Abstract
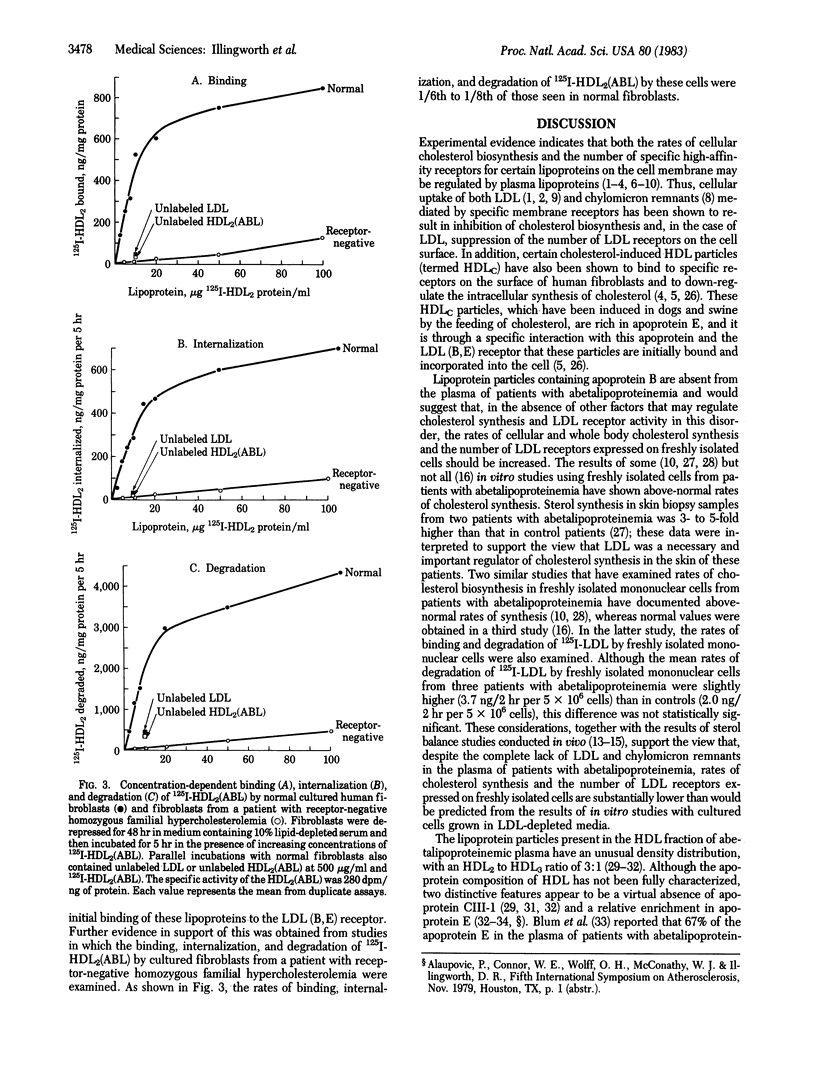
Despite an absence of low density lipoproteins (LDLs) and chylomicron remnants from plasma, the rates of cholesterol synthesis or the number of LDL receptors expressed on freshly isolated cells from patients with abetalipoproteinemia are not markedly increased. These observations suggest that other lipoprotein particles present in the plasma of patients with abetalipoproteinemia may regulate LDL receptor activity and the rates of cellular cholesterol synthesis in this disorder. In the present report we have studied the effects of lipoprotein fractions from the plasma of normal subjects, patients with abetalipoproteinemia, and a patient with dysbetalipoproteinemia on the binding, internalization, and degradation of 125I-labeled LDL (125I-LDL) by cultured human fibroblasts. LDL from normal subjects or the high density lipoprotein fraction HDL2 from the plasma of patients with abetalipoproteinemia effectively down-regulated LDL receptor activity (greater than 50% inhibition at 20 micrograms of protein per ml). HDL2 from the plasma of patients with abetalipoproteinemia also effectively reduced the binding, internalization, and degradation of 125I-LDL by cultured human fibroblasts. 125I-HDL2 from the plasma of patients with abetalipoproteinemia was bound, internalized, and degraded by cultured human fibroblasts; this process was competitively inhibited by unlabeled normal LDL or HDL2 from abetalipoproteinemic plasma and was 1/6th to 1/8th times as high when 125I-HDL2 was incubated with fibroblasts from a patient with receptor-negative homozygous familial hypercholesterolemia. We conclude that lipoproteins present in the HDL2 fraction of plasma from patients with abetalipoproteinemia (which are relatively rich in apoprotein E) are effective regulators of LDL receptor activity in normal human fibroblasts. These in vitro findings may explain why the in vivo rates of cholesterol synthesis and the number of LDL receptors expressed on freshly isolated cells from patients with abetalipoproteinemia are not markedly increased.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G. Mutations that affect membrane receptor for LDL are useful for studying normal receptor function. Am J Physiol. 1982 Jul;243(1):E5–14. doi: 10.1152/ajpendo.1982.243.1.E5. [DOI] [PubMed] [Google Scholar]
- Bierman E. L., Stein O., Stein Y. Lipoprotein uptake and metabolism by rat aortic smooth muscle cells in tissue culture. Circ Res. 1974 Jul;35(1):136–150. doi: 10.1161/01.res.35.1.136. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Brannan P. G., Bohmfalk H. A., Brunschede G. Y., Dana S. E., Helgeson J., Goldstein J. L. Use of mutant fibroblasts in the analysis of the regulation of cholesterol metabolism in human cells. J Cell Physiol. 1975 Apr;85(2 Pt 2 Suppl 1):425–436. doi: 10.1002/jcp.1040850409. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Familial hypercholesterolemia: defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci U S A. 1974 Mar;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Goldstein J. L. Receptor-mediated control of cholesterol metabolism. Science. 1976 Jan 16;191(4223):150–154. doi: 10.1126/science.174194. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Receptor-mediated uptake of lipoprotein-cholesterol and its utilization for steroid synthesis in the adrenal cortex. Recent Prog Horm Res. 1979;35:215–257. doi: 10.1016/b978-0-12-571135-7.50009-6. [DOI] [PubMed] [Google Scholar]
- Brown M. S., Kovanen P. T., Goldstein J. L. Regulation of plasma cholesterol by lipoprotein receptors. Science. 1981 May 8;212(4495):628–635. doi: 10.1126/science.6261329. [DOI] [PubMed] [Google Scholar]
- Carr B. R., Simpson E. R. Lipoprotein utilization and cholesterol synthesis by the human fetal adrenal gland. Endocr Rev. 1981 Summer;2(3):306–326. doi: 10.1210/edrv-2-3-306. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Basu S. K., Brunschede G. Y., Brown M. S. Release of low density lipoprotein from its cell surface receptor by sulfated glycosaminoglycans. Cell. 1976 Jan;7(1):85–95. doi: 10.1016/0092-8674(76)90258-0. [DOI] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- Gwynne J. T., Strauss J. F., 3rd The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr Rev. 1982 Summer;3(3):299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havel R. J., Chao Y., Windler E. E., Kotite L., Guo L. S. Isoprotein specificity in the hepatic uptake of apolipoprotein E and the pathogenesis of familial dysbetalipoproteinemia. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4349–4353. doi: 10.1073/pnas.77.7.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho Y. K., Faust J. R., Bilheimer D. W., Brown M. S., Goldstein J. L. Regulation of cholesterol synthesis by low density lipoprotein in isolated human lymphocytes. Comparison of cells from normal subjects and patients with homozygous familial hypercholesterolemia and abetalipoproteinemia. J Exp Med. 1977 Jun 1;145(6):1531–1549. doi: 10.1084/jem.145.6.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui D. Y., Innerarity T. L., Mahley R. W. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apo-E and apo-B,E receptors. J Biol Chem. 1981 Jun 10;256(11):5646–5655. [PubMed] [Google Scholar]
- Illingworth D. R., Connor W. E., Alaupovic P. High density lipoprotein metabolism in a patient with abetalipoproteninemia. Ann Nutr Metab. 1981;25(1):1–10. doi: 10.1159/000176473. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Connor W. E., Buist N. R., Jhaveri B. M., Lin D. S., McMurry M. P. Sterol balance in abetalipoproteinemia: studies in a patient with homozygous familial hypobetalipoproteinemia. Metabolism. 1979 Nov;28(11):1152–1160. doi: 10.1016/0026-0495(79)90155-0. [DOI] [PubMed] [Google Scholar]
- Illingworth D. R., Connor W. E., Lin D. S., Diliberti J. Lipid metabolism in abetalipoproteinemia: a study of cholesterol absorption and sterol balance in two patients. Gastroenterology. 1980 Jan;78(1):68–75. [PubMed] [Google Scholar]
- Illingworth D. R., Corbin D. K., Kemp E. D., Keenan E. J. Hormone changes during the menstrual cycle in abetalipoproteinemia: reduced luteal phase progesterone in a patient with homozygous hypobetalipoproteinemia. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6685–6689. doi: 10.1073/pnas.79.21.6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illingworth D. R., Kenny T. A., Connor W. E., Orwoll E. S. Corticosteroid production in abetalipoproteinemia: evidence for an impaired response ACTH. J Lab Clin Med. 1982 Jul;100(1):115–126. [PubMed] [Google Scholar]
- Illingworth D. R., Kenny T. A., Orwoll E. S. Adrenal function in heterozygous and homozygous hypobetalipoproteinemia. J Clin Endocrinol Metab. 1982 Jan;54(1):27–33. doi: 10.1210/jcem-54-1-27. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978 Apr 18;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Innerarity T. L., Pitas R. E., Mahley R. W. Receptor binding of cholesterol-induced high-density lipoproteins containing predominantly apoprotein E to cultured fibroblasts with mutations at the low-density lipoprotein receptor locus. Biochemistry. 1980 Sep 2;19(18):4359–4365. doi: 10.1021/bi00559a032. [DOI] [PubMed] [Google Scholar]
- Kostner G., Holasek A., Bohlmann H. G., Thiede H. Investigation of serum lipoproteins and apoproteins in abetalipoproteinaemia. Clin Sci Mol Med. 1974 Apr;46(4):457–468. doi: 10.1042/cs0460457. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Langer T., Strober W., Levy R. I. The metabolism of low density lipoprotein in familial type II hyperlipoproteinemia. J Clin Invest. 1972 Jun;51(6):1528–1536. doi: 10.1172/JCI106949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layman D. L., Jelen B. J., Illingworth D. R. Inability of serum from abetalipoproteinemic subjects to stimulate proliferation of human smooth muscle cells and dermal fibroblasts in vitro. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1511–1515. doi: 10.1073/pnas.77.3.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W., Hui D. Y., Innerarity T. L., Weisgraber K. H. Two independent lipoprotein receptors on hepatic membranes of dog, swine, and man. Apo-B,E and apo-E receptors. J Clin Invest. 1981 Nov;68(5):1197–1206. doi: 10.1172/JCI110365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFARLANE A. S. Efficient trace-labelling of proteins with iodine. Nature. 1958 Jul 5;182(4627):53–53. doi: 10.1038/182053a0. [DOI] [PubMed] [Google Scholar]
- Reichl D., Myant N. B., Lloyd J. K. Surface binding and catabolism of low-density lipoprotein by circulating lymphocytes from patients with abetalipoproteinaemia, with observations on sterol synthesis in lymphocytes from one patient. Biochim Biophys Acta. 1978 Jul 25;530(1):124–131. doi: 10.1016/0005-2760(78)90132-7. [DOI] [PubMed] [Google Scholar]
- Scanu A. M., Aggerbeck L. P., Kruski A. W., Lim C. T., Kayden H. J. A study of the abnormal lipoproteins in abetalipoproteinemia. J Clin Invest. 1974 Feb;53(2):440–453. doi: 10.1172/JCI107578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider W. J., Kovanen P. T., Brown M. S., Goldstein J. L., Utermann G., Weber W., Havel R. J., Kotite L., Kane J. P., Innerarity T. L. Familial dysbetalipoproteinemia. Abnormal binding of mutant apoprotein E to low density lipoprotein receptors of human fibroblasts and membranes from liver and adrenal of rats, rabbits, and cows. J Clin Invest. 1981 Oct;68(4):1075–1085. doi: 10.1172/JCI110330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd J., Caslake M., Farish E., Fleck A. Chemical and kinetic study of the lipoproteins in abetalipoproteinaemic plasma. J Clin Pathol. 1978 Apr;31(4):382–387. doi: 10.1136/jcp.31.4.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill B. C., Dietschy J. M. Characterization of the sinusoidal transport process responsible for uptake of chylomicrons by the liver. J Biol Chem. 1978 Mar 25;253(6):1859–1867. [PubMed] [Google Scholar]


