Abstract
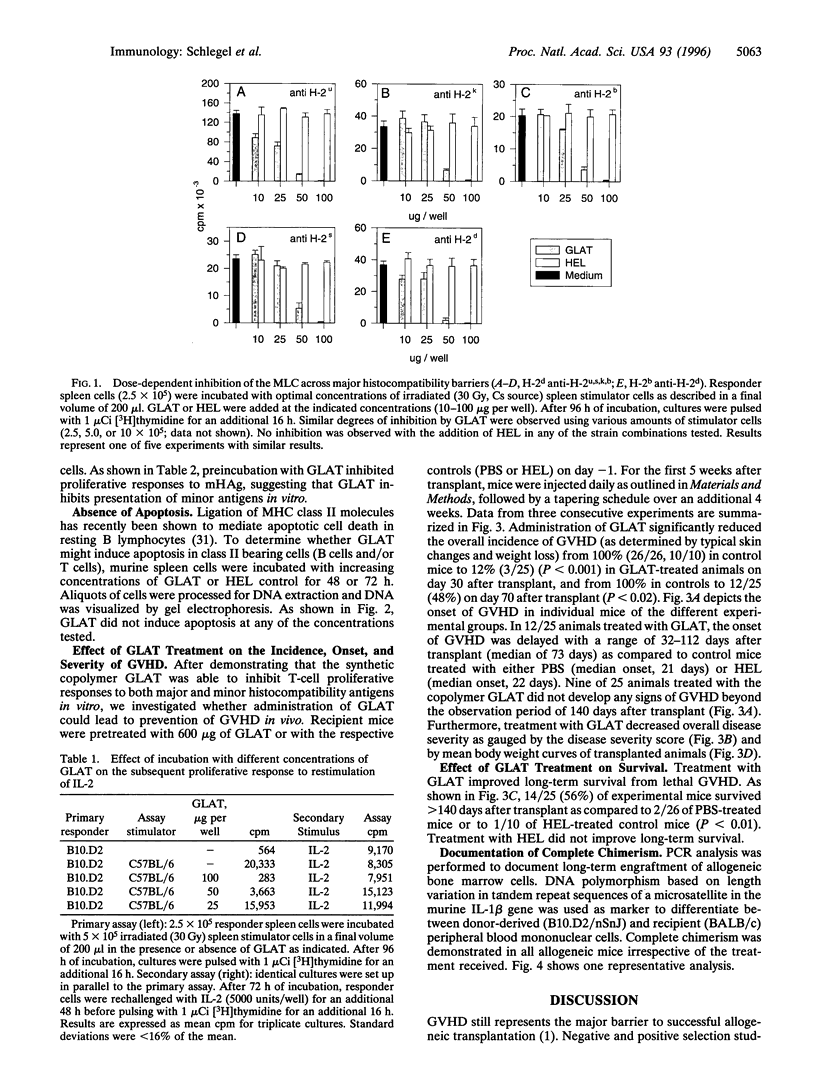
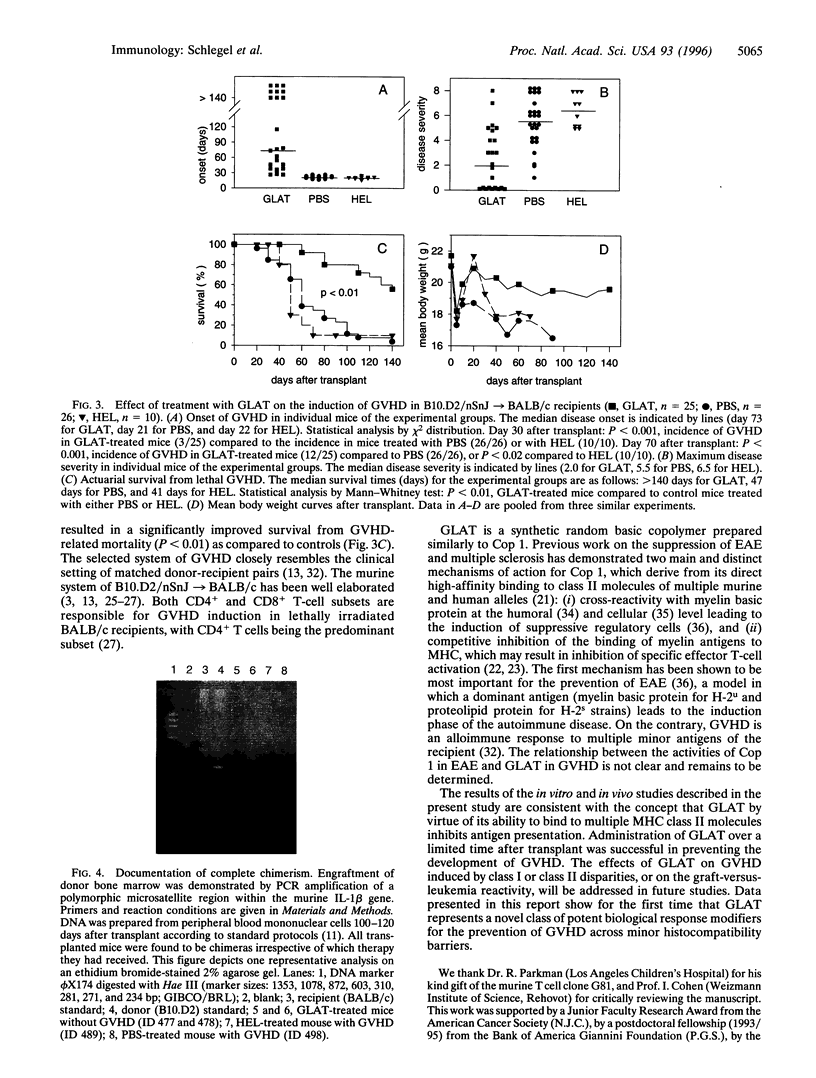
Graft-versus-host disease (GVHD) is a T-cell-mediated disease of transplanted donor T cells recognizing host alloantigens. Data presented in this report show, to our knowledge, for the first time that a synthetic copolymer of the amino acids L-Glu, L-Lys, L-Ala, and L-Tyr (molecular ratio, 1.9:6.0:4.7:1.0; Mr, 6000-8500) [corrected], termed GLAT, with promiscuous binding to multiple major histocompatibility complex class II alleles is capable of preventing lethal GVHD in the B10.D2 --> BALB/c model (both H-2d) across minor histocompatibility barriers. Administration of GLAT over a limited time after transplant significantly reduced the incidence, onset, and severity of disease. GLAT also improved long-term survival from lethal GVHD: 14/25 (56%) of experimental mice survived > 140 days after transplant compared to 2/26 of saline-treated or to 1/10 of hen egg lysozyme-treated control mice (P < 0.01). Long-term survivors were documented to be fully chimeric by PCR analysis of a polymorphic microsatellite region in the interleukin 1beta gene. In vitro, GLAT inhibited the mixed lymphocyte culture in a dose-dependent fashion across a variety of major barriers tested. Furthermore, GLAT inhibited the response of nylon wool-enriched T cells to syngeneic antigen-presenting cells presenting minor histocompatibility antigens. Prepulsing of the antigen-presenting cells with GLAT reduced the proliferative response, suggesting that GLAT inhibits antigen presentation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharoni R., Teitelbaum D., Arnon R. T suppressor hybridomas and interleukin-2-dependent lines induced by copolymer 1 or by spinal cord homogenate down-regulate experimental allergic encephalomyelitis. Eur J Immunol. 1993 Jan;23(1):17–25. doi: 10.1002/eji.1830230105. [DOI] [PubMed] [Google Scholar]
- Blazar B. R., Taylor P. A., Vallera D. A. In vivo or in vitro anti-CD3 epsilon chain monoclonal antibody therapy for the prevention of lethal murine graft-versus-host disease across the major histocompatibility barrier in mice. J Immunol. 1994 Apr 1;152(7):3665–3674. [PubMed] [Google Scholar]
- Bornstein M. B., Miller A., Slagle S., Weitzman M., Crystal H., Drexler E., Keilson M., Merriam A., Wassertheil-Smoller S., Spada V. A pilot trial of Cop 1 in exacerbating-remitting multiple sclerosis. N Engl J Med. 1987 Aug 13;317(7):408–414. doi: 10.1056/NEJM198708133170703. [DOI] [PubMed] [Google Scholar]
- Chao N. J. Graft versus host disease following allogeneic bone marrow transplantation. Curr Opin Immunol. 1992 Oct;4(5):571–576. doi: 10.1016/0952-7915(92)90028-d. [DOI] [PubMed] [Google Scholar]
- Claman H. N., Jaffee B. D., Huff J. C., Clark R. A. Chronic graft-versus-host disease as a model for scleroderma. II. Mast cell depletion with deposition of immunoglobulins in the skin and fibrosis. Cell Immunol. 1985 Aug;94(1):73–84. doi: 10.1016/0008-8749(85)90086-3. [DOI] [PubMed] [Google Scholar]
- Fridkis-Hareli M., Teitelbaum D., Arnon R., Sela M. Synthetic copolymer 1 and myelin basic protein do not require processing prior to binding to class II major histocompatibility complex molecules on living antigen-presenting cells. Cell Immunol. 1995 Jul;163(2):229–236. doi: 10.1006/cimm.1995.1121. [DOI] [PubMed] [Google Scholar]
- Fridkis-Hareli M., Teitelbaum D., Gurevich E., Pecht I., Brautbar C., Kwon O. J., Brenner T., Arnon R., Sela M. Direct binding of myelin basic protein and synthetic copolymer 1 to class II major histocompatibility complex molecules on living antigen-presenting cells--specificity and promiscuity. Proc Natl Acad Sci U S A. 1994 May 24;91(11):4872–4876. doi: 10.1073/pnas.91.11.4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griem P., Wallny H. J., Falk K., Rötzschke O., Arnold B., Schönrich G., Hämmerling G., Rammensee H. G. Uneven tissue distribution of minor histocompatibility proteins versus peptides is caused by MHC expression. Cell. 1991 May 17;65(4):633–640. doi: 10.1016/0092-8674(91)90095-g. [DOI] [PubMed] [Google Scholar]
- Hamilton B. L. L3T4-positive T cells participate in the induction of graft-vs-host disease in response to minor histocompatibility antigens. J Immunol. 1987 Oct 15;139(8):2511–2515. [PubMed] [Google Scholar]
- Hunt D. F., Michel H., Dickinson T. A., Shabanowitz J., Cox A. L., Sakaguchi K., Appella E., Grey H. M., Sette A. Peptides presented to the immune system by the murine class II major histocompatibility complex molecule I-Ad. Science. 1992 Jun 26;256(5065):1817–1820. doi: 10.1126/science.1319610. [DOI] [PubMed] [Google Scholar]
- Ishioka G. Y., Adorini L., Guery J. C., Gaeta F. C., LaFond R., Alexander J., Powell M. F., Sette A., Grey H. M. Failure to demonstrate long-lived MHC saturation both in vitro and in vivo. Implications for therapeutic potential of MHC-blocking peptides. J Immunol. 1994 May 1;152(9):4310–4319. [PubMed] [Google Scholar]
- Johnson K. P., Brooks B. R., Cohen J. A., Ford C. C., Goldstein J., Lisak R. P., Myers L. W., Panitch H. S., Rose J. W., Schiffer R. B. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995 Jul;45(7):1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- Korngold R., Sprent J. Variable capacity of L3T4+ T cells to cause lethal graft-versus-host disease across minor histocompatibility barriers in mice. J Exp Med. 1987 Jun 1;165(6):1552–1564. doi: 10.1084/jem.165.6.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConkey D. J., Hartzell P., Duddy S. K., Håkansson H., Orrenius S. 2,3,7,8-Tetrachlorodibenzo-p-dioxin kills immature thymocytes by Ca2+-mediated endonuclease activation. Science. 1988 Oct 14;242(4876):256–259. doi: 10.1126/science.3262923. [DOI] [PubMed] [Google Scholar]
- Miconnet I., Roger T., Seman M., Bruley-Rosset M. Critical role of endogenous Mtv in acute lethal graft-versus-host disease. Eur J Immunol. 1995 Feb;25(2):364–368. doi: 10.1002/eji.1830250209. [DOI] [PubMed] [Google Scholar]
- Nestel F. P., Price K. S., Seemayer T. A., Lapp W. S. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992 Feb 1;175(2):405–413. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell M. K., VanderWall J., Beard K. S., Freed J. H. Ligation of major histocompatibility complex class II molecules mediates apoptotic cell death in resting B lymphocytes. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10459–10463. doi: 10.1073/pnas.90.22.10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roopenian D. C., Christianson G. J., Davis A. P., Zuberi A. R., Mobraaten L. E. The genetic origin of minor histocompatibility antigens. Immunogenetics. 1993;38(2):131–140. doi: 10.1007/BF00190900. [DOI] [PubMed] [Google Scholar]
- Rus V., Svetic A., Nguyen P., Gause W. C., Via C. S. Kinetics of Th1 and Th2 cytokine production during the early course of acute and chronic murine graft-versus-host disease. Regulatory role of donor CD8+ T cells. J Immunol. 1995 Sep 1;155(5):2396–2406. [PubMed] [Google Scholar]
- Sakamoto H., Michaelson J., Jones W. K., Bhan A. K., Abhyankar S., Silverstein M., Golan D. E., Burakoff S. J., Ferrara J. L. Lymphocytes with a CD4+ CD8- CD3- phenotype are effectors of experimental cutaneous graft-versus-host disease. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10890–10894. doi: 10.1073/pnas.88.23.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson M. F., Smilek D. E. Reversal of acute experimental autoimmune encephalomyelitis and prevention of relapses by treatment with a myelin basic protein peptide analogue modified to form long-lived peptide-MHC complexes. J Immunol. 1995 Sep 1;155(5):2737–2746. [PubMed] [Google Scholar]
- Schlegel P. G., Aharoni R., Smilek D. E., Fernandez L. P., McDevitt H. O., Tran N., Vaysburd M., Chao N. J. Prevention of graft-versus-host disease by peptides binding to class II major histocompatibility complex molecules. Blood. 1994 Oct 15;84(8):2802–2810. [PubMed] [Google Scholar]
- Schlegel P. G., Vaysburd M., Chen Y., Butcher E. C., Chao N. J. Inhibition of T cell costimulation by VCAM-1 prevents murine graft-versus-host disease across minor histocompatibility barriers. J Immunol. 1995 Oct 15;155(8):3856–3865. [PubMed] [Google Scholar]
- Sellins K. S., Cohen J. J. Gene induction by gamma-irradiation leads to DNA fragmentation in lymphocytes. J Immunol. 1987 Nov 15;139(10):3199–3206. [PubMed] [Google Scholar]
- Speiser D. E., Zürcher T., Ramseier H., Hengartner H., Staeheli P., Haller O., Zinkernagel R. M. Nuclear myxovirus-resistance protein Mx is a minor histocompatibility antigen. Proc Natl Acad Sci U S A. 1990 Mar;87(5):2021–2025. doi: 10.1073/pnas.87.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Aharoni R., Arnon R., Sela M. Specific inhibition of the T-cell response to myelin basic protein by the synthetic copolymer Cop 1. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9724–9728. doi: 10.1073/pnas.85.24.9724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Aharoni R., Sela M., Arnon R. Cross-reactions and specificities of monoclonal antibodies against myelin basic protein and against the synthetic copolymer 1. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9528–9532. doi: 10.1073/pnas.88.21.9528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D., Meshorer A., Hirshfeld T., Arnon R., Sela M. Suppression of experimental allergic encephalomyelitis by a synthetic polypeptide. Eur J Immunol. 1971 Aug;1(4):242–248. doi: 10.1002/eji.1830010406. [DOI] [PubMed] [Google Scholar]
- Teitelbaum D., Webb C., Meshorer A., Arnon R., Sela M. Suppression by several synthetic polypeptides of experimental allergic encephalomyelitis induced in guinea pigs and rabbits with bovine and human basic encephalitogen. Eur J Immunol. 1973 May;3(5):273–279. doi: 10.1002/eji.1830030505. [DOI] [PubMed] [Google Scholar]
- Wallny H. J., Rammensee H. G. Identification of classical minor histocompatibility antigen as cell-derived peptide. Nature. 1990 Jan 18;343(6255):275–278. doi: 10.1038/343275a0. [DOI] [PubMed] [Google Scholar]
- Wang W., Meadows L. R., den Haan J. M., Sherman N. E., Chen Y., Blokland E., Shabanowitz J., Agulnik A. I., Hendrickson R. C., Bishop C. E. Human H-Y: a male-specific histocompatibility antigen derived from the SMCY protein. Science. 1995 Sep 15;269(5230):1588–1590. doi: 10.1126/science.7667640. [DOI] [PubMed] [Google Scholar]
- Webb C., Teitelbaum D., Arnon R., Sela M. In vivo and in vitro immunological cross-reactions between basic encephalitogen and synthetic basic polypeptides capable of suppressing experimental allergic encephalomyelitis. Eur J Immunol. 1973 May;3(5):279–286. doi: 10.1002/eji.1830030506. [DOI] [PubMed] [Google Scholar]
- Webb S., Morris C., Sprent J. Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 1990 Dec 21;63(6):1249–1256. doi: 10.1016/0092-8674(90)90420-j. [DOI] [PubMed] [Google Scholar]
- den Haan J. M., Sherman N. E., Blokland E., Huczko E., Koning F., Drijfhout J. W., Skipper J., Shabanowitz J., Hunt D. F., Engelhard V. H. Identification of a graft versus host disease-associated human minor histocompatibility antigen. Science. 1995 Jun 9;268(5216):1476–1480. doi: 10.1126/science.7539551. [DOI] [PubMed] [Google Scholar]