Summary
Patients with Down syndrome (DS) suffer from muscle hypotonia and an altered motor coordination whose basic mechanisms are still largely unknown. Interestingly, they show muscle weakness like healthy aged subjects, suggesting possible similarity with sarcopenia: to test this hypothesis, the Ts65Dn mouse, a suitable animal model of DS, was employed. The fine structure of skeletal fibres of the quadriceps femoris muscle was analysed in adult (12 months) and aging (19 months) animals and their age-matched euploid controls by combining morphometry and immunocytochemistry at transmission electron microscopy. Results demonstrated structural alterations of mitochondria and myonuclei reminiscent of those observed in age-related sarcopenia, supporting the hypothesis that trisomy leads to an early aging of skeletal muscle consistent with the multi-systemic premature aging typical of DS.
Keywords: aging, cell nucleus, Down syndrome, mitochondria, skeletal muscle, Ts65Dn mouse
Introduction
Down syndrome (DS) is a genetically based disease which, in humans, affects about 1 over 700 newborns and is due to the presence of all or part of an extra chromosome 21. Among their several pathological traits, DS patients suffer from muscle hypotonia and an altered motor coordination, the most typical features being a slowed voluntary movement and a high error incidence in motion1. Although difficulties in motility represent a serious problem in daily life the muscular apparatus as a whole being seriously affected in persons with DS, no data exist in the literature on the ultrastructural features of skeletal muscle of these patients.
The mechanisms responsible for the overall muscle hypotonia and motor dysfunction in DS are likely manifold and still largely unknown; defective neuromuscular transmission and central activation are probably involved, but abnormal organization and function of myofibre components can not be ruled out. It is worth noting that persons with DS undergo premature aging in multiple organs2,3, and exhibit a decrement in muscle strength compared to euploid subjects as much as it occurs in aged versus healthy young persons4,5. It is, therefore, possible that muscle hypotonia and motor dysfunction in DS share some basic mechanisms with the progressive age-related decrease in skeletal muscle mass, strength and quality known as sarcopenia6. In a sociological perspective, it is of great importance knowing the effects of aging on the already altered skeletal muscle of persons with DS owing to the present significant increase in their life expectancy.
In recent years, in situ analyses proved to be a unique approach to study the structural organization and function of skeletal muscles, and have been widely used investigating the basic mechanisms of neuromuscular disorders: in particular, light and electron microscopy immunocytochemistry allowed to detect the subcellular localization of specific muscle molecular components thus providing crucial information on the cellular basis of skeletal muscle wasting under different condition such as sarcopenia, myotonic dystrophies, or laminopathies7.
Due to obvious ethical reasons, this kind of studies are difficult to perform on muscle biopsies from DS patients, but suitable animal models are available to partly overcome this limitation. The Ts65Dn mouse strain bearing a trisomy for a segment of chromosome 16 (i.e. the homologue of human chromosome 21) is the most extensively studied murine model of DS. Ts65Dn mice display a remarkable number of phenotypic traits expressed by humans with DS, inclusive of craniofacial alterations, structural and cognitive alterations of the brain, Alzheimer’s-like brain abnormalities, and congenital heart defects; they also exhibit DS-like motor dysfunctions8,9, thus representing a suitable model for structural analysis of skeletal muscle fibres aimed at elucidating the possible mechanisms of DS-associated muscle dysfunction. In a recent study, Cowley et al.10 demonstrated functional alterations of mitochondria in the soleus muscle of Ts65Dn mice which could account for the post-fatigue muscle weakness occurring in these animals.
In the present study we used adult (12 months) and aging (19 months) Ts65Dn mice to examine, by morphometry and immunocytochemistry at transmission electron microscopy, the fine structure of skeletal myofibres of the quadriceps femoris muscle in trisomic and age-matched euploid animals. Special attention has been paid to mitochondria and myonuclei which have been demonstrated to undergo structural and functional alterations in sarcopenia11,12. The quadriceps femoris was chosen because it is mostly composed by fast type II fibres (about 90%, in the mouse) which are known to be principally affected by sarcopenia13.
Materials and Methods
Animals and tissue processing
Four adult (12 months of age) and four late adult (19 months of age) Ts65Dn mice (two trisomic and two age-matched euploid animals per age) (The Jackson Laboratory, ME, USA) were used. All animals were bred under controlled environmental conditions with a 12 h light/dark cycle, and fed ad libitum with a standard commercial chow. The experimental protocols comply with the guidelines of the Italian Ministry of Health as well as with internationally recognized guidelines.
The mice were deeply anaesthetised with 35% chloral hydrate (0.1 ml/100 g b.w.) and then transcardially perfused with a fixative solution containing 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 at 4°C. Quadriceps femoris muscles were quickly removed and placed for 2 h at 4°C either in the same fixation solution (for immunoelectron microscopy) or in a 2.5% glutaraldehyde plus 2% paraformaldehyde solution (for conventional electron microscopy).
After fixation, samples for ultrastructural morphology were rinsed with PBS, post-fixed with 1% OsO4 for 2 h at room temperature, dehydrated with acetone and embedded in Epon 812. For immunocytochemistry, samples were washed in PBS, treated with 0.5 M NH4Cl in PBS for 45 min at 4°C to block free aldehydes, dehydrated with ethanol, and embedded in LR White resin.
Morphological and morphometrical analyses
Semithin (2 μm thick) sections of Epon-embedded muscles stained with 0.1% toluidine blue were used for morphometrical evaluation of myofibre size. The cross-sectional area of fifty transversally cut fibres per sample was measured using the software Image J (NIH, USA), at ×40 on micrographs taken in an Olympus BX51 microscope with an Olympus Camedia C-5050 digital camera.
Ultrathin (70–90 nm thick) sections of Epon-embedded samples were stained with uranyl acetate, and observed in a Philips Morgagni TEM operating at 80kV and equipped with a Megaview II camera for digital image acquisition.
Morphometrical evaluations were carried out on twenty myonuclei per muscle (×11,000) from longitudinally sectioned fibres. The following parameters were considered: area of nuclei, percentage of nuclear area occupied by heterochromatin, area of nucleolus and percentage of area occupied by the nucleolar components (fibrillar centres, dense fibrillar component, granular component).
A morphometrical analysis of intermyofibrillar mitochondria, known to have size similar to the subsarcolemmal ones14, was carried out in fifty mitochondria (×28,000) per animal; mitochondrial area and the ratio between inner and outer membranes, (estimating the extension of cristae independently of mitochondrial size) were considered.
Immunocytochemical analyses
To evaluate myonuclear activity, the fine distribution of two factors involved in different steps of the mRNA synthesis process was investigated. Sections from LR White-embedded samples were treated with a mouse monoclonal antibodies directed against the phosphorylated (activated) form of RNA polymerase II (Research Diagnostic Inc., Flanders, NJ), or the (Sm)snRNP (small nuclear ribonucleoprotein) core protein (Abcam, Cambridge, MA). Briefly, sections were floated for 3 min on normal goat serum diluted 1:100 in PBS and then incubated for 17 h at 4°C with the primary antibody diluted in PBS containing 0.1% bovine serum albumin (Fluka, St. Louis, MO) and 0.05% Tween 20. After rinsing, sections were floated on normal goat serum, and then reacted for 30 min at room temperature with the anti-mouse secondary 12 nm gold-conjugated antibody (Jackson Immuno Research Laboratories, West Grove, PA) diluted 1:10 in PBS. The sections were finally rinsed, air-dried and stained by uranyl acetate.
Quantitative assessment of immunolabelling was carried out by estimating the gold grain density over selected compartments on sections treated in the same run. The nucleoplasmic area was measured on twenty randomly selected electron micrographs of myonuclei (×28,000) from each animal. For background evaluation, samples processed in the absence of the primary antibody were considered. The gold grains present over the selected areas were counted and the labelling density was expressed as number of gold grains/μm2.
Statistics
Results for each measured variable were pooled according to the experimental group and the mean±standard error of the mean (SE) value calculated. Statistical comparisons were performed by the two-way ANOVA test (significance set at P≤0.05) to evaluate the factor “trisomy” and “age” as well as the interaction term between the two factors for each variable.
Results
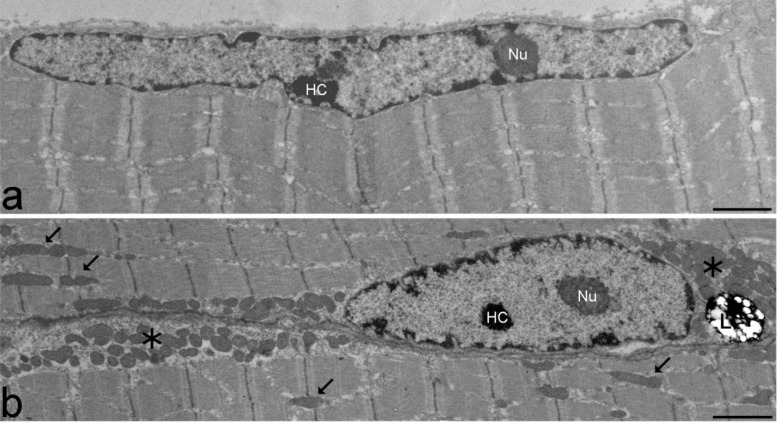
The general ultrastructural organization of quadriceps skeletal muscle cells did not apparently differ between trisomic and euploid mice (Fig. 1): the myofibres showed their typical elongated shape and contained multiple, subsarcolemmal elongated nuclei containing a few heterochromatin clumps and one or two roundish nucleoli; the cytoplasm was mostly occupied by longitudinally arrayed myofibrils with regular sarcomeres; ovoid mitochondria with transversal cristae were lined between the myofibrils as well as in close proximity of myonuclei in the subsarcolemmal region; the rough endoplasmic reticulum occurred close to the myonuclei, while the smooth endoplasmic reticulum was abundant among the myofibrils; glycogen was copious and evenly scattered in the sarcoplasm, while lipid droplets and lipofuscin deposits were scarce. No morphological evidence of apoptosis or necrosis was observed in all muscle samples.
Figure 1.
Transmission electron micrographs of myofibres from adult euploid (a) and trisomic (b) mice. The myonuclei show a finely irregular border, a few clumps of heterochromatin (HC) and one roundish nucleolus (Nu). Large subsarcolemmal (asterisks) and intermyofibrillar (arrows) mitochondria are evident in (b). L, lipofuscin deposit. Bars: 2 μm.
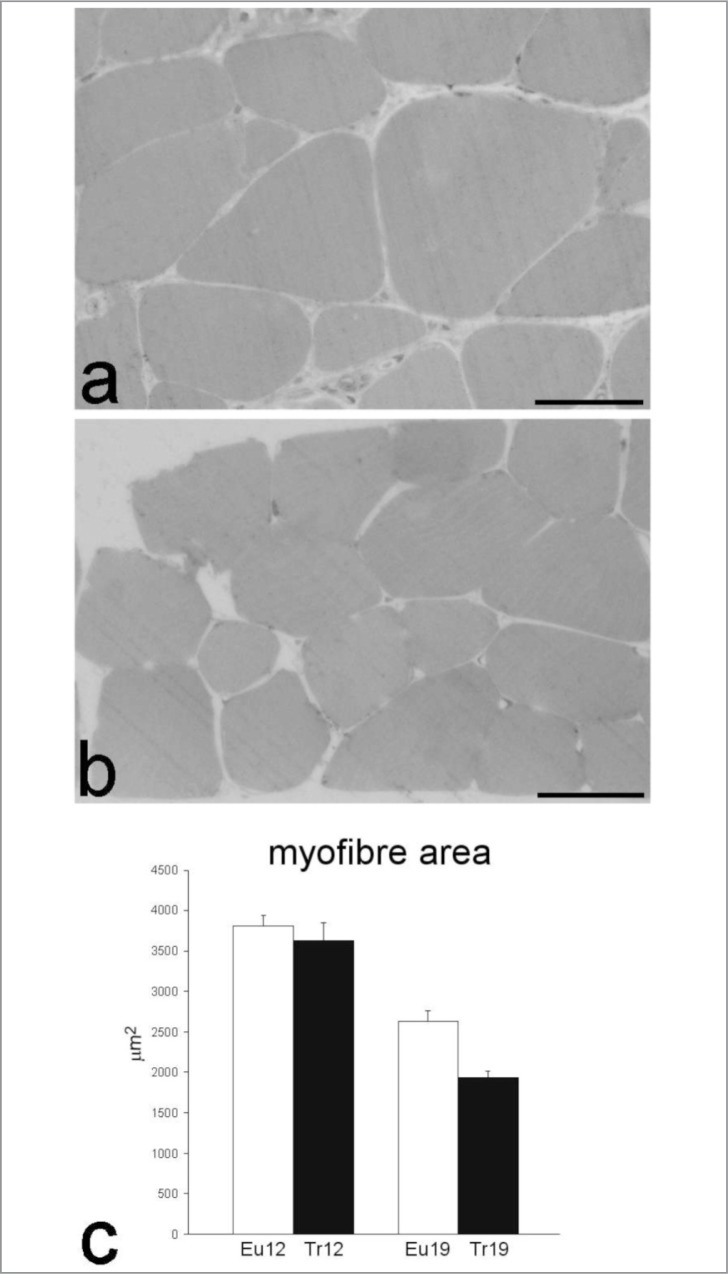
Morphometric analysis of myofibres (two-way ANOVA, Fig. 2) showed a significant reduction of the mean cross-sectional area depending on both the “trisomy” (P<0.001) and “age” (P<0.001) factors; statistical significance was also observed for the interaction term (P<0.001).
Figure 2.
(a,b) Light microscopy micrographs of transversally cut quadriceps muscles of aging euploid (a) and trisomic (b) mice. Bars: 40 μm. (c) Cross-sectional area (mean±SE) of myofibres from 12- and 19 month-old euploid (Eu) and trisomic (Tr) mice. Two-way ANOVA revealed significant effect of the factors “trisomy” (P<0.001), “age” (P<0.001) and their interaction term (P<0.001).
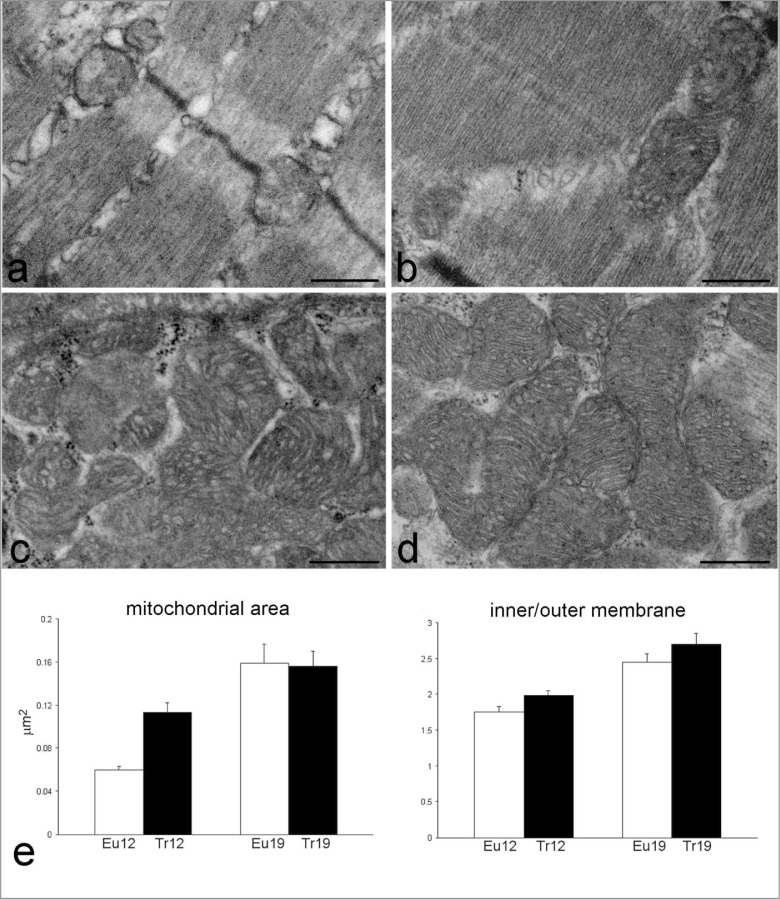
In all trisomic mice as well as aging euploid animals (Fig. 1b) clusters of large subsarcolemmal and intermyofibrillar mitochondria occurred, which were not present in euploid adult animals; morphometry (twoway ANOVA, Fig. 3) of intermyofibrillar mitochondria showed a significant effect of the “trisomy“ (P=0.043) and “age“ (P<0.001) factors, and their interaction term (P=0.035), on mitochondrial area. For the extension of the inner/outer membrane, the “trisomy” (P=0.024) and “age” (P=0.001) factors showed a significant effect, but their interaction term did not.
Figure 3.
Representative transmission electron micrographs of myofibres from adult euploid (a), adult trisomic (b), aging euploid (c) and aging trisomic (d) mice. Bars: 500 nm. (e) Results of morphometrical analysis on mitochondria: mitochondrial area and inner/outer membrane ratio (mean±SE) in 12- and 19 month-old euploid (Eu) and trisomic (Tr) mice. Two-way ANOVA revealed significant effect of the factors “trisomy” (P=0.043), “age” (P<0.001) and their interaction term (P=0.035) on the mitochondrial area; for the inner/outer membrane ratio significant effects were found for the factor “trisomy” (P=0.024) and “age” (P<0.001).
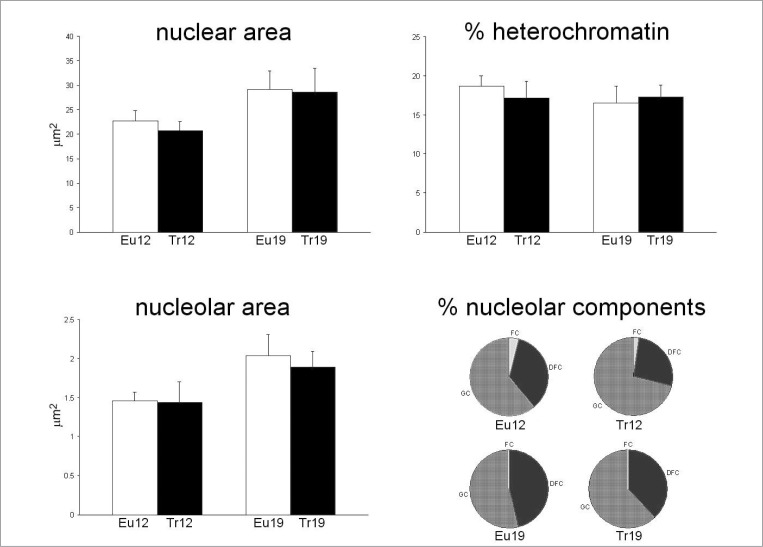
As for the myonuclei, morphometrical analysis showed similar percentages of heterochromatin in all groups; however, several differences were found in nuclear structures among the experimental groups (Fig. 4). In detail, the nuclear area significantly increased with age in both trisomic and euploid mice (P=0.006), nucleolar area increased as well (P=0.025); instead, the percentage of fibrillar centre area (where the rDNA is located) decreased with age (P=0.007), associated to an increase (P<0.001) in the percentage of the dense fibrillar component area (which is involved in the synthesis of rRNA). The “trisomy” factor also showed a significant effect on the percentage of dense fibrillar component area (P=0.002) which was lower in trisomic than in euploid animals. Instead, the percentage of granular component area (where maturation and accumulation of the pre-ribosomes take place before the ribosomal subunits being exported to cytoplasm) significantly increased in all trisomic animals (P=0.005).
Figure 4.
Electron microscopy morphometry of myonuclei of quadriceps muscles from 12- and 19 month-old euploid (Eu) and trisomic (Tr) mice (mean±SE). Two-way ANOVA revealed significant effect of the “age” factor on nuclear area (P=0.006), nucleolar area (P=0.025), and the relative areas of fibrillar centres (FCs, P=0.007) and dense fibrillar component (DFC, P<0.001). The “trisomy” factor showed significant effect on dense fibrillar component and granular component (GC) relative area (P=0.002 and P=0.005, respectively). No significant difference was found for heterochromatin percentage.
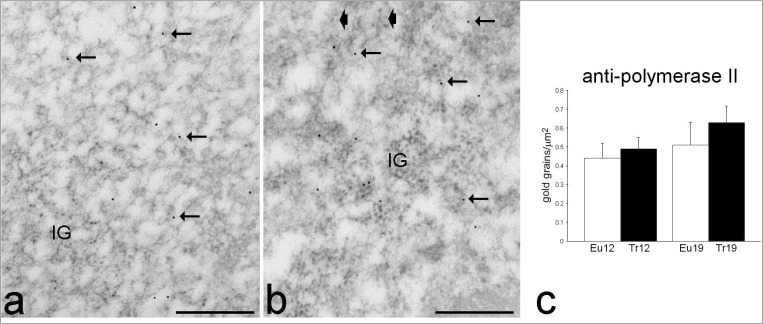
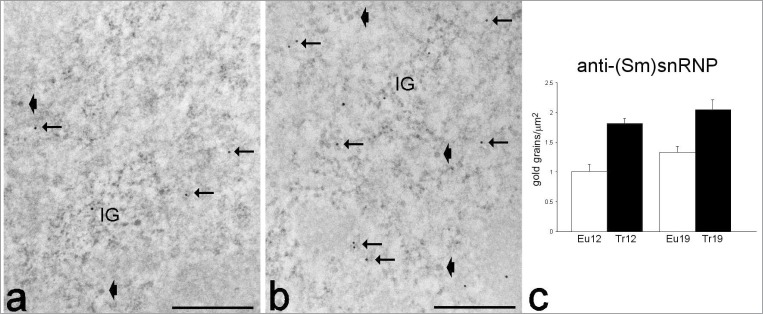
The nucleoplasmic RNP-containing components involved in mRNA transcription, processing and export (i.e., perichromatin fibrils, interchromatin granules, and perichromatin granules) were normally distributed in the nuclei of all mice. Consistently, the distribution of the anti-polymerase II and anti-(Sm)snRNP labelling was similar in all animals: both polymerase II (Fig. 5) and the Sm antigen (which is active in early splicing) (Fig. 6) were found on perichromatin fibrils (representing the sites where mRNA transcription and co-transcriptional splicing occur), and on interchromatin granules (the storage and modification sites of transcription and splicing factors). Quantitative evaluation of the immunolabelling showed that polymerase II was not affected by trisomy or aging, whereas the amount of the Sm antigen significantly increased in all trisomic as well as aging euploid mice.
Figure 5.
Transmission electron micrographs of myonuclei from adult euploid (a) and adult trisomic (b) mice; immunolabelling with anti-polymerase II antibody. The gold labelling is present on perichromatin fibrils (arrows) and interchromatin granules (IG). Arrowheads, perichromatin granules. Bars: 500 nm. (c) Quantitative evaluation of anti-polymerase II labelling density in 12- and 19 month-old euploid (Eu) and trisomic (Tr) mice (mean±SE). Two-way ANOVA did not reveal significant differences.
Figure 6.
Transmission electron micrographs of myonuclei from adult euploid (a), adult trisomic (b) mice; immunolabelling with anti-(Sm)snRNP antibody. The gold labelling occurs on perichromatin fibrils (arrows) and interchromatin granules (IG). Arrowheads, perichromatin granules. Bars: 500 nm. (c) Quantitative evaluation of anti-(Sm)snRNP density in 12- and 19 month-old euploid (Eu) and trisomic (Tr) mice (mean±SE). Two-way ANOVA revealed significant effect of the “trisomy” (P=0.006) and “age” factor (P<0.001).
Background values were negligible for both antibodies (not shown).
Discussion
The results obtained in the present study by combining ultrastructural morphology and morphometry with immunoelectron microscopy demonstrate that, in Ts65Dn mice, both the trisomic condition and aging promote structural changes in quadriceps muscle fibres. To our knowledge, this is the first structural characterization of skeletal muscle in this mouse strain which shows muscle weakness and motor alterations 8,9 comparable to those found in humans with DS, thus representing a suitable model to investigate the basic mechanisms of DS-associated motor dysfunction.
Previous observations10 have demonstrated that trisomy does not affect muscle mass in young mice; however, our results demonstrate that the age-related atrophy is more pronounced in aging trisomy animals than in age-matched euploid controls, some reduction in cross sectional area of myofibres already occurring in adult trisomic mice, thus suggesting that trisomy is associated with an earlier onset of sarcopenia in this mouse strain. Consistently, the ultrastructural analysis of myofibres of trisomic mice showed evident structural changes in the mitochondria and cell nuclei reminiscent of age-related alterations.
In fact, it is known that the size of mitochondria increases with aging in myofibres11, probably due to an imbalance in the mechanisms involved in mitochondrial fusion and fission15 as well as in inner membrane development16. Our results suggest that a similar dysfunction could occur in trisomic mice already in adulthood: actually mitochondria were found to be enlarged in 12 months old Ts65Dn mice vs. age-matched controls. The increase in size and in cristae extension of mitochondria may not correspond to higher organelle efficiency; accordingly, alterations in glucose and fat metabolism and in ATP biosynthesis suggestive of mitochondrial functional limitations have been reported in skeletal muscle of Ts65Dn mice10. This is consistent with the lower basal VO217 found in these mice as well as the mitochondrial dysfunctions reported in brain tissue from DS patients18.
Aging is likely involved in the increase in size of nuclear and nucleolar areas which, on the contrary, do not seem to be affected by trisomy. It has already been reported19 that nuclear activity slows down during aging, and this may account for the observed increase in size of nuclei and nucleoli in euploid and trisomic aging mice. Indeed, a decline in the RNA processing and export rate would determine accumulation in their functional sites of the involved nuclear structures and factors, with a concomitant enlargement of nucleoplasm and nucleoli; in the latter, the dense fibrillar component (where of rRNA synthesis and maturation take place) especially enlarges, whereas a decrease in size occurs in the fibrillar centres, where rDNA is located20,21. Consistently, (Sm)snRNPs accumulate in the nucleoplasm of aging mice as a consequence of the nuclear retention of transcripts in maturation phase, whereas the amount of heterochromatin and polymerase II does not change suggesting that the mRNA transcriptional rate is unaffected by age. It is worth noting that nuclear accumulation of (Sm)snRNPs was already evident in 12 month-old trisomic mice, thus suggesting again an early aging process.
A similar nucleoplasmic accumulation of (Sm)snRNPs has been already demonstrated in hepatocytes22 and in skeletal muscle satellite cells of aged rat23, and has been mainly related to alterations of the degradation systems, which is responsible for the accumulation of crosslinked, insoluble and oxidized proteins impairing the molecular transport mechanisms in aging cells24. The higher amount in trisomic mice of the nucleolar granular component, where the ribosomal subunits are stored before being exported the cytoplasm, confirms that the nuclear export as a whole is impaired in the myocyte nuclei of aging trisomic mice consistent with the accumulation of oxidized proteins found in skeletal muscle of this mouse strain10.
Previous reports in Wistar rats and Balb-c mice showed a significant decrease in nuclear size, and polymerase II and (Sm)snRNP amounts in the skeletal muscle from very old animals (28 months)12,25; however, these animals showed severe sarcopenia, their muscles containing atrophic myofibres with small nuclei and reduced transcriptional activity, whereas in the present study we investigated 19 month-old mice at an early phase of sarcopenia and of the general aging process.
In conclusion, the data on the myofibres of TsDn65 mice suggest that trisomy leads to some morpho-functional modifications of the quadriceps muscle which are reminiscent of those observed in age-related sarcopenia, thus supporting the view that the Down syndrome is characterized by a multi-systemic early aging2,3.
In this pilot study, where quite demanding experimental methods have been used, a small number of animals have only been considered; the original results reported, however, demonstrate that structural sarcopenia-related alterations in the myofibres of trysomic mice exist, and this provides an initial experimental basis for future investigations.
Previous studies performed by our group on the effect of mild physical exercise on some skeletal muscles of normal mice demonstrated that the RNA transcription, splicing, and export to the cytoplasm in the myonuclei of type II fibers can be reactivated even in very old mice25, while mitochondria undergo significant reduction in size and inner membrane length (unpublished results). This suggests that prevention of the sarcopenic drive can be obtained also in severely sarcopenic individuals and should stimulate further studies on the cellular bases of neuromuscular dysfunction in DS, in the attempt to design protocols of non-pharmacological therapy to attenuate the progressive motor disability and improve life quality of adult and aged persons with DS.
Acknowledgments
M.C. is a PhD student of the Doctoral Program “Multimodal Imaging in Biomedicine” (University of Verona).
References
- 1.Latash ML, Kang N, Patterson D. Finger coordination in persons with Down syndrome: atypical patterns of coordination and the effects of practice. Exp Brain Res. 2002;146:345–355. doi: 10.1007/s00221-002-1189-3. [DOI] [PubMed] [Google Scholar]
- 2.Roth GM, Sun B, Greensite FS, Lott IT, Dietrich RB. Premature aging in persons with Down syndrome: MR findings. AJNR Am J Neuroradiol. 1996;17:1283–1289. [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura E, Tanaka S. Biological ages of adult men and women with Down’s syndrome and its changes with aging. Mech Ageing Dev. 1998;105:89–103. doi: 10.1016/s0047-6374(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 4.Overend TJ, Cunningham DA, Kramer JF, Lefcoe MS, Paterson DH. Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol. 1992;47:M204–M210. doi: 10.1093/geronj/47.6.m204. [DOI] [PubMed] [Google Scholar]
- 5.Angelopoulou N, Matziari C, Tsimaras V, Sakadamis A, Souftas V, Mandroukas K. Bone mineral density and muscle strength in young men with mental retardation (with and without Down syndrome) Calcif Tissue Int. 2000;66:176–180. doi: 10.1007/s002230010035. [DOI] [PubMed] [Google Scholar]
- 6.Thompson LD. Age-related muscle dysfunction. Exp Gerontol. 2009;44:106–111. doi: 10.1016/j.exger.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Malatesta M, Meola G. Structural and functional alterations of the cell nucleus in skeletal muscle wasting:the evidence in situ. Eur J Histochem. 2010;54:e44. doi: 10.4081/ejh.2010.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa ACS, Walsh K, Davisson MT. Motor dysfunction in a mouse model for Down syndrome. Physiol Behav. 1999;68:211–220. doi: 10.1016/s0031-9384(99)00178-x. [DOI] [PubMed] [Google Scholar]
- 9.Costa AC, Stasko MR, Schmidt C, Davisson MT. Behavioral validation of the Ts65Dn mouse model for Down syndrome of a genetic background free of the retinal degeneration mutation Pde6b(rd1) Behav Brain Res. 2010;206:52–62. doi: 10.1016/j.bbr.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cowley PM, Keslacy S, Middleton FA, et al. Functional and biochemical characterization of soleus muscle in Down syndrome mice: insight into the muscle dysfunction seen in the human condition. Am J Physiol Regul Integr Comp Physiol. 2012;303:R1251–1260. doi: 10.1152/ajpregu.00312.2012. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Rev. 1997;77:425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- 12.Malatesta M, Perdoni F, Muller S, Zancanaro C, Pellicciari C. Nuclei of aged myofibres undergo structural and functional changes suggesting impairment in RNA processing. Eur J Histochem. 2009;53:97–106. doi: 10.4081/ejh.2009.e12. [DOI] [PubMed] [Google Scholar]
- 13.Lexell J. Human aging, muscle mass, and fibre type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 14.Picard M, White K, Turnbull DM. Mitochondrial morphology, topology, and membrane interactions in skeletal muscle: a quantitative three-dimensional electron microscopy study. J Appl Physiol. 2013;114:161–171. doi: 10.1152/japplphysiol.01096.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peterson CM, Johannsen DL, Ravussin E. Skeletal muscle mitochondria and aging: a review. J Aging Res. 2012;2012:194821. doi: 10.1155/2012/194821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannella CA. Structure and dynamics of the mitochondrial inner membrane cristae. Biochim Biophys Acta. 2006;1763:542–548. doi: 10.1016/j.bbamcr.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Cefalu JA, Croom WJ, Jr, Eisen EJ, Jones EE, Daniel LR, Taylor IL. Jejunal function and plasma amino acid concentrations in the segmental trisomic Ts65Dn mouse. Growth Dev Aging. 1998;62:47–59. [PubMed] [Google Scholar]
- 18.Busciglio J, Pelsman A, Wong C, et al. Altered metabolism of the amyloid beta precursor protein is associated with mitochondrial dysfunction in Down’s syndrome. Neuron. 2002;33:677–688. doi: 10.1016/s0896-6273(02)00604-9. [DOI] [PubMed] [Google Scholar]
- 19.Nagata T, Ma H. Electron microscopic autoradiographic study on nucleic acid synthesis in amitotic hepatocytes of the aging mouse. Med Electron Microsc. 2003;36:263–271. doi: 10.1007/s00795-003-0224-1. [DOI] [PubMed] [Google Scholar]
- 20.Schwarzacher HG, Wachtler F. The nucleolus. Anat Embryol (Berl) 1993;188:515–536. doi: 10.1007/BF00187008. [DOI] [PubMed] [Google Scholar]
- 21.Cisterna B, Biggiogera M. Ribosome biogenesis: from structure to dynamics. Int Rev Cell Mol Biol. 2010;284:67–111. doi: 10.1016/S1937-6448(10)84002-X. [DOI] [PubMed] [Google Scholar]
- 22.Malatesta M, Biggiogera M, Cisterna B, Balietti M, Bertoni-Freddari C, Fattoretti P. Perichromatin fibrils accumulation in hepatocyte nuclei reveals alterations of pre-mRNA processing during ageing. DNA Cell Biol. 2010;29:49–57. doi: 10.1089/dna.2009.0880. [DOI] [PubMed] [Google Scholar]
- 23.Malatesta M, Perdoni F, Muller S, Pellicciari C, Zancanaro C. Pre-mRNA processing is partially impaired in satellite cell nuclei from aged muscles. J Biomed Biotechnol. 2010;2010:410405. doi: 10.1155/2010/410405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jameson CW. Towards a unified and interdisciplinary model of ageing. Med Hypotheses. 2004;63:83–86. doi: 10.1016/j.mehy.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 25.Malatesta M, Fattoretti P, Giagnacovo M, Pellicciari C, Zancanaro C. Physical training modulates structural and functional features of cell nuclei in type II myofibers of old mice. Rejuvenation Res. 2011;14:543–552. doi: 10.1089/rej.2011.1175. [DOI] [PubMed] [Google Scholar]