are part of an experiment, and achieving ecological validity has a long track record in neuroethology. On the other hand, studies in nonhuman animals have their own problems, including lack of verbal report and explicit instruction, making it often very difficult to know how to interpret what we observe (Figure 3).
Figure 3. A Schematic Representation of the Relative Strengths and Weaknesses of Four Animal Groups Commonly Used to Study Social Neuroscience.
Relative rank ordering of the four different groups (human, nonhuman primate, rodent, and insect) for each of nine themes pertaining to social neuroscience. Darker, thicker bars indicate a higher rank order. The orderings depicted represent the authors’ sense of the field. It is the authors’ expressed opinion that no single level of study is superior to any other. Rather, all are informative and advance the cause of social neuroscience.
(B) Social Neuroscience of Psychiatric Disorders
This topic should in our view be considered simply one aspect of studying individual differences, including cultural effects. The extent to which any given social behavior is pathological or not is often relative to a particular society and is almost always on a spectrum. The recent push by the National Institute of Mental Health to discover more basic dimensions along which psychiatric illnesses can be described (Kapur et al., 2012), as opposed to the categorical classifications provided by DSM-based diagnoses, also opens up this topic to fusion with data-driven approaches (Poldrack et al., 2012). The field is especially exciting because, perhaps for the first time, we can begin to see a strong alternative to the symptom-driven classification of mental disorders provided by traditional psychiatry. Just as psychiatry has embraced approaches from molecular biology and cognitive neuroscience, it should embrace computational tools and modeling methods. If we want to be able to map disorders onto the brain, we need models that specify particular cognitive processes so that we can understand which ones are explanatory and how. Computational psychiatry, in our view, will be a major focus within social neuroscience in the near future (Montague et al., 2012).
(C) Social Neuroscience of Collective Behavior and the Internet
Over the past 25 years, the type and quality of our social interactions have undergone a profound shift as online interactions (e.g., email, instant messaging, social networks) have supplemented, and in many cases supplanted, face-to-face interactions. Indeed, one open question is how social development will be influenced by this radical shift in how we interact (e.g., without social cues that we have evolved to process). There are now several intriguing studies of the relationship between neural function and social networks (e.g., Bickart et al., 2011, 2012; Kanai et al., 2012; Meshi et al., 2013), a topic that has been explored also in monkeys (Sallet et al., 2011). One clear direction for the future of social neuroscience is the development of tools and metrics for the analysis of electronically available social data, such as online social interactions, given the ready availability of massive amounts of such data. With the substantial efforts already put into social network analysis more generally (e.g., from Google), one could think of social neuroscience as capitalizing and piggybacking on this larger enterprise. The ingredient that needs to be added, of course, is the neural data. In principle, one could imagine achieving this, at least in part, by combining MRI data acquired across thousands of people (e.g., the database that NeuroSynth provides) with their social network information. The trick would be tracking individuals across these two very different sets of data, an issue that will occupy not only database experts but also institutional review boards who protect the confidentiality of data on human subjects!
Taking stock more broadly, what has emerged from the corpus of social neuroscience research is not a single, but several, neural systems for processing social information. Correspondingly, there has been a shift from focusing on the function of structures in isolation (Figure 2A) to understanding circuits and systems, with increasing attention to connectivity (Figures 2B and 2C). To date, a number of core networks have been identified as having functional properties related to social processing; we briefly mention four (Figure 2B) (Kennedy and Adolphs, 2012). One, the “social perception” network, centered on the amygdala, has been implicated in a range of social behaviors including the influence of emotion on social decision-making, responses to socially threatening stimuli, and social saliency in general, social-affiliative behaviors and social pain. Sometimes these somewhat diverse functions fractionate into three networks involving different amygdala nuclei (Bickart et al., 2012). A second, “mentalizing,” network is engaged both when actively thinking about others and when reflecting on oneself (Mitchell et al., 2005; Saxe and Powell, 2006; Spunt and Lieberman, 2012; Van Overwalle and Baetens, 2009; Frith and Frith, 2006). Interestingly, this network shows considerable overlap with the so-called default mode network (Raichle et al., 2001), which is more active and coupled during rest, as well as with networks subserving episodic and prospective memory. This suggests that perhaps all these functions share something in common, such as an ability to shift one’s perspective away from current stimuli (Buckner and Carroll, 2007). A third network concerned with “empathy” is engaged when individuals experience vicarious emotions from observing others (de Vignemont and Singer, 2006). Finally, a fourth, “mirror,” network is activated when individuals observe the actions of others and is thought to play a role in learning through observation (Carr et al., 2003; Rizzolatti and Craighero, 2004; Spunt and Lieberman, 2012). The empathy and mirror networks are clearly related, and the mentalizing and mirror networks have in fact been combined into more global schemes for a unified model of how we think about other people (Keysers and Gazzola, 2007). However, there is certainly not unanimous agreement on precisely what the networks are, on their composition, or on how best to study them (Barrett and Satpute, 2013).
Indeed, it is likely that current beliefs about network architecture are biased, at least in part, by pre-existing theoretical divisions and distinctions in social psychology—as well as limited by data. An alternative data-driven approach that is less biased capitalizes on data mining of the literature to find relationships between the psychological concepts studied and the brain activation patterns that emerge over several thousand publications (Table 1; Figure 2C) (Yarkoni et al., 2011). Networks derived from these data-driven approaches will need to be compared and combined in some way with networks obtained from specific social neuroscience studies that use concepts from social psychology, as well as with networks obtained from model-based approaches. Yet even a cursory exploration with a data-driven approach (using NeuroSynth, see Figure 2C) yields both a confirmation of known patterns (e.g., several regions, such as medial prefrontal cortex and precuneus, feature in social cognition networks) as well as the discovery of new ones that can be further tested (e.g., the amygdala appears to participate in many social cognition processes but not mentalizing). The future approach we advocate uses such data mining not as the sole tool but precisely to test results against patterns in the literature and to motivate new hypotheses to be further tested with other approaches (cf. Table 1).
One looming question regarding the concept of the “social brain” and its modern network versions is whether any of these networks are specialized for processing social information. Plausibly, all social cognition draws on entirely domain-general processes, only applied to social stimuli. This unresolved question has been discussed in detail before (e.g., Adolphs, 2010) with the recommendation that, for methodological reasons, we should assume the existence of such specialized processes and brain networks (e.g., Kennedy and Adolphs, 2012). This assumption may in time be proved wrong, or wrong for some of the networks (e.g., Barrett and Satpute, 2013), but there are enough examples that we feel it must be at least partly right, and we just need to delineate the boundaries of the social brain rather than question the entire concept. For instance, there are uncontentious examples of systems specialized for processing social information in the case of pheromone detection in insects and in the case of the vomeronasal system in many mammals. Examples in primates are more debated, but again we would argue that there are clear studies ranging from lesion work to neuroimaging of face processing.
Although we have moved from regions to networks, the next key step is to identify the flow of information through these networks to follow social information processing from stimulus through to response. This requires an understanding of the detailed computations implemented by the different nodes in the networks as well the dynamic interplay between them. One could make the analogy of moving from words (brain areas) to sentences (networks) to propositions (arrangements of network dynamics) to conversations (brains interacting). We are still solidly in the age of sentences and are only beginning to enter the age of propositions and conversations.
III. Where Are We Going?
Social neuroscience must include a wide selection of methods, study a wide range of species, and utilize a range of concepts and theories. It is this topical and methodological breadth, combined with its interdisciplinary approach, that generates tension in the field. Psychologists often find the methods of neuroscience impressive but its concepts and theories impoverished. Neurobiologists find the questions of social psychology intriguing but its methods limited. No wonder there is often little agreement at faculty meetings on whom to hire in a “social neuroscience” search!
We believe that the single major challenge—and exciting open terrain—for the future of social neuroscience is conceptual rather than methodological. How can we parse social behavior, to begin with, and what vocabulary of concepts should we deploy in describing central processes and relating them to neurobiological constituents? This question, we believe, is also the main source of tension among different strands of social neuroscience or between those with backgrounds in different disciplines. A large part of this tension stems from the belief among some social scientists that the processes responsible for understanding both human and animal social behavior are very complex, are very context-dependent, and draw on many factors, including ones outside the brain—as such making these processes ill suited to neuroscientific study.
It is important to understand the several facets behind this tension. One difficulty is simply to discover the processes, a query that can be approached in different ways—further development of theoretical frameworks or “discovery science” based on data mining, to name just two (see Table 1). But another important worry is reductionism, the sense that neurobiological approaches will generate concepts that displace those of social psychology, as exemplified in the quote below:
...some of the topics of interest to social psychologists are not amenable to brain localization techniques because of the complexity of the processes; they have embedded in them subprocesses that interact, and such complex processes are difficult to localize. It would be a pity if, in their justifiable enthusiasm for this powerful tool, social psychologists subtly shifted their research programs to problems that are amenable to brain localization or shifted their theoretical language to constructs that are localizable. –Willingham and Dunn (2003)
Certainly, it is currently hard to see how basic computations implemented in small assemblies of neurons can be related to, say, phenomena such as stereotyping from social psychology. This threat of reductionism, properly a threat of elimination of concepts associated with more macroscopic levels of description, is however not unique to social neuroscience but pervades the study of all of cognition. As in the general case, the way forward in social neuroscience is simple enough: both micro-and macroscopic levels of analysis, as well as the development of concepts associated with each of them, should proceed in tandem. Tension can be relieved if we realize that there is no “fundamental” level of description, or ontology of concepts, that should have priority over any other; we would favor a pragmatic view that incorporates new concepts simply on the basis of their utility. Each level of description has concepts that are the most useful for that level of description. Of course, the levels describe a single reality, and so the concepts must somehow relate to one another. But reduction or elimination is not needed: what is needed is communication, so that those working at different levels of analysis can appreciate, and understand, work at different levels. We do not so much need a single language, as we need people who can speak several languages and translate easily between them.
Nowhere is the challenge of translating across languages more apparent than in comparative social neuroscience. People with backgrounds in neuroethology, animal behavior, or cellular neurobiology typically do not discuss science with those doing fMRI in humans. As we noted at the beginning, the two main societies for social neuroscience in fact reflect this rift: there are those studying humans (generally with fMRI) on the one hand and those studying nonhuman animals (generally not with fMRI) on the other. It is interesting to note that the species differences parallel the different methods used. We most strongly believe that these differences need communication. Comparisons must be made across species, and the findings in particular from fMRI studies in humans need to be related to data from other species and obtained with other methods (see Adolphs and Anderson, 2013). However, it is one thing to recommend this, and another to spell out in more detail why and how. There are strengths and weaknesses inherent in the study of different species (see Figure 3), and so it is natural to ask which should be considered most important: which are the most “social,” which the easiest to study, and which the most relevant models of human social behavior in health and in disease? These questions are not easy to answer for the simple reason that we don’t know much (yet) about the social neuroscience of any species, let alone many of them. Nonetheless, even a cursory inspection of Figure 3 highlights the fact that different animals offer very complementary opportunities: insects are tremendously useful for the study of highly specific social behaviors and their genetic basis; rodents are ideal for optogenetic manipulation; monkeys offer the best glimpse at the neurophysiology underlying complex group behaviors most similar to those of humans; and of course humans are indispensable because they can tell us about ourselves most directly.
We conclude by asking where should we invest our effort, thinking ahead to the next 25 years (see Tables 2 and 3). We highlight three especially exciting avenues for the future. Arguably, one of the most exciting methods currently in neurobiology is optogenetics. This approach, especially suitable to the circuit and small-systems level, permits inhibition or excitation of activity across large populations of cells but with precision at the level of single cells (Deisseroth, 2011; Zhang et al., 2007). As such, very precise patterns of neural activity can be manipulated in space and time—so precisely, in fact, that in principle they can perfectly emulate the patterns that actually occur in the brain normally. It is thus not just the causal aspect of the method that is so impressive but the (future) ability literally to replay the neural events that would normally constitute a cognitive event. In the near future, these techniques will likely reveal with unprecedented detail the causal relationships between sequences of neural events and social behaviors in many social species including nonhuman primates (Gerits and Vanduffel, 2013). Indeed, although optogenetic approaches are currently too invasive for use with humans, it is no longer in the realm of science fiction to consider that tools of this nature may be available for human research in the not-too-distant future as well, a prospect that opens up some very exciting possibilities (Alivisatos et al., 2012). For instance, we could (in principle) reinstantiate the neural state that corresponds to social anxiety; it would not be caused so much as constituted. One could imagine tweaking the neural state slightly, mapping out the boundaries of what people subjectively report as social anxiety, replaying the neural state as modulated by anxiolytic drugs, and so forth. There is little question that these advances will play a large role in helping to biologically constrain theories of social cognition over the next 25 years.
The second exciting future direction is not so much brand-new as greatly expanding: “discovery science” driven by mining data rather than by formulating hypotheses. Already the hallmark of genetic data and also of neurobiological data in animals (e.g., the Allen Brain Atlas for the mouse), the idea of mining fMRI data has been around for over a decade (Van Horn and Gazzaniga, 2002) but has come into its own only very recently (Yarkoni et al., 2011). With the launch of several large-scale funding efforts, such as the NIMH-funded “Human Connectome Project,” the Allen Institute for Brain Science’s “Project Mindscope,” the European “Blue Brain/ Human Brain” project, and the “BRAINS” project just recently announced by president Obama, there is no question that the next few years will see a massive ballooning of data, together with tools to mine it. Although to some extent these resources can be used simply as one component in the pipeline of an experiment, they also can be the data to be studied in their own right, revealing new patterns.
This then brings us to our final future direction: computational neuroscience that combines measures of brain function and behavior with sophisticated mathematical models. There are several advantages to building concepts based on computational models, including precision, parametric quantification, and easy expandability. But one feature stands out in particular: such models may be unique in their applicability across a very wide range of levels of analysis, from cells to brain systems to behavior. Although model-based fMRI has been quite widely adopted in studies of learning and decision making, to date, relatively few have directly applied it to social neuroscience. One early example studied learning behavior in a strategic game and fit the fMRI data to computational models; the best fitting model showed not only that participants were tracking opponents’ actions (as a poorer-performing model showed) but also that the participants understood that their opponents were tracking them (Hampton et al., 2008). The ability to link distinct computational components of a model to distinct neural regions offers tremendous promise for understanding more precisely what it is that these brain regions contribute (Behrens et al., 2009; Dunne and O’Doherty, 2013). Other studies have used computational models to identify neural correlates of tracking the quality of other peoples’ advice (Behrens et al., 2008; Boorman et al., 2013) or applied the approach to understanding dysfunction in psychiatric illness (Montague et al., 2012). The computational approach to social neuroscience questions, although brand-new, is a growing subfield with substantial activity and promise for the future.
Social neuroscience faces perennial themes of prediction and causality: fMRI, as is well known, is a purely correlational method. However, the accuracy with which neuroimaging data are related to cognition and behavior is often tested with the predictive power of the data—for instance, through training-machine learning algorithms on detailed multivoxel patterns of activation (Tong and Pratte, 2012). More powerful yet are formal computational models. Depending on the nature and fit of the model, the data together with the model can suggest more than correlation and argue for directional causal architectures. Ultimately, this is of course the kind of understanding that we want to have, and often it is already implicit in the way we think about data, even when unjustified. Modern neuroimaging combined with computational models and vetted with truly causal methods such as optogenetics could thus be the methods armamentarium for the future of social neuroscience—also making explicit the need for studies that cut across species. As we noted, we expect that computational models will help to provide an economical inventory of processes and concepts, and moreover one that will likely cut across not only species but also levels of analysis. What exactly that vocabulary will look like is a major open question and brings us back to one overarching concern: is there anything special about social neuroscience? The investigation of social behavior defines the field; we should look for an inventory of parameters in our models that define what is unique about social interactions. As we alluded to above, some prior studies have done precisely that (Hampton et al., 2008). The challenge as we see it now is to build up our inventory of processes derived from model-based and data-mining approaches, pit them against entrenched concepts already in use, and forge forward with a redefined notion of what social neuroscience is really all about.
Acknowledgments
This work was supported in part by a Conte Center (R.A.) and K01 grant (K01MH099343 to D.A.S.) from NIMH. We thank SANS (in particular Mauricio Delgado) and S4SN (in particular Larry Young) for providing metrics on the societies and their members for providing the online data used in some of our figures. We also thank Naomi Eisenberger, Keise Izuma, Catherine Hartley, Cendri Hutcherson, and Bob Spunt for comments on the manuscript. We are particularly indebted to Markus Christen for help with bibliometric data shown in Figure 1A.
References
- Adolphs R. Conceptual challenges and directions for social neuroscience. Neuron. 2010;65:752–767. doi: 10.1016/j.neuron.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Anderson DJ. Social and emotional neuroscience. Curr Opin Neurobiol. 2013;23(special issue):291–293. doi: 10.1016/j.conb.2013.04.011. [DOI] [PubMed] [Google Scholar]
- Alivisatos AP, Chun M, Church GM, Greenspan RJ, Roukes ML, Yuste R. The brain activity map project and the challenge of functional connectomics. Neuron. 2012;74:970–974. doi: 10.1016/j.neuron.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Satpute AB. Large-scale brain networks in affective and social neuroscience: towards an integrative functional architecture of the brain. Curr Opin Neurobiol. 2013;23:361–372. doi: 10.1016/j.conb.2012.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–650. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Woolrich MW, Rushworth MFS. Associative learning of social value. Nature. 2008;456:245–249. doi: 10.1038/nature07538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Hunt LT, Rushworth MFS. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Bickart KC, Wright CI, Dautoff RJ, Dickerson BC, Barrett LF. Amygdala volume and social network size in humans. Nat Neurosci. 2011;14:163–164. doi: 10.1038/nn.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Hollenbeck MC, Barrett LF, Dickerson BC. Intrinsic amygdala-cortical functional connectivity predicts social network size in humans. J Neurosci. 2012;32:14729–14741. doi: 10.1523/JNEUROSCI.1599-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, O’Doherty JP, Adolphs R, Rangel A. The behavioral and neural mechanisms underlying the tracking of expertise. 2013 doi: 10.1016/j.neuron.2013.10.024. Neuron Published online December 18 2013 http://dx.doi.org/10.1016/j.neuron.2013.10.024. [DOI] [PMC free article] [PubMed]
- Brothers L. The social brain: a project for integrating primate behavior and neurophysiology in a new domain. Concepts Neurosci. 1990;1:27–51. [Google Scholar]
- Buckner RL, Carroll DC. Self-projection and the brain. Trends Cogn Sci. 2007;11:49–57. doi: 10.1016/j.tics.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Social psychological contributions to the decade of the brain. Doctrine of multilevel analysis. Am Psychol. 1992;47:1019–1028. doi: 10.1037//0003-066x.47.8.1019. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Amaral DG, Blanchard JJ, Cameron JL, Carter CS, Crews D, Fiske S, Heatherton T, Johnson MK, Kozak MJ, et al. Social neuroscience: progress and implications for mental health. Perspect Psychol Sci. 2007;2:99–123. doi: 10.1111/j.1745-6916.2007.00032.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Adolphs R, Carter CS, Davidson RJ, McClintock MK, McEwen BS, Meaney MJ, Schacter DL, Sternberg EM, et al., editors. Foundations in Social Neuroscience. Cambridge, MA: MIT Press; 2001. [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proc Natl Acad Sci USA. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vignemont F, Singer T. The empathic brain: how, when and why? Trends Cogn Sci. 2006;10:436–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewall CN, Macdonald G, Webster GD, Masten CL, Baumeister RF, Powell C, Combs D, Schurtz DR, Stillman TF, Tice DM, Eisenberger NI. Acetaminophen reduces social pain: behavioral and neural evidence. Psychol Sci. 2010;21:931–937. doi: 10.1177/0956797610374741. [DOI] [PubMed] [Google Scholar]
- Dunne S, O’Doherty JP. Insights from the application of computational neuroimaging to social neuroscience. Curr Opin Neurobiol. 2013;23:387–392. doi: 10.1016/j.conb.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev Neurosci. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Cole SW. Social neuroscience and health: neurophysiological mechanisms linking social ties with physical health. Nat Neurosci. 2012;15:669–674. doi: 10.1038/nn.3086. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happé F, Frith U, Baker SC, Dolan RJ, Frackowiak RS, Frith CD. Other minds in the brain: a functional imaging study of “theory of mind” in story comprehension. Cognition. 1995;57:109–128. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Francis G. Publication bias and the failure of replication in experimental psychology. Psychon Bull Rev. 2012;19:975–991. doi: 10.3758/s13423-012-0322-y. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. The neural basis of mentalizing. Neuron. 2006;50:531–534. doi: 10.1016/j.neuron.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Gerits A, Vanduffel W. Optogenetics in primates: a shining future? Trends Genet. 2013;29:403–411. doi: 10.1016/j.tig.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Green AE, Munafò MR, DeYoung CG, Fossella JA, Fan J, Gray JR. Using genetic data in cognitive neuroscience: from growing pains to genuine insights. Nat Rev Neurosci. 2008;9:710–720. doi: 10.1038/nrn2461. [DOI] [PubMed] [Google Scholar]
- Hampton AN, Bossaerts P, O’Doherty JP. Neural correlates of mentalizing-related computations during strategic interactions in humans. Proc Natl Acad Sci USA. 2008;105:6741–6746. doi: 10.1073/pnas.0711099105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happé F, Ehlers S, Fletcher P, Frith U, Johansson M, Gillberg C, Dolan R, Frackowiak R, Frith C. ‘Theory of mind’ in the brain. Evidence from a PET scan study of Asperger syndrome. Neuroreport. 1996;8:197–201. doi: 10.1097/00001756-199612200-00040. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Annu Rev Neurosci. 2004;27:697–722. doi: 10.1146/annurev.neuro.27.070203.144148. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kanai R, Bahrami B, Roylance R, Rees G. Online social network size is reflected in human brain structure. Proc Biol Sci. 2012;279:1327–1334. doi: 10.1098/rspb.2011.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur S, Phillips AG, Insel TR. Why has it taken so long for biological psychiatry to develop clinical tests and what to do about it? Mol Psychiatry. 2012;17:1174–1179. doi: 10.1038/mp.2012.105. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci. 2012;16:559–572. doi: 10.1016/j.tics.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cogn Sci. 2007;11:194–196. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Lindquist MA, Nichols TE, Poldrack RA, Vul E. Everything you never wanted to know about circular analysis, but were afraid to ask. J Cereb Blood Flow Metab. 2010;30:1551–1557. doi: 10.1038/jcbfm.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman MD. A geographical history of social cognitive neuroscience. Neuroimage. 2012;61:432–436. doi: 10.1016/j.neuroimage.2011.12.089. [DOI] [PubMed] [Google Scholar]
- Matusall S, Kaufmann I, Christen M. The emergence of social neuroscience as an academic discipline. In: Decety J, Cacioppo J, editors. The Oxford Handbook of Social Neuroscience. Oxford: Oxford University Press; 2011. pp. 9–27. [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15:681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- Meshi D, Morawetz C, Heekeren HR. Nucleus accumbens response to gains in reputation for the self relative to gains for others predicts social media use. Front Hum Neurosci. 2013;7:439. doi: 10.3389/fnhum.2013.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. Neuroimage. 2005;28:757–762. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dolan RJ, Friston KJ, Dayan P. Computational psychiatry. Trends Cogn Sci. 2012;16:72–80. doi: 10.1016/j.tics.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, Rowland D, Young AW, Calder AJ, Dolan RJ. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383:812–815. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Lieberman MD. The emergence of social cognitive neuroscience. Am Psychol. 2001;56:717–734. [PubMed] [Google Scholar]
- Poldrack RA. Inferring mental states from neuroimaging data: from reverse inference to large-scale decoding. Neuron. 2011;72:692–697. doi: 10.1016/j.neuron.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Schonberg T, Kalar D, Barman B, Yarkoni T. Discovering relations between mind, brain, and mental disorders using topic mapping. PLoS Comput Biol. 2012;8:e1002707. doi: 10.1371/journal.pcbi.1002707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rule NO, Freeman JB, Ambady N. Culture in social neuroscience: a review. Soc Neurosci. 2013;8:3–10. doi: 10.1080/17470919.2012.695293. [DOI] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Andersson JL, O’Reilly JX, Jbabdi S, Croxson PL, Jenkinson M, Miller KL, Rushworth MFS. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- Saxe R, Powell LJ. It’s the thought that counts: specific brain regions for one component of theory of mind. Psychol Sci. 2006;17:692–699. doi: 10.1111/j.1467-9280.2006.01768.x. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, Costall A, Bente G, Schlicht T, Vogeley K. Toward a second-person neuroscience. Behav Brain Sci. 2013;36:393–414. doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Singer T. The past, present and future of social neuroscience: a European perspective. Neuroimage. 2012;61:437–449. doi: 10.1016/j.neuroimage.2012.01.109. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. Dissociating modality-specific and supramodal neural systems for action understanding. J Neurosci. 2012;32:3575–3583. doi: 10.1523/JNEUROSCI.5715-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong F, Pratte MS. Decoding patterns of human brain activity. Annu Rev Psychol. 2012;63:483–509. doi: 10.1146/annurev-psych-120710-100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn JD, Gazzaniga MS. Opinion: Databasing fMRI studies towards a ‘discovery science’ of brain function. Nat Rev Neurosci. 2002;3:314–318. doi: 10.1038/nrn788. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others’ actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–584. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci. 2009;4:274–290. doi: 10.1111/j.1745-6924.2009.01125.x. [DOI] [PubMed] [Google Scholar]
- Willingham DT, Dunn EW. What neuroimaging and brain localization can do, cannot do and should not do for social psychology. J Pers Soc Psychol. 2003;85:662–671. doi: 10.1037/0022-3514.85.4.662. [DOI] [PubMed] [Google Scholar]
- Yamasue H, Yee JR, Hurlemann R, Rilling JK, Chen FS, Meyer-Lindenberg A, Tost H. Integrative approaches utilizing oxytocin to enhance prosocial behavior: from animal and human social behavior to autistic social dysfunction. J Neurosci. 2012;32:14109–14117. doi: 10.1523/JNEUROSCI.3327-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8:665–670. doi: 10.1038/nmeth.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]