Abstract
Patients with chronic kidney disease (CKD) have increased risk of fractures, yet the optimal treatment is unknown. In secondary analyses of large randomized trials, bisphosphonates have been shown to improve bone mineral density and reduce fractures. However, bisphosphonates are currently not recommended in patients with advanced kidney disease due to concern about over-suppressing bone remodeling, which may increase the risk of developing arterial calcification. In the present study we used a naturally occurring rat model of CKD with secondary hyperparathyroidism, the Cy/+ rat, and compared the efficacy of treatment with zoledronic acid, calcium given in water to simulate a phosphate binder, and the combination of calcium and zoledronic acid. Animals were treated beginning at 25 weeks of age (approximately 30% of normal renal function) and followed for ten weeks. The results demonstrate that both zoledronic acid and calcium improved bone volume by microCT and both equally suppressed mineral apposition rate, bone formation rate, and mineralizing surface of trabecular bone. In contrast, only calcium treatment with or without zoledronic acid improved cortical porosity and cortical biomechanical properties (ultimate load and stiffness) and lowered parathyroid hormone (PTH). However, only calcium treatment led to the adverse effects of increased arterial calcification and fibroblast growth factor 23 (FGF23). These results suggest zoledronic acid may improve trabecular bone volume in CKD in the presence of secondary hyperparathyroidism, but does not benefit extraskeletal calcification or cortical biomechanical properties. Calcium effectively reduces PTH and benefits both cortical and trabecular bone yet increases the degree of extra skeletal calcification.
Introduction
Chronic kidney disease – mineral and bone disorder (CKD-MBD) is a disorder of abnormal biochemistries, bone fragility and arterial calcification in patients with advanced CKD(1). Patients with CKD have increased risk of bone fracture compared to the general population(2). The increased fracture risk is due to a combination of abnormal bone quantity and quality(3,4).
The pathophysiology of bone loss associated with CKD-MBD is different than post- menopausal osteoporosis suggesting that extrapolation of data from trials of treatments in post-menopausal or corticosteroid-induced osteoporosis may not be appropriate. Secondary analyses of randomized controlled trials of bisphosphonates in post-menopausal women subsequently identified to have kidney disease demonstrated reduced fracture risk and improved bone mineral density without adverse consequences(5-7). However, these patients had neither advanced kidney disease nor elevated PTH levels. These differences provided the rationale for global clinical practice guidelines to recommend against treating patients with CKD stage 3b-5 with bisphosphonates without a bone biopsy(8). We have previously used our slowly progressive animal model of CKD-MBD to test different doses of the bisphosphonate zoledronic acid on bone mass, turnover, and biomechanical properties in animals with hyperparathyroidism and moderate renal disease(9). In the present study, we extend these findings to compare the skeletal (both cortical and cancellous) and vascular effects of zoledronic acid with and without calcium in the setting of high and low PTH levels in CKD animals with more advanced renal disease.
Materials and Methods
Animal model and experimental design
Male Cy/+ rats, Han:SPRD rats with autosomal dominant polycystic kidney disease, and their non-affected (normal) littermates were used for this study. Male heterozygous rats (Cy/+) develop characteristics of CKD (azotemia) around 10 weeks of age which progresses to terminal uremia by about 40 weeks. This animal model spontaneously develops all three manifestations of CKD-MBD: biochemical abnormalities, extra skeletal calcification, and abnormal bone(10,11).
At 25 weeks of age, animals were assigned to treatment groups. In the CKD (Cy/+) animals, this age represents approximately 30-40% of the kidney function of the normal littermates. This was chosen to simulate late stage 3 CKD, a stage at which there is elevated PTH, yet normal calcium and phosphorus levels, and a stage at which clinical practice guidelines do not recommend treatment with bisphosphonates(8). The CKD treatment groups (n=10 per group) were given 1) a single subcutaneous (SQ) dose of vehicle as control (CTL) and normal deionized drinking water, 2) a single SQ dose of zoledronic acid (ZOL) (20 μg/kg body weight) and normal deionized drinking water, 3) no injection but administered 3% calcium gluconate (3% Ca) in the drinking water, or 4) Zoledronic acid plus 3% Ca in the drinking water (Ca + ZOL). The calcium gluconate group was used to simulate calcium administration as a phosphate binder. In addition, we studied age-matched normal (NL) littermate animals (n = 10) to determine if treatments normalized bone manifestations or extra skeletal calcification. All animals were fed a casein diet (Purina AIN-76A; 0.53% Ca and 0.56% P) during the experiment which has been shown to produce a more consistent kidney disease in this model(10). Two weeks prior to the end of the study, all animals were given an intraperitoneal injection of calcein (1% concentration, 0.1mL/100g body weight); a second injection was given 10 days later. At 35 weeks of age all animals were euthanized by an overdose of sodium pentobarbital. All procedures were reviewed and approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Tissues collection and analysis
At sacrifice at 35 weeks, blood and urine were collected by cardiac and bladder puncture, respectively. The heart and aorta arch were excised and weighed. Left ventricular mass index (LVMI) was determined by dividing total heart weight by body weight. To quantify aorta and heart calcification, proximal segments of the ascending aorta and a segment of the inferior apex of the left ventricle of the heart were snap frozen and the degree of calcification determined biochemically as previously described (12). Left tibiae were placed in 10% neutral buffered formalin for 48 hours and then changed to 70% ethanol for imaging followed by histological processing.
Serum and urine biochemical measurements
Blood plasma was analyzed for BUN, calcium, phosphorus, and creatinine using colorimetric assays (Point Scientific, Canton, MI, USA, or Sigma kits). Intact PTH was determined by ELISA (Alpco, Salem, NH, USA). FGF23 was assessed with a two-site assay (Immunotopics, San Clemente, CA, USA). Urine was analyzed for creatinine, calcium, and phosphorus using the colorimetric methods described above (11).
Computed tomography (CT)
Morphological parameters of the proximal tibia were assessed using high-resolution microCT (Skyscan 1172). Bones were wrapped in parafilm to prevent drying during scanning. Scans were obtained using an x-ray source, set at 60kV and 167 μA over an angular range of 180 degrees (rotational steps of 0.70 degrees) with a 12-μm pixel size. Projection images were reconstructed using standard Skyscan software (NRecon). A 1mm region of interest of the proximal tibia (located ∼ 0.5 mm distal to the growth plate) was analyzed by segmenting the trabecular bone from the cortical shell and calculating trabecular bone volume per total volume (BV/TV) in accordance with recommended guidelines (13). On the most distal slice of the 1 mm region of interest, the cortical shell was manually isolated from the trabecular bone by tracing the periosteal and endocortical edges. Porosity of the cortical shell was calculated as total bone area within the cortex divided by total area of bone plus void space within the cortex.
Bone Histomorphometry
Tibiae were embedded in methylmethacrylate for sectioning as previously described (9,14). The proximal tibial metaphysis was thin sectioned (4 μm) and mounted unstained using non-fluorescent medium. Sections were analyzed using a microscope interfaced with a semiautomatic analysis system (Bioquant OSTEO 7.20.10, Bioquant Image Analysis Co.). Two of ten CKD vehicle-treated animals, and two of nine CKD calcium-treated animals did not have double labels in the proximal tibia section region of interest. In these animals, tibia midshafts were sectioned and also found to contain no double label. This suggests these animals were not properly administered both labels (likely due to injection directly into bladder) and thus these animals were excluded from the histological analysis, according to previously published recommendations(15). For trabecular bone analyses, a region of interest of approximately 8 mm2 within the secondary spongiosa (∼ 0.5 mm distal to the growth plate) was defined, and then measures of single- and double-label perimeter (sL.Pm, dL.Pm), total bone perimeter (B.Pm) and interlabel width (Ir.L.Wi) were conducted. From these primary measurements, derived parameters were calculated as: mineralizing surface (MS/BS = [1/2sL.Pm + dL.Pm]/B.Pm; %), mineral apposition rate (MAR =Ir.l.W/days between labels; μm/day), and bone formation rate (BFR/BS = MAR × MS/BS × 3.65; μm3/μm2/yr). All parameters were measured and calculated in accordance with ASBMR recommended standards(16).
Bone Mechanics
Femora were tested via three-point bending using standard methods as previously described for the rat.(9). Femora were thawed to room temperature, hydrated in 0.9% saline, and placed on the bottom support of a servo-hydraulic test system (Test Resources). All bones were loaded to failure in an anterior-posterior direction using a displacement rate of 2 mm/min with force vs. displacement data collected at 10 Hz. Structural mechanical properties, ultimate load, stiffness, and energy to failure were determined from the load-deformation curves using standard definitions.
Statistics
All analyses were run using SigmaStat software. The five groups were compared using a one-way ANOVA with Fisher's post-hoc analyses for within group comparisons. Correlations were examined by the Pearson product-moment algorithm. A priori α-levels were set at 0.05. Data are presented as means and standard errors.
Results
Biochemical outcomes
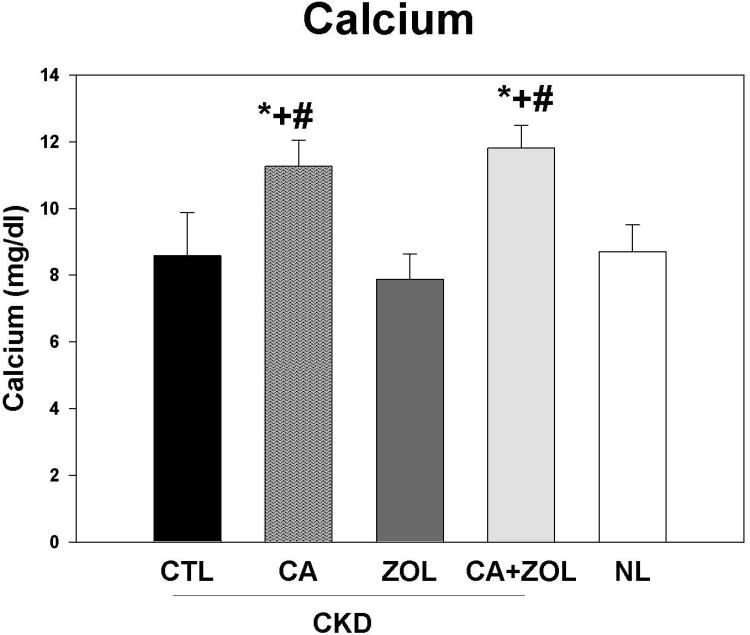
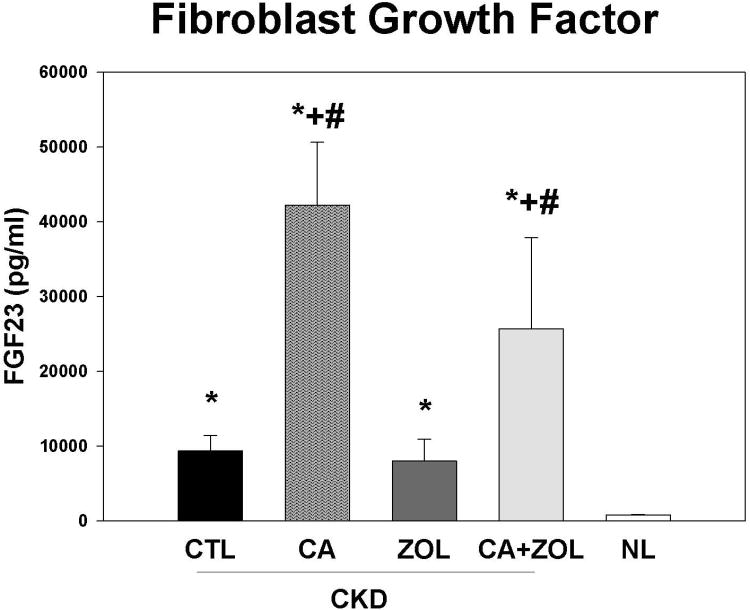
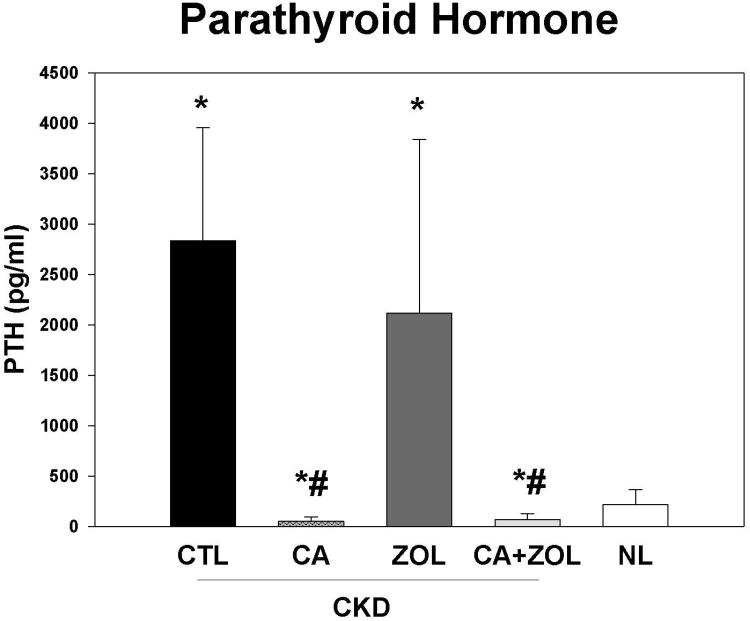
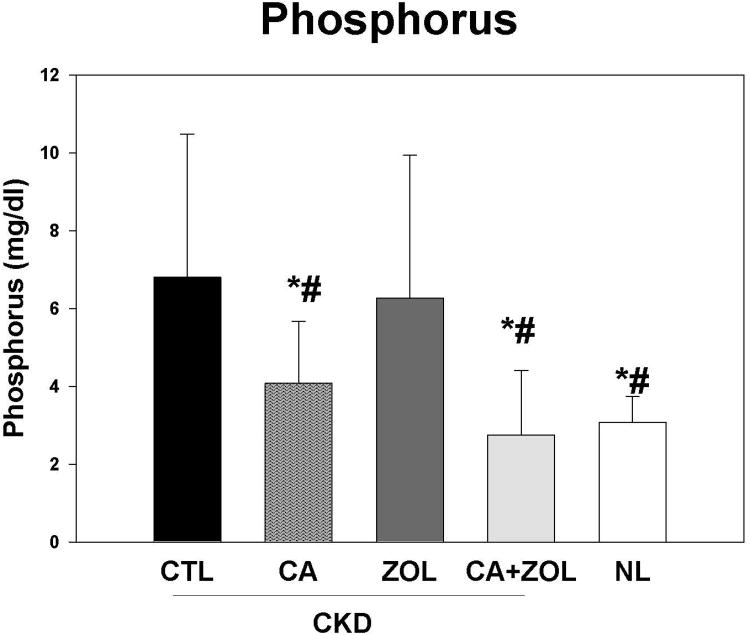
At 35 weeks, there was no difference in the plasma BUN in the CKD animal treatment groups, but values for all CKD animals were higher than normal animals as expected (CKD BUN 59.6 ± 1.5 vs. NL BUN 19.9 ± 0.63 mg/dl). The total body weight was not different between any of the 5 groups. There were overall differences in the other blood results (Figure 1 A-D): In the CKD rats, treatment with calcium, both alone and in combination with zoledronic acid, led to an increase in plasma calcium (p < 0.001) and FGF23 (p < 0.005) levels and a decrease in PTH (p < 0.001) and phosphorus (p < 0.05) levels compared to CKD-CTL. In contrast, treatment with zoledronic acid alone had no significant effect on these biochemical measures. The effect of the combination of zoledronic acid with calcium was not different than calcium alone for any of these plasma biochemistry results. The plasma calcium level was negatively correlated with PTH (r = -0.53, p < 0.001) and positively correlated with FGF23 (r = 0.44, p < 0.002). The plasma phosphorus level was more strongly related to the PTH (r = 0.79, p < 0.001) than FGF23 (r = 0.33, p < 0.003). The urine calcium/creatinine ratio was significantly increased in both calcium treatment groups (Vehicle = 0.27 ± 0.09; Zol = 0.27 ± 0.12; Ca = 0.70 ± 0.22; Zol + Ca = 0.50 ± 0.20 mg/mg, p < 0.0001). The urine phosphorus/creatinine ratio was decreased in both calcium treatment groups (Vehicle = 1.33 ± 0.56; Zol = 1.75 ± 0.44; Ca = 1.08 ± 0.49; Ca + Zol = 0.79 ± 0.35 mg/mg, overall p = 0.002). The albumin/creatinine ratio was not different among the groups.
Figure 1. Biochemical changes in response to therapies.
CKD animals were treated beginning at 25 weeks of age with control vehicle (CTL), calcium in drinking water daily for 10 weeks (Ca), zoledronic acid 20 ug/kg given subcutaneously once at 25 weeks (ZOL), or the combination of Ca + ZOL. The results were compared to normal animals (NL) treated with vehicle. Blood was drawn at 35 weeks of age. The results demonstrate that treatment with calcium, with or without zoledronic acid, led to increased calcium levels (A), increased fibroblast growth factor 23 (B), decreased parathyroid hormone levels to those in normal animals (C), and decreased phosphorus levels (D). *= different than NL; + = different than CKD-CTL; # = different than CKD + ZOL; all p < 0.05. Graphs are mean ± SEM, n = 8 to 10 per group.
Cardiovascular outcomes
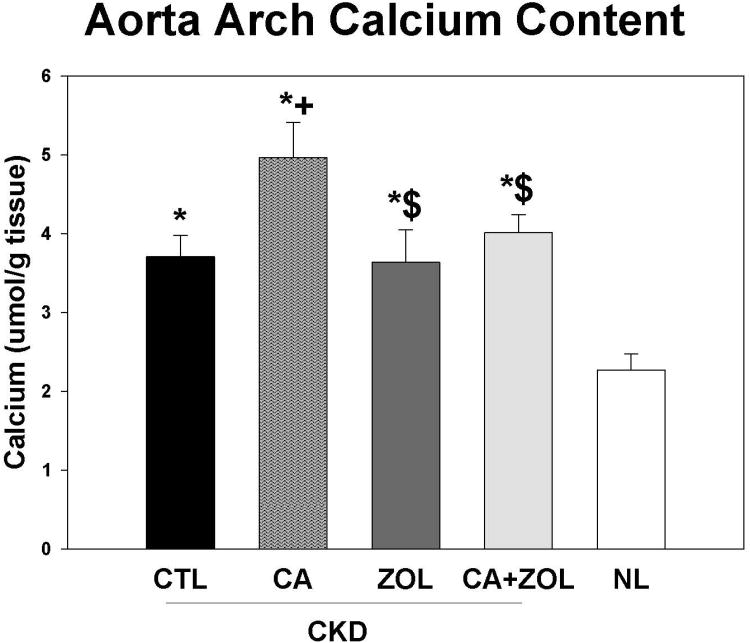
At 35 weeks, the CKD animals had increased left ventricular mass index (LVMI) compared to normal animals (p<0.001) that was unaffected by treatments (CKD + CTL = 3.8 ± 0.19, CKD + Ca = 3.9 ± 1.2, CKD + ZOL = 4.0 ± 0.4, CKD + Ca + ZOL = 4.0 ± 0.4, Nl +Vehicle 3.2 ± 0.06, overall p = 0.035). However, treatment with calcium increased aortic arch calcium content, and this adverse effect was mitigated by zoledronic acid (Figure 2). The aorta arch calcium content was significantly (all p < 0.01) correlated with LVMI (r = 0.48), BUN (r = 0.47), calcium (r = 0.43), and FGF23 (r = 0.51) but not phosphorus or PTH. In contrast to our previous studies in this model (12), there was no difference in the heart calcification between any of the groups.
Figure 2. Aorta arch calcification in response to therapies.
CKD animals were treated beginning at 25 weeks of age with control vehicle (CTL), calcium in drinking water daily for 10 weeks (Ca), zoledronic acid 20 ug/kg given subcutaneously once at 25 weeks (ZOL), or the combination of Ca + ZOL. The results were compared to normal animals (NL) treated with vehicle. The aorta calcium content was determined biochemically. The results demonstrated that CKD animals given any treatment had increased aorta calcification compared to NL animals, and that the treatment with calcium increased calcification further among the CKD animals. The co-administration of calcium with zoledronic acid led to a reduction of the calcium induced arterial calcification compared to calcium alone. *= different than NL; + = different than CKD-CTL; $ = different than CKD + Ca; all p < 0.05. Graphs are mean ± SEM, n = 8 to 10 per group.
Bone outcomes
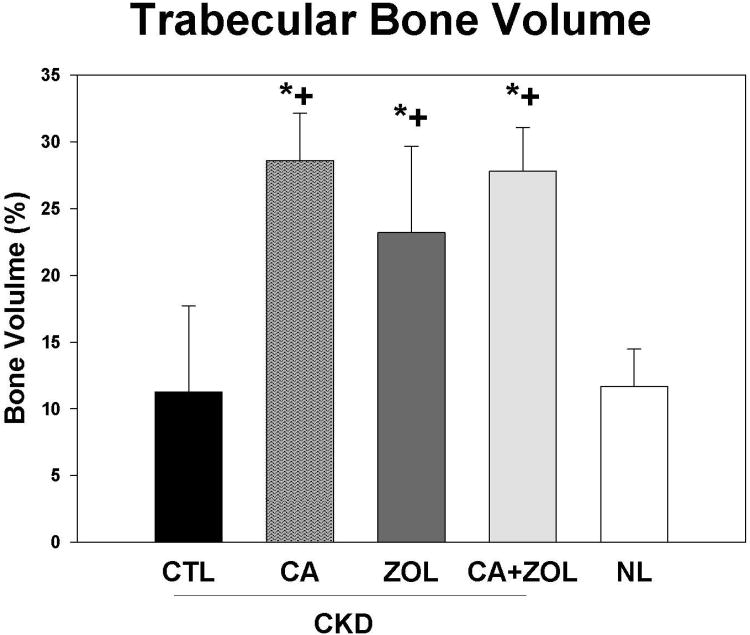
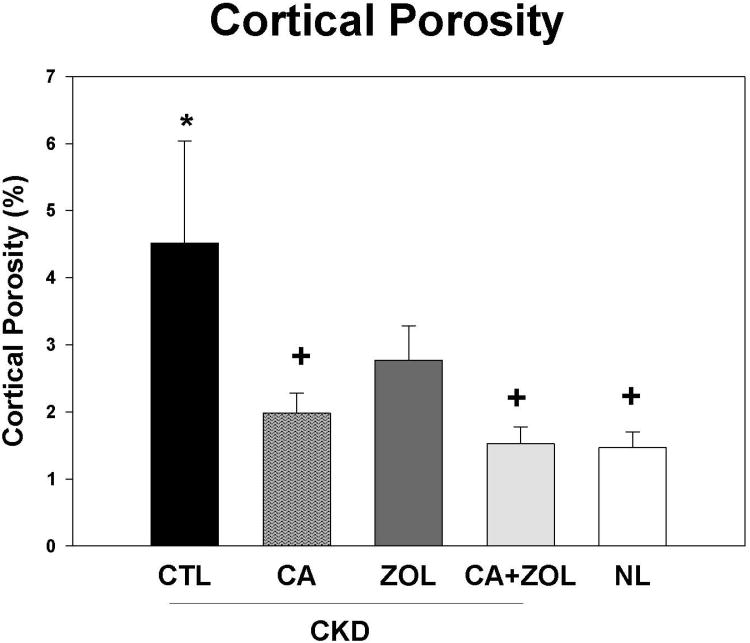
By microCT, there was no difference in the trabecular bone volume between CKD and NL animals. ZOL, calcium, and calcium + ZOL treatment significantly increased percent bone volume (p<0.0001) in CKD rats but there was no difference among these three treatment groups (Figure 3A). The bone volume was strongly correlated with trabecular number (r = 0.77, p < 0.001).The trabecular bone volume was positively correlated with the calcium (r = 0.53) and FGF23 (r = 0.42), negatively correlated with PTH (r = -0.47; all p < 0.01), but not correlated with phosphorus. Cortical porosity was increased in CKD animals compared to normal animals, with significant reduction in animals treated with calcium and calcium plus ZOL; the ZOL treatment showed a non-significant reduction (p = 0.13;Figure 3B). Corresponding three-dimensional reconstruction of the microCT images are shown in Figure 3C. The magnitude of cortical porosity was strongly associated with phosphorus (r = 0.80) and PTH (r = 0.70, both p < 0.001), but not associated with FGF23, calcium or bone volume.
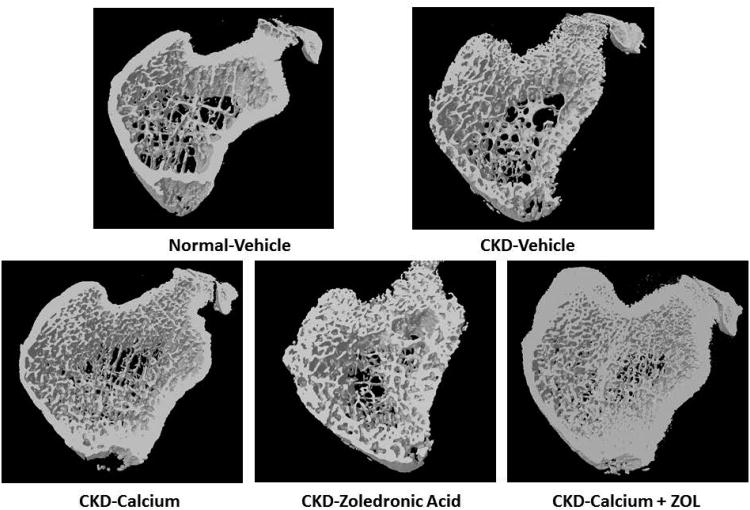
Figure 3. Trabecular bone volume and cortical porosity in response to therapies.
CKD animals were treated beginning at 25 weeks of age with control vehicle (CTL), calcium in drinking water daily for 10 weeks (Ca), zoledronic acid 20 ug/kg given subcutaneously once at 25 weeks (ZOL), or the combination of Ca + ZOL. The results were compared to normal animals (NL) treated with vehicle. At sacrifice at 35 weeks, the tibiae were assessed by microCT for trabecular bone volume (A) and cortical porosity (B). The results demonstrate that there was no difference in trabecular bone volume between CKD and NL animals treated with vehicle. However, all of the treatments led to higher bone volume in the CKD animals compared to CKD-vehicle. In contrast, the cortical porosity was increased in vehicle treated CKD animals compared to NL animals. Treatment with calcium, or calcium plus zoledronic acid, but not ZOL alone, reduced cortical porosity to NL levels. The 3D reconstructions (C) provide visualization of these trabecular and cortical effects across groups.*= different than NL; + = different than CKD-CTL; all p < 0.05. Graphs are mean ± SEM, n = 7 to 10 per group.
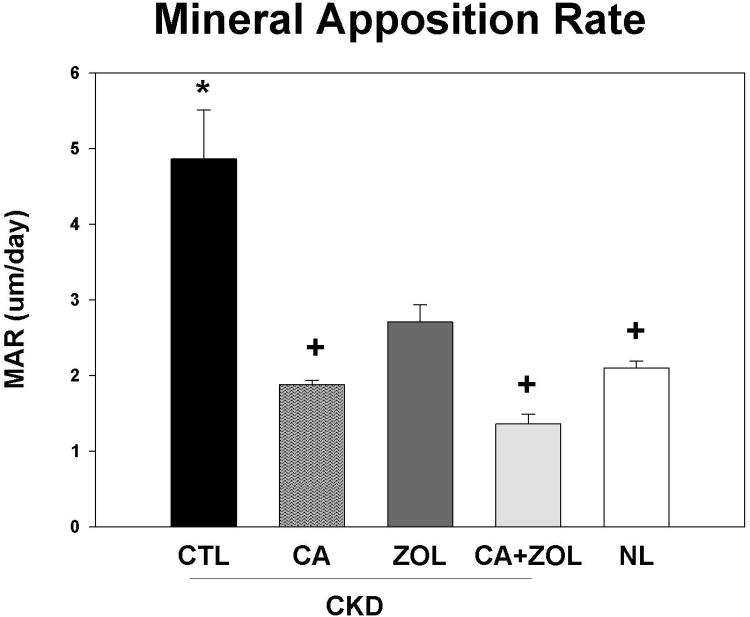
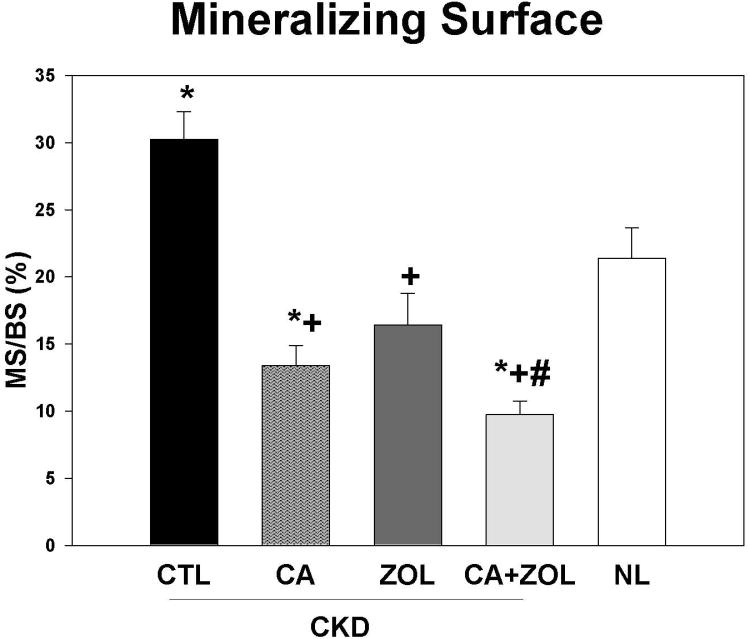
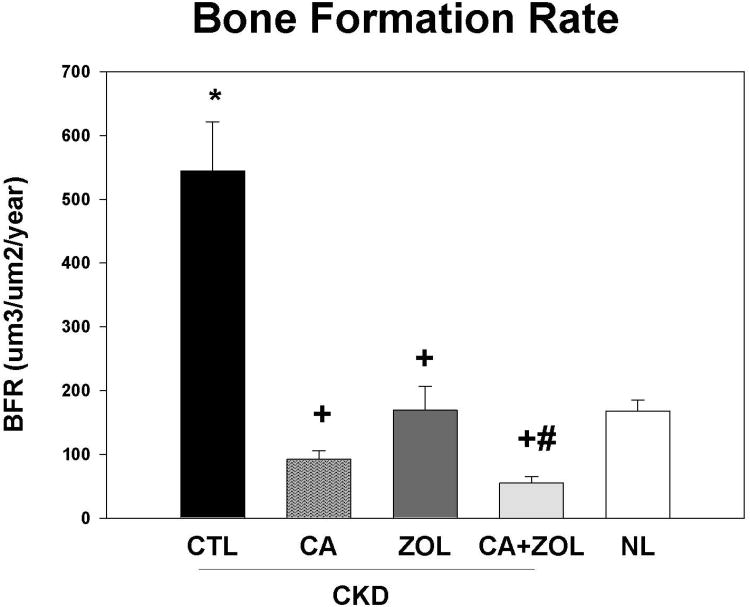
Dynamic bone histomorphometry demonstrated that the mineralization apposition rate (MAR) was increased in the CKD animals compared to normal, and decreased by all treatments in the CKD animals to levels similar to that observed in NL animals (Figure 4A). Similar results were observed for mineralizing surface (MS/BS; Figure 4B) and BFR (Figure 4C). PTH and phosphorus were positively associated with all three histomorphometric indices with the strongest relationship with MAR (r = 0.61 for PTH and r = 0.42 for phosphorus, both p < 0.01). The calcium level was negatively correlated only with BFR (r = -0.35, p = 0.03) and MS/BS (r =-0.53, p < 0.001).
Figure 4. Bone histomorphometry in response to therapies.
CKD animals were treated beginning at 25 weeks of age with control vehicle (CTL), calcium in drinking water daily for 10 weeks (Ca), zoledronic acid 20 ug/kg given subcutaneously once at 25 weeks (ZOL), or the combination of Ca + ZOL. The results were compared to normal animals (NL) treated with vehicle. At sacrifice at 35 weeks, the tibiae were processed for bone histomorphometry. The results for mineral apposition rate (MAR; A), mineralizing surface as a percentage of bone surface (MS/BS; B), and bone formation rate (BFR; C). The results demonstrate that there was higher MAR, BFR, and MS/BS in the CKD animals treated with vehicle compared to NL animals. Treatment with calcium orzoledronic acid reduced all parameters to levels similar in the NL animals with additive reduction in the calcium plus zoledronic acid group. The *= different than NL; + = different than CKD-CTL; # = different than CKD + ZOL; all p < 0.05. Graphs are mean ± SEM, n = 7 to 10 per group.
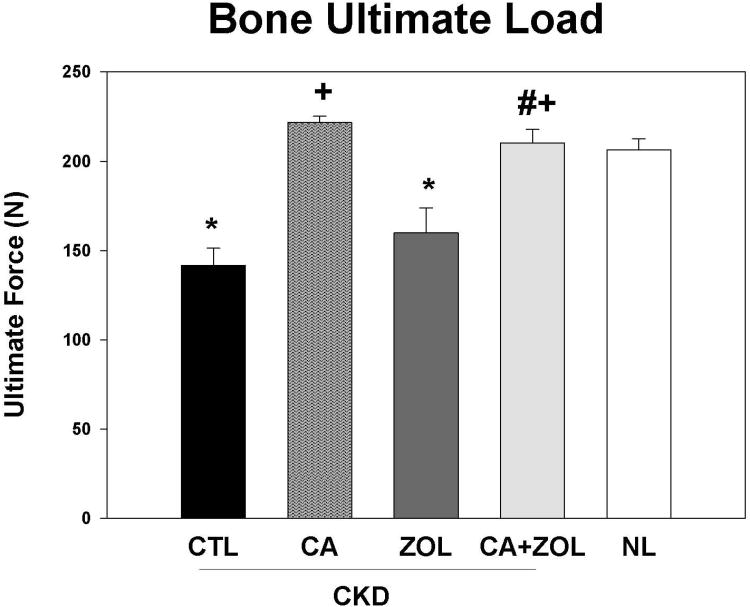
Ultimate load and stiffness of the femoral diaphysis were both significantly lower in untreated CKD animals compared to NL (Table 1, Figure 5). CKD animals treated with ZOL had properties similar to untreated CKD (and lower than NL-VEH), while those treated with calcium, or the combination of ZOL and calcium had mechanical properties significantly higher than CKD and similar to NL.
Table 1. Biomechanical properties of femoral diaphysis.
| CKD-CTL (n=10) | CKD-Ca (n=9) | CKD-ZOL (n=9) | CKD-Ca + ZOL(n=6) | NL | |
|---|---|---|---|---|---|
| Ultimate Load, N | 142 ± 31 * | 222 ± 11 +# | 160 ± 42 * | 219 ± 18 +# | (n=10) |
| Stiffness, N/mm | 395 ± 55 * | 512 ± 20 +# | 378 ± 90 * | 473 ± 32 +# | 206 ± 20 |
| Energy to Failure, mJ | 72 ± 31 | 83 ± 12 | 80 ± 37 | 85 ± 18 | 473 ± 32 |
Data presented as mean ± SD.
p< 0.05 vs NL,
CKD-CTL
and CKD-ZOL.
Figure 5. Bone biomechanics in response to therapies.
CKD animals were treated beginning at 25 weeks of age with control vehicle (CTL), calcium in drinking water daily for 10 weeks (Ca), zoledronic acid 20 ug/kg given subcutaneously once at 25 weeks (ZOL), or the combination of Ca + ZOL. The results were compared to normal animals (NL) treated with vehicle. At sacrifice at 35 weeks, the femora were tested via three-point bending. The results demonstrate that the CKD animals had reduced ultimate load (fracture predisposition) than normal animals and this was improved by calcium treatment with or without zoledronic acid The *= different than NL; + = different than CKD-CTL; # = different than CKD + ZOL; all p < 0.05. Graphs are mean ± SEM, n = 7 to 10 per group.
Relationship of bone to cardiovascular outcomes
The aortic arch calcification was correlated with trabecular bone volume (r= 0.43, p = 0.005) and cortical porosity (r = 0.34, p = 0.03), but not with any of the three histormorphometry measures.
Discussion
Multiple studies have documented increased fractures in patients with CKD due to a combination of abnormal bone volume and bone quality(17,18). While secondary analyses support the use of bisphosphonates in post-menopausal women with moderate to advanced kidney disease, these studies did not enroll individuals with elevated PTH levels (5,7). Clinical practice guidelines recommend lowering PTH as a primary treatment approach in CKD(8). There is no evidence that bisphosphonates directly reduce PTH; however their potent bone resorption inhibition could effectively offset the osteoclast-medicated effects of high PTH. We have previously shown efficacy of zoledronic acid in improving trabecular bone volume in animals with earlier stages of CKD and mild hyperparathyroidism(9). In the present study we tested the hypothesis that zoledronic acid would be efficacious in animals with more advanced CKD, severe hyperparathyroidism, and even when combined with oral calcium supplementation. The latter has been shown to induce low bone turnover in patients on dialysis(19). Our results demonstrate that individually, zoledronic acid and calcium each improved trabecular bone volume and reduced bone formation rate and the mineralizing surface to a similar magnitude, but only the calcium treatment improved cortical porosity and cortical biomechanical properties. In addition, only the calcium treatment induced hypercalcemia and increased arterial calcification, although hypercalcemia was not a prerequisite for aortic calcification. These results demonstrate that lowering PTH is more effective at improving cortical bone porosity and biomechanical integrity, however, doing so with calcium may adversely impact arterial calcification risk. The elevations in plasma calcium levels may have a played a role in inducing aorta calcification, and it is possible that lower doses of calcium would not results in this adverse effect. In our previous study in this animal model we demonstrated that the administration of calcium at similar doses to the current study led to aorta calcification. This occurred even if the calcium was given together with a calcimimetic that resulting in normal plasma calcium levels resulting in increased calcium load but normal calcium levels(12). These results would imply that it is the calcium load, not the level itself that contributes to calcification. However, dose finding studies would be required to fully assess the importance of elevated calcium levels versus calcium load or decreased bone formation.
The concern over the use of bisphosphonates in CKD has been the potential induction of low turnover bone disease(8). In the present study, zoledronic acid did not suppress BFR any more than calcium alone. In humans, zoledronic acid has been shown to improve bone mineral density and reduce bone fractures in both primary and secondary prevention trials in patients with post-menopausal osteoporosis(20-22). In contrast, in post-menopausal osteoporosis the efficacy of calcium alone on fracture prevention is minimal to uncertain(23) and its adverse effects have recently come under scrutiny(24). We cannot definitively determine if the efficacy of calcium on bone in the present study was due to calcium itself, or suppression of PTH, but based on the strong correlation of PTH, but not calcium, with cortical porosity it is most likely PTH-mediated. Interestingly, the effects of zoledronic acid on improving trabecular bone volume in the present study was less than compared to our previous study(9) when the drug was given at an earlier time point with less severe hyperparathyroidism and over a 5 week duration. One possible explanation is that the severe hyperparathyroidism induced such a profound increase in bone resorption that the zoledronic acid bound to bone was actually resorbed out of bone with the ‘first pass’ effect of the osteoclasts, leaving little drug bound for continued effect as the drug was only dosed one time. This dosing schedule was used to mimic the dosing to osteoporosis patients, where a single dose is given once per year – about two remodeling cycles in humans. Ten weeks in a rat is also equivalent to about two remodeling cycles. If true that a single dose was inefficient to control osteoclasts over this duration, this could be overcome by using a less potent bisphosphonate, such as alendronate or risedronate, normally administered on a more regular basis (daily/weekly). Current clinical practice guidelines recommend dose reduction of bisphosphonates in the setting of CKD(8). Our data suggests that the pharmacokinetics could be altered in the setting of significant secondary hyperparathyroidism requiring increased dosing frequency although perhaps a lower dose could still be given. Thus, further studies are needed to evaluate pharmacokinetics of bisphosphonates in CKD, and further studies should determine if the efficacy and adverse effects of zoledronic acid are different when given to CKD animals and humans with and without hyperparathyroidism. It should also be emphasized that changes in bone volume may not reflect an improvement in biomechanical properties/strength. Zoledronic acid did not improve cortical measures of strength, but similar measures to test strength of trabecular bone are not available.
In our CKD animals, the hyperparathyroidism produced profound changes in cortical bone porosity and biomechanical properties that were corrected with the administration of calcium. Cortical bone is more adversely affected than trabecular bone in hyperparathyroidism(25,26). However, traditional assessment of renal osteodystrophy by bone biopsy in CKD patients has been with trabecular bone. Our results suggest that the apparent disconnect between PTH and bone turnover assessed by histology in cross sectional studies(27) may be partly due to the use of trabecular bone. Supporting this is that in patients with CKD, the distal radius X-ray absorptiometry (DXA), which is nearly entirely cortical bone, is more predictive of fractures than other sites(28). These data, and our results in the present study, support current clinical practice guidelines that emphasize treating hyperparathyroidism as first line agent in patients with CKD stages 3-5D(8). However, in a recent study of nearly 4000 hemodialysis patients, the largest fracture assessment study to date in CKD, there was no effect of the calcimimetic cinacalcet on fractures despite improved PTH(29). However, this study only evaluated clinical (reported) fractures and thus may have underestimated true fracture prevalence and efficacy of lowering PTH. Thus more studies specifically designed to compare the treatment of hyperparathyroidism versus anti-resorptive agents on fracture prevention in CKD are required.
Previous studies have demonstrated that bisphosphonates reduce arterial calcification in CKD animal models. Tamura found that etidronate decreased arterial calcification in 5/6th nephrectomy rats also given calcitriol(30). Price found similar efficacy in the adenine model of CKD treated with alendronate or ibandronate(31). Lomashevilli found that pamidronate reduced arterial calcification in 5/6th nephrectomy animals treated with high phosphate diet, but also found suppression of bone turnover and concluded the drug may be efficacious but not safe, although there was no comparison of the degree of bone turnover to normal animals (32). Bisphosphonates work by suppression of remodeling and therefore induce lower bone formation rates are expected. In our previous study, the level of BFR suppression was no different in CKD animals treated with zoledronic acid compared to normal animals treated with zoledronic acid(9). In the current study, the turnover was equally suppressed with zoledronic acid compared to calcium given in the drinking water (to act as a phosphate binder, a treatment used worldwide).
A major concern about inducing suppressed bone turnover is the association of such suppression with arterial calcification in patients with CKD and in dialysis patients(19,33,34). This has been hypothesized to be due to the inability of bone to take up excess calcium in the setting of low bone formation rates(35), predisposing to extra skeletal deposition. In the present study we found that despite similar suppression of BFR with zoledronic acid and calcium, only the calcium induced arterial calcification and suppression of PTH and zoledronic acid appears to ameliorate this effect but had no efficacy by itself. Thus, positive calcium balance which occurs in CKD(36) may be a critical factor involved in the development of arterial calcification. The calcium treatment also increased FGF23 as has been previously noted in animals(37). In other studies of human and animal arteries, FGF23 appeared to be protective against arterial calcification, but only when klotho was present(38). We have previously shown in our animal model that klotho expression in the kidney is markedly decrease as early as 20 weeks(11) and thus, assuming similar suppression in arteries, this potential positive effect of FGF23 may have been negated with advanced kidney disease. The mechanism by which calcium stimulates FGF23 is likely direct on the osteocytes, as calcium altered FGF23 secretion in rats who had undergone a parathyroidectomy and then were infused with calcium or given a high calcium diet (37). This increase in FGF23 may have adverse cardiac consequences in rodents(33,39), and higher FGF23 levels have been found to predict progression of LVH in patients with CKD 3-4(39). In the current study, we did not observe differences in the LVH in animals with the various treatments, but the duration of therapy was relatively short.
In humans with ESRD, there are only small studies examining the role of bisphosphonates in the prevention of arterial calcification. In small cohort or observational studies from Japan, etidronate reduced arterial calcification(40-42). In contrast, in a randomized controlled trial of 50 patients, Toussaint et al found that alendronate had no effect on arterial calcification(43). These differences may be explained by the increased mineral dissolution properties of etridronate compared to alendronate and other nitrogen containing bisphosphonates, although a number of other explanations such as dosing and disease stage are also plausible.
In summary, we found similar efficacy of calcium and a single dose of zoledronic acid on improving trabecular bone volume in animals with advanced CKD and severe hyperparathyroidism. However, only the calcium treatment improved cortical porosity and lowered PTH. Unfortunately, calcium also induced arterial calcification whereas zoledronic acid did not despite similar suppression of bone remodeling. These results suggest that suppression of bone remodeling with bisphosphonates may not have the same adverse consequences as suppression of bone remodeling with calcium. It is important to caution on the extrapolation of changes in bone remodeling or bone volume in animal studies to human disease. However, the data supports the conduct of a study in CKD patients directly testing whether suppression of PTH with non-calcium containing agents (e.g. calcimimetics) are more effective than anti-resorptive agents for fracture prevention.
Acknowledgments
This work was supported by the NIH NIAMS R015R01 AR058005 (SMM) and S10-RR023710 (microCT equipment grant). We thank Drew Brown for tissue dissections, CT scanning and analysis.
Footnotes
Disclosures: SM has received honoraria and grant support from Sanofi, and stock in Eli Lilly. All other authors report no conflicts of interest. MRA has received consultation honoraria from Merck and research support from Merck and Eli Lilly.
References
- 1.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G. Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69(11):1945–53. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 2.Nickolas TL, Leonard MB, Shane E. Chronic kidney disease and bone fracture: a growing concern. Kidney Int. 2008;74(6):721–31. doi: 10.1038/ki.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nickolas T, Stein E, Cohen A, Staron R, McMahon DJ, Leonard MB, Shane E. Bone mass and microarchitecture in CKD patients with fracture. Journal of the American Society of Nephrology. 2010 doi: 10.1681/ASN.2009121208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lindberg JS, Moe SM. Osteoporosis in end-state renal disease. Semin Nephrol. 1999;19(2):115–22. [PubMed] [Google Scholar]
- 5.Miller PD, Roux C, Boonen S, Barton IP, Dunlap LE, Burgio DE. Safety and efficacy of risedronate in patients with age-related reduced renal function as estimated by the Cockcroft and Gault method: a pooled analysis of nine clinical trials. J Bone Miner Res. 2005;20(12):2105–15. doi: 10.1359/JBMR.050817. [DOI] [PubMed] [Google Scholar]
- 6.Miller PD, Schwartz EN, Chen P, Misurski DA, Krege JH. Teriparatide in postmenopausal women with osteoporosis and mild or moderate renal impairment. Osteoporos Int. 2007;18(1):59–68. doi: 10.1007/s00198-006-0189-8. [DOI] [PubMed] [Google Scholar]
- 7.Jamal SA, Bauer DC, Ensrud KE, Cauley JA, Hochberg M, Ishani A, Cummings SR. Alendronate treatment in women with normal to severely impaired renal function: an analysis of the fracture intervention trial. J Bone Miner Res. 2007;22(4):503–8. doi: 10.1359/jbmr.070112. [DOI] [PubMed] [Google Scholar]
- 8.KDIGO. Clinical Practice Guidelines for the Management of CKD-MBD. Kidney International. 2009;76(S113):S1–S130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 9.Allen MR, Chen NX, Gattone VH, 2nd, Chen X, Carr AJ, Leblanc P, Brown D, Moe SM. Skeletal effects of zoledronic acid in an animal model of chronic kidney disease. Osteoporos Int. 2012 doi: 10.1007/s00198-012-2103-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moe SM, Chen NX, Seifert MF, Sinders RM, Duan D, Chen X, Liang Y, Radcliff JS, White KE, Gattone VH., 2nd A rat model of chronic kidney disease-mineral bone disorder. Kidney Int. 2009;75(2):176–84. doi: 10.1038/ki.2008.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moe SM, Radcliffe JS, White KE, Gattone VH, 2nd, Seifert MF, Chen X, Aldridge B, Chen NX. The pathophysiology of early-stage chronic kidney disease-mineral bone disorder (CKD-MBD) and response to phosphate binders in the rat. J Bone Miner Res. 2011;26(11):2672–81. doi: 10.1002/jbmr.485. [DOI] [PubMed] [Google Scholar]
- 12.Moe SM, Seifert MF, Chen NX, Sinders RM, Chen X, Duan D, Henley C, Martin D, Gattone VH., 2nd R-568 reduces ectopic calcification in a rat model of chronic kidney disease-mineral bone disorder (CKD-MBD) Nephrol Dial Transplant. 2009 doi: 10.1093/ndt/gfp078. [DOI] [PubMed] [Google Scholar]
- 13.Marra M, Salzano G, Leonetti C, Tassone P, Scarsella M, Zappavigna S, Calimeri T, Franco R, Liguori G, Cigliana G, Ascani R, La Rotonda MI, Abbruzzese A, Tagliaferri P, Caraglia M, De Rosa G. Nanotechnologies to use bisphosphonates as potent anticancer agents: the effects of zoledronic acid encapsulated into liposomes. Nanomedicine. 2011;7(6):955–64. doi: 10.1016/j.nano.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Allen MR, Burr DB. The pathogenesis of bisphosphonate-related osteonecrosis of the jaw: so many hypotheses, so few data. J Oral Maxillofac Surg. 2009;67(5 Suppl):61–70. doi: 10.1016/j.joms.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Patntirapong S, Singhatanadgit W, Chanruangvanit C, Lavanrattanakul K, Satravaha Y. Zoledronic acid suppresses mineralization through direct cytotoxicity and osteoblast differentiation inhibition. J Oral Pathol Med. 2012;41(9):713–20. doi: 10.1111/j.1600-0714.2012.01154.x. [DOI] [PubMed] [Google Scholar]
- 16.Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone & Mineral Research. 1987;2(6):595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- 17.Nickolas TL, Cremers S, Zhang A, Thomas V, Stein E, Cohen A, Chauncey R, Nikkel L, Yin MT, Liu XS, Boutroy S, Staron RB, Leonard MB, McMahon DJ, Dworakowski E, Shane E. Discriminants of prevalent fractures in chronic kidney disease. J Am Soc Nephrol. 2011;22(8):1560–72. doi: 10.1681/ASN.2010121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ensrud KE, Lui LY, Taylor BC, Ishani A, Shlipak MG, Stone KL, Cauley JA, Jamal SA, Antoniucci DM, Cummings SR. Renal function and risk of hip and vertebral fractures in older women. Arch Intern Med. 2007;167(2):133–9. doi: 10.1001/archinte.167.2.133. [DOI] [PubMed] [Google Scholar]
- 19.Barreto DV, Barreto Fde C, de Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, Moyses RM, Neves KR, Jorgetti V, Miname M, Santos RD, Canziani ME. Phosphate binder impact on bone remodeling and coronary calcification--results from the BRiC study. Nephron Clin Pract. 2008;110(4):c273–83. doi: 10.1159/000170783. [DOI] [PubMed] [Google Scholar]
- 20.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 21.Brown JE, Ellis SP, Lester JE, Gutcher S, Khanna T, Purohit OP, McCloskey E, Coleman RE. Prolonged efficacy of a single dose of the bisphosphonate zoledronic acid. Clin Cancer Res. 2007;13(18 Pt 1):5406–10. doi: 10.1158/1078-0432.CCR-07-0247. [DOI] [PubMed] [Google Scholar]
- 22.Calis KA, Pucino F. Zoledronic acid and secondary prevention of fractures. N Engl J Med. 2007;357(18):1861–2. doi: 10.1056/NEJMe078192. [DOI] [PubMed] [Google Scholar]
- 23.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, Burckhardt P, Li R, Spiegelman D, Specker B, Orav JE, Wong JB, Staehelin HB, O'Reilly E, Kiel DP, Willett WC. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86(6):1780–90. doi: 10.1093/ajcn/86.5.1780. [DOI] [PubMed] [Google Scholar]
- 24.Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, Reid IR. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parfitt AM. The hyperparathyroidism of chronic renal failure: a disorder of growth. Kidney International. 1997;52(1):3–9. doi: 10.1038/ki.1997.297. [DOI] [PubMed] [Google Scholar]
- 26.Parfitt AM. Misconceptions (2): turnover is always higher in cancellous than in cortical bone. Bone. 2002;30(6):807–9. doi: 10.1016/s8756-3282(02)00735-4. [DOI] [PubMed] [Google Scholar]
- 27.Sprague SM, Moe SM. The Case for Routine Parathyroid Hormone Monitoring. Clin J Am Soc Nephrol. 2012 doi: 10.2215/CJN.04650512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leonard MB. A structural approach to skeletal fragility in chronic kidney disease. Semin Nephrol. 2009;29(2):133–43. doi: 10.1016/j.semnephrol.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix CH, Moe SM, Trotman ML, Wheeler DC, Parfrey PS. A Randomized Trial of Cinacalcet in Patients on Hemodialysis with Secondary Hyperparathyroidism. N Engl J Med 2012 [Google Scholar]
- 30.Michaelsson K, Melhus H, Warensjo Lemming E, Wolk A, Byberg L. Long term calcium intake and rates of all cause and cardiovascular mortality: community based prospective longitudinal cohort study. BMJ. 2013;346:f228. doi: 10.1136/bmj.f228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arterioscler Thromb Vasc Biol. 2001;21(5):817–24. doi: 10.1161/01.atv.21.5.817. [DOI] [PubMed] [Google Scholar]
- 32.Lomashvili KA, Monier-Faugere MC, Wang X, Malluche HH, O'Neill WC. Effect of bisphosphonates on vascular calcification and bone metabolism in experimental renal failure. Kidney Int. 2009 doi: 10.1038/ki.2008.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B, Bonewald LF, Stubbs JR, Wacker MJ. FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab. 2013;304(8):E863–73. doi: 10.1152/ajpendo.00596.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moe SM. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. Eur J Clin Invest. 2006;36(2):51–62. doi: 10.1111/j.1365-2362.2006.01665.x. [DOI] [PubMed] [Google Scholar]
- 35.Kurz P, Monier-Faugere MC, Bognar B, Werner E, Roth P, Vlachojannis J, Malluche HH. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney International. 1994;46(3):855–61. doi: 10.1038/ki.1994.342. [DOI] [PubMed] [Google Scholar]
- 36.Hill KM, Martin BR, Wastney ME, McCabe GP, Moe SM, Weaver CM, Peacock M. Oral calcium carbonate affects calcium but not phosphorus balance in stage 3-4 chronic kidney disease. Kidney Int. 2012 doi: 10.1038/ki.2012.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez-Ortiz ME, Lopez I, Munoz-Castaneda JR, Martinez-Moreno JM, Ramirez AP, Pineda C, Canalejo A, Jaeger P, Aguilera-Tejero E, Rodriguez M, Felsenfeld A, Almaden Y. Calcium deficiency reduces circulating levels of FGF23. J Am Soc Nephrol. 2012;23(7):1190–7. doi: 10.1681/ASN.2011101006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim K, Lu TS, Molostvov G, Lee C, Lam FT, Zehnder D, Hsiao LL. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125(18):2243–55. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 39.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nitta K, Akiba T, Suzuki K, Uchida K, Watanabe R, Majima K, Aoki T, Nihei H. Effects of cyclic intermittent etidronate therapy on coronary artery calcification in patients receiving long-term hemodialysis. Am J Kidney Dis. 2004;44(4):680–8. [PubMed] [Google Scholar]
- 41.Hashiba H, Aizawa S, Tamura K, Shigematsu T, Kogo H. Inhibitory effects of etidronate on the progression of vascular calcification in hemodialysis patients. Ther Apher Dial. 2004;8(3):241–7. doi: 10.1111/j.1526-0968.2004.00136.x. [DOI] [PubMed] [Google Scholar]
- 42.Quinn SJ, Thomsen AR, Pang JL, Kantham L, Brauner-Osborne H, Pollak M, Goltzman D, Brown EM. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am J Physiol Endocrinol Metab. 2013;304(3):E310–20. doi: 10.1152/ajpendo.00460.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. Am J Kidney Dis. 2010;56(1):57–68. doi: 10.1053/j.ajkd.2009.12.039. [DOI] [PubMed] [Google Scholar]