Abstract
Therapeutic strategy for cardiac arrhythmias has undergone a remarkable change during the last decades. Currently implantable cardioverter defibrillator therapy is considered to be the most effective therapeutic method to treat malignant arrhythmias. Some even argue that there is no room for antiarrhythmic drug therapy in the age of implantable cardioverter defibrillators. However, in clinical practice, antiarrhythmic drug therapies are frequently needed, because implantable cardioverter defibrillators are not effective in certain types of arrhythmias (i.e. premature ventricular beats or atrial fibrillation). Furthermore, given the staggering cost of device therapy, it is economically imperative to develop alternative effective treatments.
Cardiac ion channels are the target of a number of current treatment strategies, but therapies based on ion channel blockers only resulted in moderate success. Furthermore, these drugs are associated with an increased risk of proarrhythmia, systemic toxicity, and increased defibrillation threshold. In many cases, certain ion channel blockers were found to increase mortality. Other drug classes such as β-blockers, angiotensin-converting enzyme inhibitors, aldosterone antagonists, and statins appear to have proven efficacy for reducing cardiac mortality. These facts forced researchers to shift the focus of their research to molecular targets that act upstream of ion channels. One of these potential targets is calcium/calmodulin-dependent kinase II (CaMKII).
Several lines of evidence converge to suggest that CaMKII inhibition may provide an effective treatment strategy for heart diseases. (1) Recent studies have elucidated that CaMKII plays a key role in modulating cardiac function and regulating hypertrophy development. (2) CaMKII activity has been found elevated in the failing hearts from human patients and animal models. (3) Inhibition of CaMKII activity has been shown to mitigate hypertrophy, prevent functional remodeling and reduce arrhythmogenic activity.
In this review, we will discuss the structural and functional properties of CaMKII, the modes of its activation and the functional consequences of CaMKII activity on ion channels.
Keywords: Heart, calmodulin kinase, arrhythmia, hypertrophy, ion channels
1. BACKGROUND
Cardiac arrhythmias are the leading cause of death in the Western world. The prevalence of lethal arrhythmias is not precisely known but can be estimated from the incidence of cardiac arrest and sudden cardiac death. According to the American Heart Association statistics, sudden cardiac death caused by malignant ventricular arrhythmias is responsible for more than 300,000 mortalities in the United States alone. The risk for malignant ventricular arrhythmias is higher in patients with structural heart disease or heart failure. Although not all forms of arrhythmias are lethal, some can be debilitating. Atrial fibrillations are linked to high incidence of stroke, and the risk increases with aging. Atrial fibrillations and ventricular ectopies are common in patients who have myocardial ischemia, cardiomyopathy or heart failure. Sometimes arrhythmias also suddenly occur in people without any known pathological background. The prevalence of the non-malignant arrhythmias is surprisingly high. In a study conducted in 1992, 33% of subjects who had no previously known heart disease presented premature ventricular complexes.
Arrhythmias have been in the focus of cardiovascular research for several decades, yet the mechanisms underlying the generation of premature heartbeats remain unclear. Earlier studies focused on individual ion channels to identify the factor(s) responsible for irregular heartbeats. However, the therapeutic strategies based on single channel targeting have proven to be ineffective or even proarrhythmic. Therefore new therapeutic strategies are urgently needed.
One promising candidate for new therapeutic target is Ca2+/calmodulin dependent protein kinase II (CaMKII). CaMKII plays a pivotal role in many regulatory processes of cardiac myocyte. In this article we provide a succinct review of the current state of knowledge on the physiologic and pathologic role of CaMKII as well as its therapeutic potential.
2. MOLECULAR BIOLOGY OF CaMKII
CaMKIIs are serine/threonine kinases that modulate many substrates. CaMKIIδ is the predominant isoform in the heart [1]. Two splice variants δB and δC have been detected at the protein level in the adult mammalian myocardium [2, 3]. The δB isoform contains an 11 amino acid Nucleus Localization Signal that is absent from δC; hence, δB is localized in the nucleus and δC is localized in cytosol and cell membrane. Recent studies also suggest that both isoforms can be found in the nucleus and the cytosol compartments, due to heteromultimerization of the different isoforms into the holoenzyme (each composed of 6–12 CaMKII monomers) [4]. Both δB and δC are activated by the Ca2+/calmodulin binding that trigger the autophosphorylation of CaMKIIδ at Thr286/287 [5].
2.1. Structure and Function
CaMKII forms a three dimensional cogwheel or cartwheel like superstructure in the cell built from 8–12 monomers linked by the C terminus [1, 6]. Each monomer consists (from N terminus to C) of a catalytic region with ATP and substrate binding site, a regulatory region with a calmodulin binding, an autoinhibitory domain and an association region [7]. Without activation, the autoinhibitory domain blocks the catalytic domain preventing it from substrate binding. When it is activated, the conformation change will free the catalytic region to transfer phosphate from ATP to substrates. Sustained activation signal can induce intersubunit autophosphorylation of the enzyme allowing for maintained activity even after activation signal is ceased. The autophosphorylated enzyme shows marked differences in deactivation kinetics. When CaMKII is not autophosphorylated, the enzyme releases the Ca2+/calmodulin complex and inactivates rapidly when cytosolic Ca2+ concentration drops down to resting level. In contrast to this rapid inactivation, calmodulin stays bound to the autophosphorylated enzyme (calmodulin trapped state) for long time independently from the Ca2+ concentration [5, 8]. This sustained activity in the absence of stimulatory signal (diastolic cytosolic Ca2+ concentration) explains why CaMKII is called a ‘memory molecule’ [1]. CaMKII integrates stimulatory signals and maintains its activity according to the Ca2+ concentration it was exposed to previously (‘remembers’). Autophosphorylated CaMKII can be dephosphorylated by phosphatases PP2A and PP1 [9].
Activated CaMKII can phosphorylate, thus modulate the function of wide array of proteins. According to their differential localization, δB modulates gene transcription and hypertrophic growth, and δC modulates ion channels and Ca2+ handling molecules in the E-C coupling machinery of cardiac muscle.
2.2. Regulation of CaMKII
Ca2+/Calmodulin dependent activation is the central pathway to activate CaMKII. Ca2+/Calmodulin complex binds to the regulatory region of the monomer and induces conformation changes which will remove the inhibitory effect of the regulatory domain on the kinase domain resulting in activation of the enzyme. The activated enzyme can phosphorylate various target proteins including other subunits of the same holoenzyme. CaMKII is shown to be localized in close proximity of L-type Ca2+ channels in cardiac myocardium [10–12]. The role of Ca2+ entering via L-type channel in activation of CaMKII is not completely understood. CaMKII activation has been shown to be independent from extracellular Ca2+ concentration in guinea pig heart [13, 14]. On the other hand, activation of L-type Ca2+ channels by S(−)-Bay K8644 was shown to activate CaMKII in cultured neonatal rat cardiomyocytes [15]. This activation is attenuated by depletion of intracellular Ca2+ stores, which indicate that Ca2+ entry via L-type channels can be an initial event in the activation of CaMKII but Ca2+ release from sarcoplasmic reticulum is also required [15]. Cytosolic Ca2+ concentration is influenced by several factors (channels, pumps, Ca2+ stores) in cardiac myocytes. CaMKII responding to both magnitude and frequency of Ca2+ signal integrates these signals targeting numerous cytosolic and membrane bound proteins.
The δB isoform of CaMKII is localized in the nucleus and has been implicated in the regulation of various transcription factors leading to structural heart disease. The nucleus, surrounded by a double membrane (nuclear envelope) is separated from the cytoplasm. This nuclear envelope contains nuclear pores having diameter of 9 nm, which is relatively large compared to the ionic radius of Ca2+ allowing for free diffusion. However, experimental evidences show that conductance through pores can be significantly reduced by nuclear accumulation of Ca2+ or by macromolecules blocking pores [16]. The significant diffusion barrier may explain that why nuclear Ca2+ changes much slower than the cytosolic Ca2+ concentration [17–19]. The concentration of Ca2+ in the cytosol and nucleus might be similar in non excitable cells or at resting state excitable cell, but can become very different during excitation in cardiac myocytes. Since the elements of the machinery required for independent control of nuclear Ca2+ concentration has been shown to exist in the nuclear membrane in several cell types [1, 20], it is likely that the nucleus of cardiac myocytes forms a Ca2+ microdomain which is linked to the cytoplasm, but regulated by independent mechanisms.
Situated at the pivotal point of Ca2+ signaling pathway CaMKII is a mediator of several intracellular signaling pathways upstream to Ca2+, including catecholamine or angiotensin receptor signaling. During β-adrenergic stimulation Ca2+ concentration increases in both dyadic cleft and nuclear envelope, which may activate CaMKII [21, 22]. Other observations suggest that CaMKII can be activated by cAMP activated exchange protein (EPAC) in PKA/Ca2+ independent manner [23, 24]. CaMKII also targets many of the same proteins as PKA (L-type Ca2+ channel, phospholamban, ryanodine receptor… etc), and hence forms a signaling pathway in parallel with PKA. The details of this parallel signaling are not yet completely understood, but complexity of the system is shown by the observations that although CaMKII inhibition or gene deletion has very little (if any) effects on physiologic cardiac function it can prevent cardiac diseases induced by β-adrenergic stimulation [25]. CaMKII was also shown to mediate α-adrenergic signaling. KN-93, an inhibitor of CaMKII, was reported to prevent phenylephrine induced reduction of IK1 in canine myocardium [26], indicating a role of CaMKII in cathecolaminergic signalization.
CaMKII was found to play a critical role in renin-angiotensin-aldosterone signaling pathway in non cardiac cells [27]. Recently Zhao et al. reported that Angiotensin II activated CaMKII and induced KN-93 suppressible afterdepolarizations in rabbit ventricular cells [28].
Besides these signaling pathways other factors can also activate CaMKII. Acidification of the cytoplasma has been associated with elelvated CaMKII activity [29, 30] and inhibition of CaMKII reduced ventricular tachycardia and spontaneous action potentials in Langendorff-perfused heart [31]. Importantly, changes in the redox state of the cell can activate CaMKII as well. Oxidation of Met residues (Met281/282) located in the regulatory domains results in persistent activation of the enzyme similar to that seen in autophosphorylated state [32, 33]. CaMKII activation by oxidation requires initial Ca2+/calmodulin binding, but can shift the Ca2+ dependence to low levels even towards the diastolic Ca2+ concentration [34]. This would cause elevated basal level CaMKII activity under oxidative stress.
Endocrine state has been reported to modulate CaMKII in non cardiac tissues. Downregulation of the expression and function of the enzyme was observed in skeletal muscle of hyperthyroid rabbits [35], while elevated expression level with increased activity was reported from diabetic rat brain [36].
2.3. Pharmacology of CaMKII
The great potential of using CaMKII as a therapeutic target stems from the central position that CaMKII occupies in the cell signaling network. CaMKII is a Ca2+ signal transducer situated at a converging point for multiple signaling pathways that are known to induce cardiac hypertrophy, arrhythmias and heart failure [1, 7]. CaMKII inhibition has shown beneficial effects on mitigating heart disease development and arrhythmias in animal models suggesting a promising and powerful new strategy for further exploration of drug treatment.
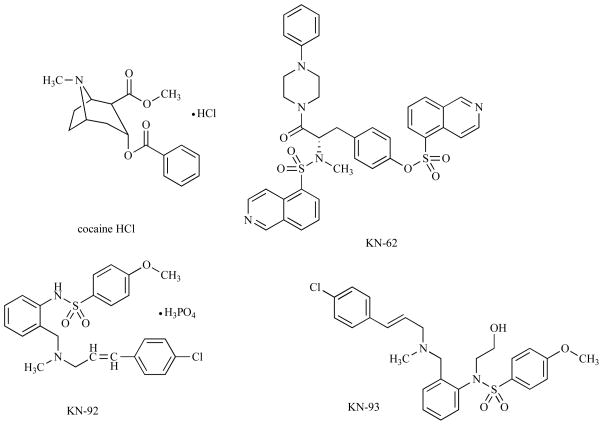
However, the number of drugs available for modulating CaMKII activity is surprisingly low. Fig. (1) and Table 1 summarize the pharmacological agents used in experiments to modify CaMKII activity. The only drug reported to increase CaMKII activity in cardiac myocyte is cocaine [1], but cocaine is not a “pure” activator of CaMKII, it also has several other effects on cardiac myocytes.
Fig. (1).
Structures of organic compounds that modulate CaMKII.
Table 1.
List of Organic Compounds Used in Cardiac Research Modulating CaMKII activity. Cocaine is the Only Known Exogenous Activator; all other Listed Compounds are Inhibitors. Amino Acid Sequence of Peptide Inhibitors are Shown in Brackets. KN-92 and AC3-C are Inactive (Non Inhibitory) Analogues of KN-93 and AC3-I Respectively
| Drug | Effective concentration |
|---|---|
| cocaine | 1 μmol/L [1] |
| KN-62 | 10 μmol/L [79] |
| KN-92 | 1 μmol/L [80] |
| KN-93 | 1 μmol/L [39], [80] |
| AIP (Auto-camtide-2-related inhibitory peptide) [KKALRRQEAVDAL] | 2 μmol/L [44] |
| CK291–317* [KKRNARRKLKGAILTTMLATRNFSVGK] | 10–20 μmol/L [45],[1] |
| CK281–302* [MHRQETVDCLKKRNARRKLKGA] | 10–20 μmol/L [45],[1] |
| AC3-I* (Auto-camtide-3-inhibitor) [KKALHRQEAVDCL] | 10–20 μmol/L [45],[46] |
| AC3-C* (Auto-camtide-3-control) [KKALHAQERVDAL] | 10–20 μmol/L [45] |
denotes non membrane permeable agents.
CaMKII inhibitors successfully prevented development of cardiac structural remodeling [37, 38] and arrhythmia [39] in heart disease models. Unfortunately, the number of membrane permeable CaMKII inhibitors is very low (see Fig. 1 and Table 1). KN-93 is the most frequently used organic CaMKII inhibitor which has provided essential information on the physiological and pathological role of CaMKII in the heart.
Nevertheless, KN-93 was found to block several voltage gated K+ channels in the heart [31, 40]. Hence, the effect of KN-93 on the action potential cannot be solely attributed to CaMKII inhibition. The regulatory role of CaMKII on sarcolemmal ionic channels and action potential need to be further studied using more specific inhibitors of CaMKII.
In recent publications in Bioorganic and Medicinal Chemistry Letters synthesis of a new class of CaMKII inhibitors was reported [42, 43]. Using KN-93 (one of the most popular CaMKII inhibitor) as starting point, a new putative structure (5,6,7,8-tetrahydropyrido[ 4,3-d]pyrimidin) was identified by high throughput screening, then more than 30 daughter derivatives tested with enzyme assays against other kinases. Several derivatives of this new class were found to be highly selective to CaMKII in these reports, but further studies and tests are needed to examine the side effects of the new compounds.
A different approach was used in studies utilizing highly specific peptide-based inhibitors to investigate CaMKII function [1, 44–46]. In most cases these small peptides are impermeable to cell membrane and difficult to deliver into the cell. Myristoylation makes AIP membrane permeable [44], but all other inhibitory peptides require microinjection or microporation for delivering into the cell. To circumvent this difficulty, transgenic mice expressing AIP, AC3-I (both of them are inhibitory peptides) and AC3-C (inactive analog of AC3-I) have been used to provide valuable information on the role of CaMKII in structural heart diseases and arrhythmia [37, 47].
3. ALTERED CaMKII FUNCTION IN CARDIAC DISEASE
Increases in CaMKII expression and activity were reported from different structural and functional heart diseases in human and animal models [7, 48]. CaMKII modulates many proteins and plays an integrative role in the adaptation and remodeling of cardiac myocytes during heart disease development. Excessive activation of signaling pathways upstream to CaMKII (e.g. catecholaminergic or renin-angiotensin systems) are known to be detrimental and β blockers or angiotensin converting enzyme (ACE) inhibitors are proven to ameliorate heart diseases. CaMKII inhibition is also shown to prevent pathologic remodeling of the heart.
3.1. Structural Heart Diseases and Heart Failure
The chronic effects of CaMKII activation are more complicated than the acute effects, because many changes in Ca2+ handling molecules and modulators occur during prolonged exposure to elevated CaMKII activity. Localized in the nucleus, CaMKIIδB causes HDAC phosphorylation and translocation [4,49] which modulates transcription factors including MEF2 [4], NFκB [50] and CREB [51] to trigger gene transcription and hypertrophic growth in cardiomyocytes. Over-expression of CaMKIIδB in transgenic mouse is sufficient to cause cardiac hypertrophy, chamber dilation and impaired heart function [52]. CaMKIIδC, although preferentially expressed in the cytosol, can also cause HDAC4 translocation and MEF2 activation and induction of hypertrophic gene expression [4]. It is hypothesized that CaMKIIδC might form heteromultimer with CaMKIIδB and thus enter the nucleus. Alternatively, CaMKIIδC might phosphorylate HDAC in the cytosol and activate MEF2. CaMKII is also implicated in cardiac remodeling leading to ventricular dilation and abnormal contractile function [53]. Abnormal activation of β-adrenergic pathway via EPAC can lead to necrotic and apoptotic cell death [54, 55]. This signaling cascade involves CaMKII dependent phosphorylation of proteins in the sarcoplasmic reticulum (SR), Ca2+ overload of mitochondria and caspase 3 activation [54]. The resultant cardiomyopathy and heart failure is characterized by impaired contractility and reduced ejection fraction. Experimental data show that contractility of failing myocardium can be improved by inhibition of CaMKII [56].
3.2. Cardiac Arrhythmias
Cardiac electric activity is governed by concerted action of more than twenty ion channels in cardiac cells. Disruption of the delicate balance of these ionic currents can lead to cardiac arrhythmias. Many of the ion channels are modulated by CaMKII. Apart from modulating ion channel activities, CaMKII also regulates the expression of channel subunits. CaMKII (and CaMKIV) upregulation is associated with increased propensity of arrhythmias in structural heart diseases. Inhibition of CaMKII has been shown to attenuate the development of structural heart disease and arrhythmias [37, 39].
3.2.1. Voltage Gated Calcium Channel
In cardiac muscle, Ca2+ entry through the sarcolemmal L-type Ca2+ channel (LTCC) during the action potential triggers Ca2+ release from sarcoplasmic reticulum (SR) via the ryanodine receptor, causing muscle contraction. The L-type Ca2+ current (ICa,L) is the main depolarizing current for maintaining action potential plateau; it also plays a central role in regulating the Ca2+ homeostasis of cardiac myocytes in both physiologic and pathologic conditions. LTCC is regulated by numerous signaling pathways, including PKA, PKC and CaMKII [57].
CaMKII binds to the α1C and β2a subunits of LTCC and phosphorylates multiple sites. This phosphorylation profoundly alters the gating of LTCC. Rate of recovery from inactivation of the channel determines its response to frequency changes. Without CaMKII phosphorylation, high frequency (>1 Hz) activation of LTCC results in reduction of the magnitude of ICa,L (called negative staircase phenomenon). When LTCC is phosphorylated by CaMKII, the channel switches to a high activity mode (mode-2) [58]. In this mode high frequency stimulation results in enhancement of the magnitude of ICa,L, a phenomenon called Ca2+ induced (or use dependent) facilitation of LTCC [44]. This facilitation causes increased magnitude of ICa,L during consecutive stimulation (called positive staircase phenomenon) [59].
Therefore, increased CaMKII activity in cardiac diseases can increase the Ca2+ influx and cause SR Ca2+ overload. Increased ICa,L and maximal activation of LTCC by CaMKII were reported in animal model of heart failure [59]. SR Ca2+ overload is known to increase the spontaneous Ca2+ waves giving rise to delayed afterdepolarizations (DAD) [60]. Prolonged opening of LTCC due to mode-2 gating and/or accelerated recovery from inactivation can increase the probability of early afterdepolarization (EAD) [61]. Both EAD and DAD are known to induce triggered action potentials and premature beats, leading to arrhythmogenic activity in the heart. Hence, CaMKII modulation of ICa,L plays an important role in the genesis of cardiac arrhythmias.
3.2.2. Voltage Activated Sodium Channel
Voltage activated Na+ channel is responsible for the upstroke of action potential (AP). Following activation, most channels inactivate within milliseconds, resulting in a rapid decay of the current. Although most channels remain in non-conductive state after inactivation, a small percentage of channels may remain open or recover from inactivation, causing a sustained ‘late Na+ current’ during the AP plateau phase. The molecular mechanism underlying the late Na+ current is not completely understood, however the presence of late Na+ current has been reported in a number of mammalian species including human heart [62–65]. The late Na+ current is small under physiological conditions, and its role in normal heart function is unclear. However, increased late Na+ current was observed in diseased hearts [66–68]. Recent experimental data suggest its possible involvement in arrhythmias. Increased Na+ influx may elevate the intracellular Na+ concentration which reduces the forward mode Na+/Ca2+ exchanger current, leading to SR Ca2+ overload and arrhythmias in failing hearts [65]. This inward Na+ current during the AP plateau phase may also contribute to prolonging the action potential and leading to arrhythmias.
Na+ channel is known to be modulated by CaMKII [69]. CaMKII phosphorylation exerts complex effects on the Na channel: steady state inactivation of the current is shifted to more negative potentials; recovery from inactivation is delayed and intermediate inactivation is facilitated. Importantly, CaMKII phosphrylation of Na+ channel slows down its fast inactivation, enhances the late Na+ current, and elevates the cytosolic Na+ concentration [69]. These changes have been observed in the failing hearts, and can be attributed to an upregulation of CaMKII in these hearts. Not surprisingly, inhibition of the late Na current has been proposed as a therapeutic strategy to preventing arrhythmias and normalizing E-C coupling in the heart [63–70].
3.2.3. Potassium Channels
Several K+ channels contribute to repolarizing the cardiac action potential. CaMKII is known to modulate the expression level and the gating kinetics of Ito, IK1 and IK,ATP [71–73]. In a transgenic mouse model of CaMKII inhibition (with AC3-I peptide expression), IK1 and Ito were found to be upregulated and their current densities elevated. Upregulation of K+ channels was shown to shorten the action potential duration and the QT interval on ECG. Interestingly, these changes were not seen when AC3-I was expressed in phospholamban knock out mouse [72].
CaMKII modulates the two components of Ito, Kv4.3 and Kv4.2, with different Ca2+ sensitivity. Elevated cytosolic Ca2+ concentration facilitates the association between these channels and CaMKII with resultant channel phosporylation. CaMKII can phosphorylate Kv4.3 at both diastolic and systolic Ca2+ concentration. However, Kv4.2 does not associate with CaMKII at diastolic Ca2+ concentration. Phosphorylation slows down channel inactivation in both types of channels. Inhibition of CaMKII with KN-93 accelerates inactivation of the fast component of Ito [74]. CaMKII can also modulate K+ channel function by interfering the regulatory pathways upstream to a given K+ channel. α1-adrenoceptor stimulation is known to reduce Ito and IK1 in cardiac myocytes. Inhibition of CaMKII with KN-93 was found to abolish α1-adrenoceptor induced reduction of IK1 but not Ito [26]. Special caution is needed in interpreting these data, because KN93 also directly affects several voltage gated channels besides inhibiting CaMKII [40, 41].
KATP channels serve pivotal function in the adaptation of cardiac myocytes to hypoxic conditions. Upregulation of IK,ATP was reported from transgenic mice with genetic inhibition (AC3-I) of CaMKII, which resulted in an improved cardiomyocyte survival after ischemia [75]. Furthermore, CaMKII was shown to mediate the effects of hypoxia in increasing IK,ATP [71].
3.2.4. Chloride Channels
Cardiac chloride channels, apart from contributing to shaping AP, are involved in regulating a large repertoire of cellular functions, including the intracellular pH, the cell volume, as well as apoptosis, differentiation and proliferation. Nevertheless, chloride channels are probably the least studied ion channels of the heart. Several subtypes of chloride channels were identified in the heart including CFTR, ClC-2, ClC-3, ClCA-1, Bestrophin and TMEM16, but the exact physiologic roles of these channels remain unclear. Since the equilibrium potential for chloride ion is estimated to be somewhere between −40 to −65 mV, chloride channels have the unique ability to generate both inward and outward currents depending on the membrane potential during AP [76]. When membrane potential is depolarized, chloride current is outward and contributes to repolarization; when membrane potential is close to the resting potential, chloride current turns inward and can depolarize the membrane.
Chloride channels are modulated by several signaling pathways including PKA, PKC, cAMP and Ca2+, suggesting their involvement in pathological conditions when changes occur in these signaling cascades. Ca2+ activated chloride channels are gated directly by Ca2+, but evidence (from non cardiac cells) also suggest involvement of CaMKII in modulating the channel gating [77]. CaMKII has been shown to be important in the recovery of contraction rate following inhibition of CFTR chloride channel in cultured neonatal cardiac myocytes [78]. The need for better understanding the role and the regulation of chloride channels in heart diseases will make cardiac chloride channels an exciting area of research in the coming years.
4. CONCLUDING REMARKS
In conclusion, experimental data and clinical observations show that abnormal activation of CaMKII plays a critically important role in the pathologic remodeling of the heart. Still, many questions on CaMKII function and modulation have yet to be answered. The role of altered CaMKII activity is well supported by studies of various heart diseases, but only sporadic reports are available regarding the benefits of targeting CaMKII as therapeutic strategy. The lack of specific inhibitors of CaMKII is unquestionably an important factor limiting the progress in the field. Another limiting factor comes from the tremendous complexity of the signaling pathways and regulatory processes that involve CaMKII. However, the urgent need to solve one of the greatest public health problems of the western world – cardiac arrhythmias and heart failure – will keep CaMKII in the focus of research in the forthcoming years.
Acknowledgments
This work was supported by National Institutes of Health USA (R01-HL90880, R03-AG031944) and Hungarian Research Fund (OTKA-K73160).
References
- 1.Tobimatsu T, Fujisawa H. Tissue-specific expression of four types of rat calmodulin-dependent protein kinase II mRNAs. J Biol Chem. 1989;264:17907–17912. [PubMed] [Google Scholar]
- 2.Edman CF, Schulman H. Identification and characterization of delta B-CaM kinase and delta C-CaM kinase from rat heart, two new multifunctional Ca2+/calmodulin-dependent protein kinase isoforms. Biochim Biophys Acta. 1994;1221:89–101. doi: 10.1016/0167-4889(94)90221-6. [DOI] [PubMed] [Google Scholar]
- 3.Hagemann D, Hoch B, Krause EG, Karczewski P. Developmental changes in isoform expression of Ca2+/calmodulin-dependent protein kinase II delta-subunit in rat heart. J Cell Biochem. 1999;74:202–210. [PubMed] [Google Scholar]
- 4.Zhang T, Kohlhaas M, Backs J, Mishra S, Phillips W, Dybkova N, Chang S, Ling H, Bers DM, Maier LS, Olson EN, Brown JH. CaMKIIδ Isoforms Differentially Affect Calcium Handling but Similarly Regulate HDAC/MEF2 Transcriptional Responses. J Bio Chem. 2007;282(48):35078–35087. doi: 10.1074/jbc.M707083200. [DOI] [PubMed] [Google Scholar]
- 5.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364:593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kolodziej SJ, Hudmon A, Waxham MN, Stoops JK. Three-dimensional reconstructions of calcium/calmodulin-dependent (CaM) kinase IIα, and truncated CaM kinase IIα reveal a unique organization for its structural core and functional domains. J Biol Chem. 2000;19:14354–14359. doi: 10.1074/jbc.275.19.14354. [DOI] [PubMed] [Google Scholar]
- 7.Zhang T, Brown JH. Role of Ca2+/calmodulin-dependent protein kinase II in cardiac hypertrophy and heart failure. Cardiovasc Res. 2004;63:476–486. doi: 10.1016/j.cardiores.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 8.Meyer T, Hanson PI, Stryer L, Schulman H. Calmodulin Trapping by Calcium-Calmodulin-Dependent Protein Kinase. Science. 1992;256:1199–1202. doi: 10.1126/science.256.5060.1199. [DOI] [PubMed] [Google Scholar]
- 9.Erickson JR, Anderson ME. CaMKII and Its Role in Cardiac Arrhythmia. Mol Persp. 2008;19:1332–1336. doi: 10.1111/j.1540-8167.2008.01295.x. [DOI] [PubMed] [Google Scholar]
- 10.Dzhura I, Wu Y, Colbran RJ, Corbin JD, Balser JR, Anderson ME. Cytoskeletal disrupting agents prevent calmodulin kinase, IQ domain and voltage-dependent facilitation of L-type Ca2+ channels. J Physiol. 2002;545:399–406. doi: 10.1113/jphysiol.2002.021881. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.O-Uchi J, Sasaki H, Morimoto S, Kusakari Y, Shinji H, Obata T, Hongo K, Komukai K, Kurihara S. Interaction of α1-adrenoceptor subtypes with different G proteins induces opposite effects on cardiac L-type Ca2+ channel. Circ Res. 2008;102(11):1378–1388. doi: 10.1161/CIRCRESAHA.107.167734. [DOI] [PubMed] [Google Scholar]
- 12.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171(3):537–547. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lindemann JP, Watanabe AM. Phosphorylation of phospholamban in intact myocardium. Role of Ca2+-calmodulin-dependent mechanisms. J Bil Chem. 1985;260(7):4516–4525. [PubMed] [Google Scholar]
- 14.Wegener AD, Simmerman HKB, Lindemann JP, Jones LR. Phospholamban phosphorylation in intact ventricles. Phosphorylation of serine 16 and threonine 17 in response to β-adrenergic stimulation. J Biol Chem. 1989;264(19):11468–11474. [PubMed] [Google Scholar]
- 15.Bartel S, Vetter D, Schlegel W-P, Wallukat G, Krause E-G, Karczewski P. Phosphorylation of phospholamban at threonine-17 in the absence and presence of β-adrenergic stimulation in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2000;32(12):2173–2185. doi: 10.1006/jmcc.2000.1243. [DOI] [PubMed] [Google Scholar]
- 16.Bustamante JO, Michelette ER, Geibel JP, Dean JDA, Hanover A, McDonnell TJ. Calcium ATP and nuclear pore channel gating. Pflugers Arch. 2000;439:433–444. doi: 10.1007/s004249900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badminton MN, Campbell AK, Rembold CM. Differential regulation of nuclear and cytosolic Ca2+ in HeLa cells. J Biol Chem. 1996;271:31210– 31214. doi: 10.1074/jbc.271.49.31210. [DOI] [PubMed] [Google Scholar]
- 18.Badminton MN, Kendall JM, Rembold CM, Campbell AK. Current evidence suggests independent regulation of nuclear calcium. Cell Calcium. 1998;23:79–86. doi: 10.1016/s0143-4160(98)90105-1. [DOI] [PubMed] [Google Scholar]
- 19.Lui PP, Kong SK, Fung KP, Lee CY. The rise of nuclear and cytosolic Ca2+ can be uncoupled in HeLa cells. Pflugers Arch. 1998;436:371–376. doi: 10.1007/s004240050645. [DOI] [PubMed] [Google Scholar]
- 20.Bootman MD, Fearnley C, Smyrnias I, MacDonald F, Roderick HL. An update on nuclear calcium signaling. J Cell Sci. 2009;122(14):2337–2350. doi: 10.1242/jcs.028100. [DOI] [PubMed] [Google Scholar]
- 21.Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+-cardiac myocytes. Biophys J. 2008;95(10):4597–4612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, Olson EN, Chen J, Brown JH, Bers DM. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116(3):675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Métrich M, Lucas A, Gastineau M, Samuel JL, Heymes C, Morel E, Lezoualc’h F. Epac mediates beta-adrenergic receptor-induced cardiomyocyte hypertrophy. Circ Res. 2008;102(8):959–965. doi: 10.1161/CIRCRESAHA.107.164947. [DOI] [PubMed] [Google Scholar]
- 24.Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase Cepsilon regulate Ca2+ release in the heart by activation of protein kinase Cepsilon and calcium-calmodulin kinase II. J Biol Chem. 2009;284(3):1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grimm M, Brown JH. β-Adrenergic receptor signaling in the heart: Role of CaMKII. J Mol Cel Cardiol. 2010;48:322–330. doi: 10.1016/j.yjmcc.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Yang B, Zhang Y, Han H, Wang J, Shi H, Wang Z. Different Subtypes of α1-Adrenoceptor Modulate Different K+ Currents via Different Signaling Pathways in Canine Ventricular Myocytes. J Biol Chem. 2001;44:40811–40816. doi: 10.1074/jbc.M105572200. [DOI] [PubMed] [Google Scholar]
- 27.Pezzi V, Clyne CD, Ando S, Mathis JM, Rainey WE. Ca2+- regulated expression of aldosterone synthase is mediated by calmodulin and calmodulin-dependent protein kinases. Endocrinology. 1997;138:835–838. doi: 10.1210/endo.138.2.5032. [DOI] [PubMed] [Google Scholar]
- 28.Zhao Z, Fefelova N, Shanmugam M, Bishara P, Babu GJ, Xie L-H. Angiotensin II induces afterdepolarizations via reactive oxygen species and calmodulin kinase II signaling. J Mol Cell Cardiol. 2010;50(1):128–136. doi: 10.1016/j.yjmcc.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeSantiago J, Maier LS, Bers DM. Phospholamban is required for CaMKII-dependent recovery of Ca2+ transients and SR Ca2+ reuptake during acidosis in cardiac myocytes. J Mol Cell Cardiol. 2004;36:67–74. doi: 10.1016/j.yjmcc.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 30.Komukai K, Pascarel C, Orchard CH. Compensatory role of CaMKII on ICa and SR function during acidosis in rat ventricular myocytes. Pflügers Arch. 2001;442:353–361. doi: 10.1007/s004240100549. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen TH, Gurung IS, Grace A, Huang1 CLH. Calmodulin kinase II initiates arrhythmogenicity during metabolic acidification in murine hearts. Acta Physiol. 2009;197:13–25. doi: 10.1111/j.1748-1716.2009.01991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O’Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462– 474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie L-H, Chen F, Karagueuzian HS, Weiss JN. Oxidative stress-induced afterdepolarizations and calmodulin kinase II signaling. Circ Res. 2009;104(1):79–86. doi: 10.1161/CIRCRESAHA.108.183475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palomeque J, Rueda OV, Sapia L, Valverde CA, Salas M, Petroff MV, Mattiazzi A. Angiotensin II-Induced Oxidative Stress Resets the Ca2+ Dependence of Ca2+-Calmodulin Protein Kinase II and Promotes a Death Pathway Conserved Across Different Species. Circ Res. 2009;105(12):1204–1212. doi: 10.1161/CIRCRESAHA.109.204172. [DOI] [PubMed] [Google Scholar]
- 35.Jiang M, Xu A, Jones DL, Narayanan N. Coordinate downregulation of CaM kinase II and phospholamban accompanies contractile phenotype transition in the hyperthyroid rabbit soleus. Amer J Physiol. 2004;287:C622–C632. doi: 10.1152/ajpcell.00352.2003. [DOI] [PubMed] [Google Scholar]
- 36.Bhardwaj SK, Kaur G. Effect of diabetes on calcium/calmodulin dependent protein kinase-II from rat brain. Neurochem Int. 1999;35(4):329–335. doi: 10.1016/s0197-0186(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 37.Khoo MSC, Grueter CE, Eren M, Yang J, Zhang R, Bass MA, Lwin ST, Mendes LA, Vaughan DE, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition disrupts cardiomyopathic effects of enhanced green fluorescent protein. J Mol Cell Cardiol. 2008;44:405–410. doi: 10.1016/j.yjmcc.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang R, Khoo MSC, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 39.Lo LW, Chen YC, Chen Y-J, Wongcharoen W, Lin Cheng-I, Chen S-A. Calmodulin kinase II inhibition prevents arrhythmic activity induced by alpha and beta adrenergic agonists in rabbit pulmonary veins. Eur J Pharm. 2007;571:197–208. doi: 10.1016/j.ejphar.2007.05.066. [DOI] [PubMed] [Google Scholar]
- 40.Anderson ME. KN-93, an inhibitor of multifunctional Ca++/calmodulin-dependent protein kinase, decreases early afterdepolarizations in rabbit heart. J Pharm Exp Ther. 1998;287(3):996–1006. [PubMed] [Google Scholar]
- 41.Rezazadeh S, Claydon TW, Fedida D. KN-93 (2-[N-(2-Hydroxyethyl)]- N-(4-methoxybenzenesulfonyl)]- amino-N-(4-chlorocinnamyl)-N-methylbenzylamine), a Calcium/Calmodulin-Dependent Protein Kinase II Inhibitor, Is a Direct Extracellular Blocker of Voltage-Gated Potassium Channels. J Pharm Exp Ther. 2006;317(1):292–299. doi: 10.1124/jpet.105.097618. [DOI] [PubMed] [Google Scholar]
- 42.Asano S, Komiya M, Koike N, Koga E, Nakatani S, Isobe Y. 5,6,7,8- Tetrahydropyrido[4,3-d]pyrimidines as novel class of potent and highly selective CaMKII inhibitors. Bioorg Med Chem Let. 2010;20(22):6696–6698. doi: 10.1016/j.bmcl.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 43.Komiya M, Asano S, Koike N, Koga E, Igarashi J, Nakatani S, Isobe Y. Structure and activity relationship of 2-(substituted benzoyl)- hydroxyindoles as novel CaMKII inhibitors. Bioorg Med Chem Let. 2011;21(5):1456–1458. doi: 10.1016/j.bmcl.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 44.Guo J, Duff HJ. Calmodulin kinase II accelerates L-type Ca2+ current recovery from inactivation and compensates for the direct inhibitory effect of [Ca2+]i in rat ventricular myocytes. J Physiol. 2006;574:509–518. doi: 10.1113/jphysiol.2006.109199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Y, Roden DM, Anderson ME. Calmodulin Kinase Inhibition Prevents Development of the Arrhythmogenic Transient Inward Current. Circ Res. 1999;84:906–912. doi: 10.1161/01.res.84.8.906. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y, Dzhura I, Colbran RJ, Anderson ME. Calmodulin kinase and a calmodulin-binding ‘IQ’ domain facilitate L-type Ca2+ current in rabbit ventricular myocytes by a common mechanism. J Physiol. 2001;535:679–687. doi: 10.1111/j.1469-7793.2001.t01-1-00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Pitch E, DeSantiago J, Huke S, Kaetzel MA, Dedman JR, Bers DM. CaMKII inhibition targeted to the sarcoplasmic reticulum inhibits frequency-dependent acceleration of relaxation and Ca2+ current facilitation. J Mol Cell Cardiol. 2007;42:196–205. doi: 10.1016/j.yjmcc.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Couchonnal LF, Anderson ME. The role of calmodulin kinase II in myocardial physiology. Physiology. 2008;23:151–159. doi: 10.1152/physiol.00043.2007. [DOI] [PubMed] [Google Scholar]
- 49.Backs J, Olson EN. Control of cardiac growth by histone acetylation/ deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 50.Meffert MK, Chang JM, Wiltgen BJ, Fanselow MS, Baltimore D. NF-kappa B functions in synaptic signaling and behavior. Nat Neurosci. 2003;6:1072–1078. doi: 10.1038/nn1110. [DOI] [PubMed] [Google Scholar]
- 51.Wu X, McMurray CT. Calmodulin kinase II attenuation of gene transcription by preventing cAMP response element-binding protein (CREB) dimerization and binding of the CREB-binding protein. J Biol Chem. 2001;276:1735–1741. doi: 10.1074/jbc.M006727200. [DOI] [PubMed] [Google Scholar]
- 52.Zhang T, Johnson EN, Gu Y, Morissette MR, Sah VP, Gigena MS, Belke DD, Dillmann WH, Rogers TB, Schulman H, Ross J, Brown JH. The Cardiac-specific Nuclear delta B Isoform of Ca2+/Calmodulin-dependent Protein Kinase II Induces Hypertrophy and Dilated Cardiomyopathy Associated with Increased Protein Phosphatase 2A Activity. J Biol Chem. 2002;277:1261–1267. doi: 10.1074/jbc.M108525200. [DOI] [PubMed] [Google Scholar]
- 53.Anderson ME. Multiple downstream proarrhythmic targets for calmodulin kinase II: moving beyond an ion channel-centric focus. Cardiovasc Res. 2007;73:657–666. doi: 10.1016/j.cardiores.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 54.Salas MA, Valverde CA, Sánchez G, Said M, Rodriguez JS, Portiansky EL, Kaetzel MA, Dedman JR, Donoso P, Kranias EG, Mattiazzi A. The signalling pathway of CaMKII-mediated apoptosis and necrosis in the ischemia/reperfusion injury. J Mol Cell Cardiol. 2010;48(6):1298–1306. doi: 10.1016/j.yjmcc.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulman H, Anderson ME. Ca2+/calmodulin-dependent protein kinase II in heart failure. Drug Disc Today: Disease Mech. 2010;7(2):e117–e122. doi: 10.1016/j.ddmec.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sossalla S, Fluschnik N, Schotola H, Ort KR, Neef S, Schulte T, Wittköpper K, Renner A, Schmitto JD, Gummert J, El-Armouche A, Hasenfuss G, Maier LS. Inhibition of elevated Ca2+/calmodulin-dependent protein kinase II improves contractility in human failing myocardium. Circ Res. 2010;107(9):1150–1161. doi: 10.1161/CIRCRESAHA.110.220418. [DOI] [PubMed] [Google Scholar]
- 57.Benitah JP, Alvarez JL, Gomez AM. L-type Ca2+ current in ventricular cardiomyocytes. J Mol Cell Cardiol. 2010;48:26–36. doi: 10.1016/j.yjmcc.2009.07.026. [DOI] [PubMed] [Google Scholar]
- 58.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2(3):173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 59.Bers DM, Grandi E. CaMKII Regulation of Cardiac Ion Channels. J Cardiovasc Pharmacol. 2009;54(3):180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maltsev VA, Vinogradova TM, Bogdanov KY, Lakatta EG, Stern MD. Diastolic calcium release controls the beating rate of rabbit sinoatrial node cells: numerical modeling of the coupling process. Biophys J. 2004;86:2596–2605. doi: 10.1016/S0006-3495(04)74314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.January CT, Riddle JM. Early afterdepolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res. 1989;64:977–990. doi: 10.1161/01.res.64.5.977. [DOI] [PubMed] [Google Scholar]
- 62.Maltsev VA, Kyle JW, Mishra S, Undrovinas A. Molecular identity of the late sodium current in adult dog cardiomyocytes identified by Nav1.5 antisense inhibition. Amer J Physiol. 2008;295(2):H667–H676. doi: 10.1152/ajpheart.00111.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hale SL, Shryock JC, Belardinelli L, Sweeney M, Kloner RA. Late sodium current inhibition as a new cardioprotective approach. J Mol Cell Cardio. 2008;44:954–967. doi: 10.1016/j.yjmcc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Maltsev VA, Kyle JW, Undrovinas A. Late Na+ current produced by human cardiac Na+ channel isoform Nav1.5 is modulated by its β1 subunit. J Physiol Sci. 2009;59(3):217–225. doi: 10.1007/s12576-009-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Undrovinas AA, Maltsev VA, Belardinelli L, Sabbah HN, Undrovinas A. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Undrovinas AI, Maltsev VA, Silverman NA. Transmuralfunctional expression of Na+ channel in normal and failing myocardium. Circulation. 2000;102:II-594. [Google Scholar]
- 67.Maltsev VA, Silverman N, Sabbah HN, Undrovinas AI. Chronic heart failure slows late sodium current in human and canine ventricular myocytes: Implications for repolarization variability. Eur J Heart Fail. 2007;9(3):219–227. doi: 10.1016/j.ejheart.2006.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Undrovinas AI, Maltsev VA, Kyle JW, Silverman N, Sabbah HN. Gating of the late Na+ channel in normal and failing human myocardium. J Mol Cell Cardiol. 2002;34(11):1477–1489. doi: 10.1006/jmcc.2002.2100. [DOI] [PubMed] [Google Scholar]
- 69.Wagner S, Dybkova N, Rasenack ECL, Jacobshagen C, Fabritz L, Kirchhof P, Maier SKG, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J. Clin. Invest. 2006;116(12):3127–3138. doi: 10.1172/JCI26620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shryock JC, Belardinelli L. Inhibition of late sodium current to reduce electrical and mechanical dysfunction of ischaemic myocardium. British J Pharm. 2008;153:1128–1132. doi: 10.1038/sj.bjp.0707522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yan X-S, Ma J-H, Zhang P-H. Modulation of KATP currents in rat ventricular myocytes by hypoxia and a redox reaction. Acta Pharm Sin. 2009;30(10):1399–1414. doi: 10.1038/aps.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Li J, Marionneau C, Zhang R, Shah V, Hell JW, Nerbonne JM, Anderson ME. Calmodulin kinase II inhibition shortens action potential duration by upregulation of K+ currents. Circ. Res. 2006;99(10):1092–1099. doi: 10.1161/01.RES.0000249369.71709.5c. [DOI] [PubMed] [Google Scholar]
- 73.Wagner S, Hacker E, Grandi E, Weber SL, Dybkova N, Sossalla S, Sowa T, Fabritz L, Kirchhof P, Bers DM, Maier LS. Ca/calmodulin kinase II differentially modulates potassium currents. Circ Arrh Electrophys. 2009;2(3):285–294. doi: 10.1161/CIRCEP.108.842799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colinas O, Gallego M, Setién R, López-López JR, Pérez-García MT, Casis O. Differential modulation of Kv4.2 and Kv4.3 channels by calmodulin-dependent protein kinase II in rat cardiac myocytes. Amer J Physiol. 2006;291(4):H1978–H1987. doi: 10.1152/ajpheart.01373.2005. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Marionneau C, Koval O, Zingman L, Mohler PJ, Nerbonne JM, Anderson ME. Calmodulin kinase II inhibition enhances ischemic preconditioning by augmenting ATP-sensitive K+ current. Chann. 2007;1(5):387–394. doi: 10.4161/chan.5449. [DOI] [PubMed] [Google Scholar]
- 76.Duan D. Phenomics of cardiac chloride channels: the systematic study of chloride channel function in the heart. J Physiol. 2009;587(10):2163–2177. doi: 10.1113/jphysiol.2008.165860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hartzell C, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- 78.Sellers ZM, De Arcangelis V, Xiang Y, Best PM. Cardiomyocytes with disrupted CFTR function require CaMKII and Ca2+-activated Cl− channel activity to maintain contraction rate. J Physiol. 2010;588(13):2417–2429. doi: 10.1113/jphysiol.2010.188334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hussain M, Drago GA, Colyer J, Orchard CH. Rate-dependent abbreviation of Ca2+ transient in rat heart is independent of phospholamban phpsphprylation. Am J Physiol. 1997;42:H695–H706. doi: 10.1152/ajpheart.1997.273.2.H695. [DOI] [PubMed] [Google Scholar]
- 80.Curran J, Hinton MJ, Rios E, Bers DM, Shannon TR. β-Adrenergic Enhancement of Sarcoplasmic Reticulum Calcium Leak in Cardiac Myocytes Is Mediated by Calcium/Calmodulin-Dependent Protein Kinase. Circ Res. 2007;100:391–398. doi: 10.1161/01.RES.0000258172.74570.e6. [DOI] [PubMed] [Google Scholar]