To the Editor:
Dabigatran etexilate is a direct thrombin inhibitor approved to reduce the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation.1 Dabigatran has many potential advantages as an anticoagulant agent when compared to warfarin. Dabigatran is active when taken orally, is administered in a fixed dose, and has predictable pharmacokinetics that do not require routine laboratory monitoring.1 Unlike warfarin, dabigatran does not interact with the cytochrome P450 system and is associated with far fewer drug interactions.1 However, dabigatran requires twice-daily dosing, an ideal reversal agent is lacking, and dose adjustments are needed for patients with impaired renal function.1
We conducted a cross-sectional study at Lutheran Hospital and the Lutheran Hospital Anticoagulation Clinic in Ft. Wayne, Indiana. Patients who were admitted to the hospital or seen in the anticoagulation clinic between October 2011 and April 2013 were included in the analysis. Patients were eligible if they had, at any time, been receiving dabigatran for any indication and for whom the decision was made to discontinue dabigatran and initiate warfarin. Information as to why dabigatran was being discontinued in favor of warfarin was collected via patient interviews, when available, and a review of patient charts.
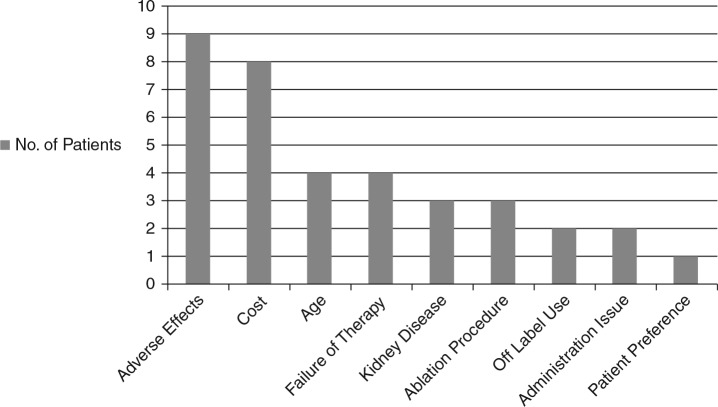
We identified 9 inpatients and 22 outpatients who had been switched from dabigatran to warfarin during the specified time frame. A summary of the reasons for dabigatran discontinuation is provided in Figure 1. The most common reasons for discontinuation of dabigatran were adverse effects (n = 9) and cost (n = 8). Gastrointestinal distress and dermatologic reactions (pruritus, rash, and skin blotching) led to discontinuation in 3 patients each. Other adverse effects identified included confusion, sore throat, and minor bleeding.
Figure 1. Summary of reasons for dabigatran discontinuation.
As with any new medication, the full impact and issues surrounding the use of dabigatran may not be known for several years after its introduction. In this study, reasons for dabigatran discontinuation in the clinical setting were highly variable. Eight of the 31 patients (26%) who discontinued dabigatran did so because of cost. This number is alarming and should be a consideration when clinicians are deciding between dabigatran and warfarin, as noncompliance may lead to worse outcomes.2 Open discussion between health care providers and patients may allow for the selection of the most appropriate medication for the patient, both clinically and financially. Three patients discontinued dabigatran secondary to a gastrointestinal adverse effect. All 3 of these patients had intolerable gastrointestinal distress, while 1 patient also complained of diarrhea. Skin disorders led to dabigatran discontinuation just as commonly as gastrointestinal complaints. Dermatologic adverse reactions,3 including toxic epidermal necrolysis,4 have been reported in observational studies and case reports; however, the precise incidence should be further evaluated. Further study is warranted to evaluate adverse effects and practice issues surrounding dabigatran therapy over the entire treatment course. This study suggests that prescribers should be most aware of potential adverse effects and cost prior to the start of dabigatran for atrial fibrillation.
Acknowledgments
No financial support was provided for this article.
Dr. Jacobs was a pharmacy practice resident at Lutheran Hospital and Dr. Linn was a clinical pharmacist at Lutheran Hospital at the time of initiation of this project.
References
- 1.Pradaxa (dabigatran) package insert Ridgefield, CT: Boehringer Ingelheim; 2012 [Google Scholar]
- 2.Ho PH, Bryson CL, Rumsfeld JS. Medication adherence: Its importance in cardiovascular outcomes. Circulation. 2009;119:3028–3035 [DOI] [PubMed] [Google Scholar]
- 3.Tsoumpris A, Tzimas T, Gkabrelas K, Akritidis N. Iron complex, dabigatran, and toxic epidermal necrolysis: A case report. J Clin Pharm Ther. 2013;38:177–178 [DOI] [PubMed] [Google Scholar]
- 4.To K, Reynolds C, Spinler SA. Rash associated with dabigatran etexilate. Pharmacotherapy. 2013;33(3):e23–e27 [DOI] [PubMed] [Google Scholar]