Abstract
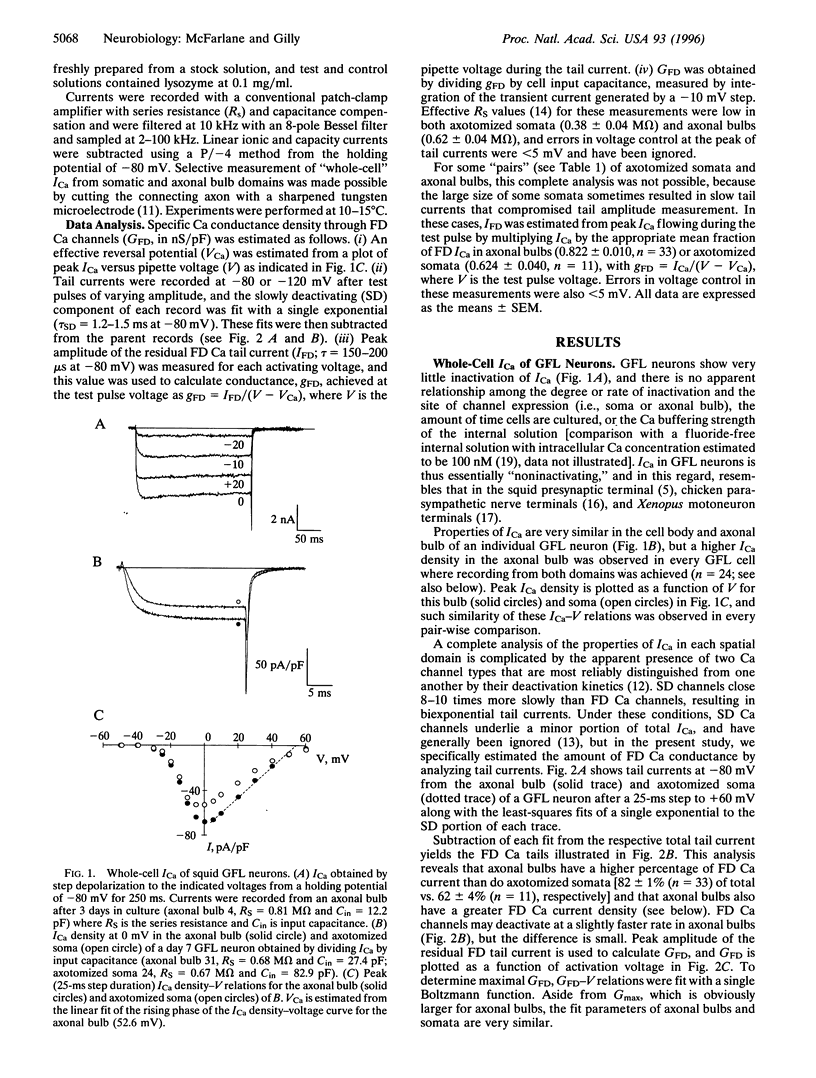
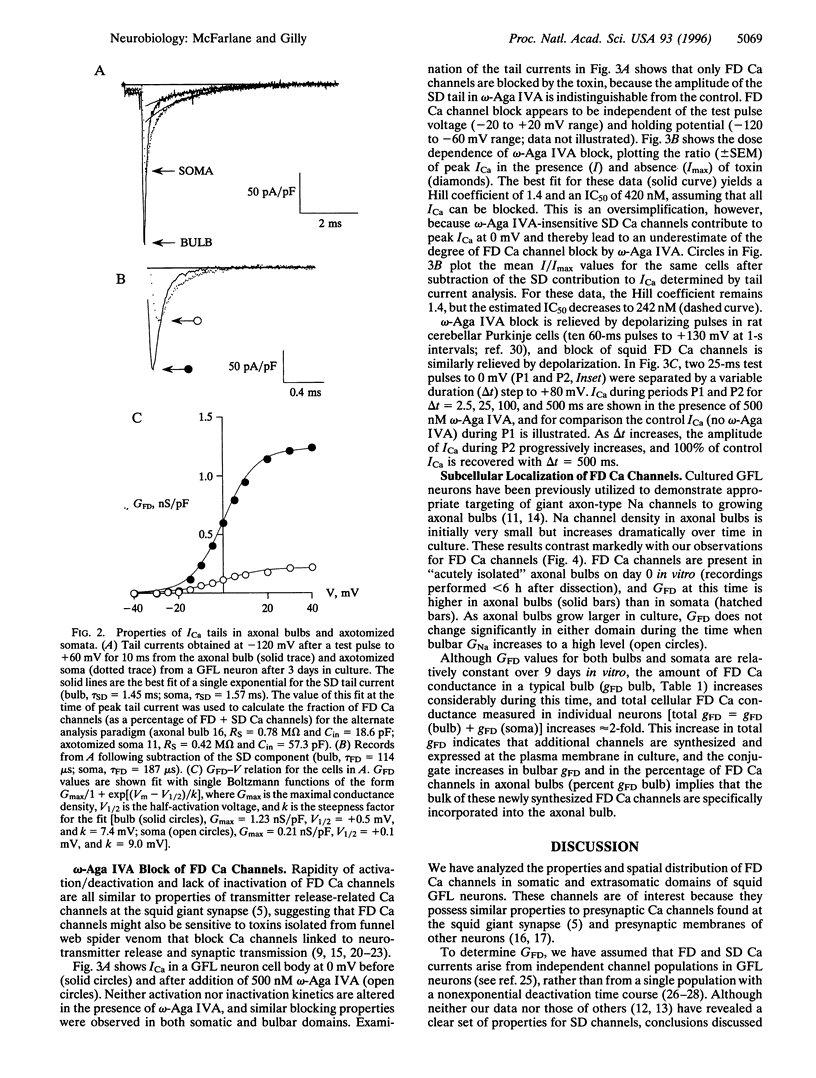
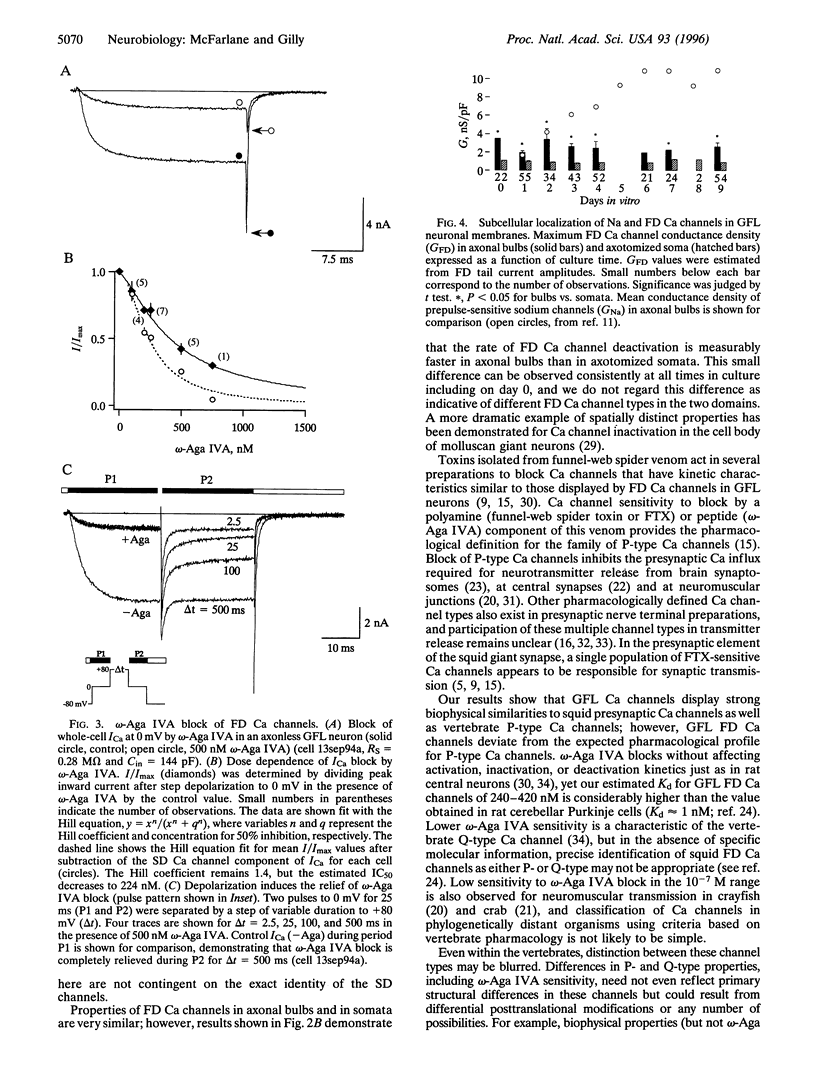
Whole-cell voltage clamp was used to investigate the properties and spatial distribution of fast-deactivating (FD) Ca channels in squid giant fiber lobe (GFL) neurons. Squid FD Ca channels are reversibly blocked by the spider toxin omega-Agatoxin IVA with an IC50 of 240-420 nM with no effect on the kinetics of Ca channel gating. Channels with very similar properties are expressed in both somatic and axonal domains of cultured GFL neurons, but FD Ca channel conductance density is higher in axonal bulbs than in cell bodies at all times in culture. Channels presumably synthesized during culture are preferentially expressed in the growing bulbs, but bulbar Ca conductance density remains constant while Na conductance density increases, suggesting that processes determining the densities of Ca and Na channels in this extrasomatic domain are largely independent. These observations suggest that growing axonal bulbs in cultured GFL neurons are not composed entirely of "axonal" membranes because FD Ca channels are absent from the giant axon in situ but, rather, suggest a potential role for FD Ca channels in mediating neurotransmitter release at the motor terminals of the giant axon.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araque A., Clarac F., Buño W. P-type Ca2+ channels mediate excitatory and inhibitory synaptic transmitter release in crayfish muscle. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4224–4228. doi: 10.1073/pnas.91.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. M., Tsuda Y., Wilson D. L. A description of activation and conduction in calcium channels based on tail and turn-on current measurements in the snail. J Physiol. 1983 Nov;344:549–583. doi: 10.1113/jphysiol.1983.sp014956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly L., Chase P. B., Stimers J. R. Calcium current activation kinetics in neurones of the snail Lymnaea stagnalis. J Physiol. 1984 Mar;348:187–207. doi: 10.1113/jphysiol.1984.sp015105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton M. P., Augustine G. J. Classification of presynaptic calcium channels at the squid giant synapse: neither T-, L- nor N-type. Brain Res. 1990 Aug 13;525(1):133–139. doi: 10.1016/0006-8993(90)91328-e. [DOI] [PubMed] [Google Scholar]
- Chow R. H. Cadmium block of squid calcium currents. Macroscopic data and a kinetic model. J Gen Physiol. 1991 Oct;98(4):751–770. doi: 10.1085/jgp.98.4.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Caputo C., Bezanilla F. Voltage-dependent calcium channel in the squid axon. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1743–1745. doi: 10.1073/pnas.80.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M. L., Garcia-Calvo M., Hidalgo P., Lee A., MacKinnon R. Purification and characterization of three inhibitors of voltage-dependent K+ channels from Leiurus quinquestriatus var. hebraeus venom. Biochemistry. 1994 Jun 7;33(22):6834–6839. doi: 10.1021/bi00188a012. [DOI] [PubMed] [Google Scholar]
- Gilly W. F., Brismar T. Properties of appropriately and inappropriately expressed sodium channels in squid giant axon and its somata. J Neurosci. 1989 Apr;9(4):1362–1374. doi: 10.1523/JNEUROSCI.09-04-01362.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilly W. F., Lucero M. T., Horrigan F. T. Control of the spatial distribution of sodium channels in giant fiber lobe neurons of the squid. Neuron. 1990 Nov;5(5):663–674. doi: 10.1016/0896-6273(90)90220-a. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Heidelberger R., Matthews G. Calcium influx and calcium current in single synaptic terminals of goldfish retinal bipolar neurons. J Physiol. 1992 Feb;447:235–256. doi: 10.1113/jphysiol.1992.sp019000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess P. Calcium channels in vertebrate cells. Annu Rev Neurosci. 1990;13:337–356. doi: 10.1146/annurev.ne.13.030190.002005. [DOI] [PubMed] [Google Scholar]
- Lemos J. R., Nowycky M. C. Two types of calcium channels coexist in peptide-releasing vertebrate nerve terminals. Neuron. 1989 May;2(5):1419–1426. doi: 10.1016/0896-6273(89)90187-6. [DOI] [PubMed] [Google Scholar]
- Llano I., Bookman R. J. Ionic conductances of squid giant fiber lobe neurons. J Gen Physiol. 1986 Oct;88(4):543–569. doi: 10.1085/jgp.88.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Steinberg I. Z., Walton K. Presynaptic calcium currents in squid giant synapse. Biophys J. 1981 Mar;33(3):289–321. doi: 10.1016/S0006-3495(81)84898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Hillman D. E., Cherksey B. Distribution and functional significance of the P-type, voltage-dependent Ca2+ channels in the mammalian central nervous system. Trends Neurosci. 1992 Sep;15(9):351–355. doi: 10.1016/0166-2236(92)90053-b. [DOI] [PubMed] [Google Scholar]
- Llinás R., Sugimori M., Lin J. W., Cherksey B. Blocking and isolation of a calcium channel from neurons in mammals and cephalopods utilizing a toxin fraction (FTX) from funnel-web spider poison. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1689–1693. doi: 10.1073/pnas.86.5.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matteson D. R., Armstrong C. M. Properties of two types of calcium channels in clonal pituitary cells. J Gen Physiol. 1986 Jan;87(1):161–182. doi: 10.1085/jgp.87.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz I. M., Adams M. E., Bean B. P. P-type calcium channels in rat central and peripheral neurons. Neuron. 1992 Jul;9(1):85–95. doi: 10.1016/0896-6273(92)90223-z. [DOI] [PubMed] [Google Scholar]
- Randall A., Tsien R. W. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995 Apr;15(4):2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr W. G., Mintz I. M. Participation of multiple calcium channel types in transmission at single climbing fiber to Purkinje cell synapses. Neuron. 1994 Mar;12(3):605–613. doi: 10.1016/0896-6273(94)90216-x. [DOI] [PubMed] [Google Scholar]
- Robitaille R., Adler E. M., Charlton M. P. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990 Dec;5(6):773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- Smith S. J., Buchanan J., Osses L. R., Charlton M. P., Augustine G. J. The spatial distribution of calcium signals in squid presynaptic terminals. J Physiol. 1993 Dec;472:573–593. doi: 10.1113/jphysiol.1993.sp019963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley E. F., Goping G. Characterization of a calcium current in a vertebrate cholinergic presynaptic nerve terminal. J Neurosci. 1991 Apr;11(4):985–993. doi: 10.1523/JNEUROSCI.11-04-00985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stea A., Tomlinson W. J., Soong T. W., Bourinet E., Dubel S. J., Vincent S. R., Snutch T. P. Localization and functional properties of a rat brain alpha 1A calcium channel reflect similarities to neuronal Q- and P-type channels. Proc Natl Acad Sci U S A. 1994 Oct 25;91(22):10576–10580. doi: 10.1073/pnas.91.22.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor W. R. Two-suction-electrode voltage-clamp analysis of the sustained calcium current in cat sensory neurones. J Physiol. 1988 Dec;407:405–432. doi: 10.1113/jphysiol.1988.sp017423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S., Coombs J. Spatial distribution of Ca currents in molluscan neuron cell bodies and regional differences in the strength of inactivation. J Neurosci. 1988 Jun;8(6):1929–1939. doi: 10.1523/JNEUROSCI.08-06-01929.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner T. J., Adams M. E., Dunlap K. Calcium channels coupled to glutamate release identified by omega-Aga-IVA. Science. 1992 Oct 9;258(5080):310–313. doi: 10.1126/science.1357749. [DOI] [PubMed] [Google Scholar]
- Uchitel O. D., Protti D. A., Sanchez V., Cherksey B. D., Sugimori M., Llinás R. P-type voltage-dependent calcium channel mediates presynaptic calcium influx and transmitter release in mammalian synapses. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3330–3333. doi: 10.1073/pnas.89.8.3330. [DOI] [PMC free article] [PubMed] [Google Scholar]


