Abstract
Background
Antimicrobial resistance in hospital pathogens is an important concern. It can cause longer hospital stays, increase costs, and contribute to increased mortality and morbidity in hospitalized patients. The aim of this study was to categorize and identify gram-negative bacilli capable of ESBLs production and to study the effect of MIC silver nanoparticles on bacteria strains and then study them in Wistar rats.
Material/Methods
A total of 186 clinical samples in 3 hospital of Isfahan city was studied during 8 months. The ESBL assay was performed by disk diffusion method. Minimum inhibitory concentration (MIC) values were determined by agar dilution method. Additionally, ESBLs production was examined by using the standard ESBL disc and DDT (double disk approximation test) procedures. Student’s T-test and ANOVA were used for statistical analysis of the data. The ESBL-producing bacteria were then subjected to minimum concentrations of silver nanoparticles and then examined in Wistar rats.
Results
Of the 186 patients studied, 140 (75.3%) had gram-negative bacilli containing ESBL and 46 (24.7%) had gram-negative bacilli without ESBL and the most prevalent bacteria was identified as Klebsiella pneumonia, with especially strong resistance to cefotaxime. All of these bacteria were sensitive to the silver nanoparticle solution with density of 100 ppm, but the 4 nm size did not show any significant difference from control group Wistar rats at 6 months.
Conclusions
The results seem to indicate a direct correlation between silver nanoparticle solution concentration and the diameter of growth zone for ESBL-producing bacteria. Assays in our study were in vitro; if use of silver nanoparticle particles in vivo proves to be with adverse effects, it could be a valuable alternative to antibiotics.
Keywords: gram-negative bacilli, ESBLs, silver nanoparticle particles, Wistar rat
Background
Patients attending hospitals are exposed to hospital and non-hospital acquired infections (particularly multi-drug-resistant bacteria). One of the most important factors involved in nosocomial infections and related death is infection with gram-negative bacilli, affected by medical intervention type and patient-related factors [1]. Prescription of antibiotics is usually the first treatment attempted (with some exceptions). Bacterial resistance to these drugs and beta-lactamase and inhibitors are growing, and the clinical benefits of these drugs are endangered [2]. In gram-negative bacilli, the most important means of bacterial resistance against beta-lactam antibiotics is the production and release of various beta-lactamase enzymes. Among these bacteria, more than 340 types of enzymes have been detected and identified [3].
These enzymes are carried by the genes located on the bacterial chromosome or, alternatively, the genes on the plasmid and transposons [4]. ESBL-producing microorganisms challenge physicians responsible for infection control, clinical microbiologists, and researchers working on new antibiotics). These enzymes can hydrolyze the third- and fourth- generation cephalosporins such as ceftazidime, cefotaxime, cefepime, and aztreonam. Currently, imipenem has been considered the drug of choice for treating infections caused by ESBL-producing microorganisms [3].These enzymes are inhibited by beta-lactamase inhibitors such as clavulanic acid, tazobactam, and sulbactam. The best tool for assessment of ESBL-producing bacteria is the reduction of their susceptibility to cefotaxime, ceftriaxone, ceftazidime, and aztreonam. After performing phenotypic confirmatory testing, which is specified by viewing the synergistic effect between an indicator and a cephalosporin lactamase inhibitor (usually clavulanic acid), it can be used by screening with the MIC method. Less than 2 Mu grams per milliliters will be considered as a measure for the concentration of producing ESBL. An appropriate criterion for testing has been presented by the National Committee for Clinical Laboratory Standards (NCCLS) [5]. However, ESBL production in bacteria can be confirmed only by performing the above experiments. Since the emergence of resistant microorganisms with ESBL is currently increasing world-wide, there is great demand for improved methods for controlling these bacteria. The characteristics and antimicrobial properties of silver ions have been identified in the past. These ions have been used largely in anti-bacterial catheters, burn bandages, and dental procedures [6]. The advent of nanotechnology has created opportunities to explore the anti-bacterial effects of metal nanoparticles. The antibacterial effects of these particles, due to their small size and large surface: volume ratio, make more contact and exert more impact over the surrounding environment. Silver nanoparticles have greater antibacterial activity in comparison to other metal nanoparticles [7] and there are many theories on the mechanism of anti-bacterial action of silver nanoparticles. It is widely believed that silver nanoparticles penetrate into the bacterial cell membrane, causing leakage and removal of intracellular material, ultimately causing bacteria death. It has been reported that antibacterial effects of silver nanoparticles are reduced when their size is increased. This change in position is related to the particle shape. Spherical particles were used in most of the studies, but it was observed that triangular-shaped particles have more antibacterial properties than the spherical and rod-shaped particles. It has also been reported that the impact and effectiveness of antibacterial properties of silver nanoparticles are also influenced by the type of microorganisms [6]. However, there is currently little evidence to support such a conclusion. Most of these studies were performed on the antibacterial effects of silver nanoparticles in various ways on a very limited number of bacterial species. The purpose of this study was 3-fold. Firstly, we sought to determine the frequency of gram-negative bacilli producing extended spectrum beta-lactamase enzymes (ESBLs) among the pathogenic bacteria-resistant isolates from patients referred to wards in Gharazi, Sina, and Alzahra (SA) hospitals in Isfahan. Secondly, we investigated the anti-bacterial effect of silver nanoparticles on these resistant bacteria with ESBL. Thirdly, we studied the minimum effective concentration of the silver nanoparticles solution on the above-mentioned bacteria in vitro and its effect on Wistar rats in vivo, and effects of their long-term usage in treatment of resistant bacteria with ESBL in the hospital infections if no toxic effects were reported.
Material and Methods
Sampling
Different clinical samples were studied, including sputum, urine, blood, stool, wound secretions, throat secretions, catheter samples, cerebrospinal fluid, and peritoneal and ascites fluid from patients in various departments of Gharazi, Sina, and Alzahra (SA) hospitals in Isfahan from 23/07/2010 through 10/03/2011. The samples were obtained by simple random sampling method. All of the above clinical samples were from patients referred to these hospitals for treatment of their diseases or who were hospitalized.
Identification of strains
The strains obtained from the patients by using the routine laboratory culture medium were isolated and identified. All of the media used in this section were obtained from Merck Co.
Susceptibility testing against antimicrobial agents
Susceptibility of the strains was studied against various antimicrobial agents by using the agar disk diffusion method. The antibiotics used in this experiment included: ciprofloxacin and norfloxacin (BBL Company), ofloxacin, cefotaxime, ceftazidime, ceftriaxone, cefuroxime, cefixime, tetracycline, tobramycin, amikacin, cotrimoxazole, piperacillin, and ticarcillin (Mast, UK). The antibiotics were identical for all of the isolates.
Two disks for synergism test
First the studied bacteria was spread in 3 directions on the surface of Mueller-Hinton agar (MHA; Merck), similar to methods used in the agar disk diffusion test. The discs used were 20 μ per 10 μg amoxicillin/clavulanate disk (Iran Daroo) and 30 μg cefotaxime, ceftazidime, and ceftriaxone (Mast). The amoxicillin/clavulanate disk was placed 20 mm from the center of MHA-coated plates in the medium containing the studied bacteria and other antibiotics in the plates. A positive result was defined as presence of an increased growth inhibition halo by creating synergy around the disk containing oxy immunoassay beta-lactam in the vicinity of the disk containing amoxicillin / clavulanate after 24 hours of incubation [8].
Two disks combined test
this test was performed by using the 30 μg ceftazidime and cefotaxime disk, plus 20 μ per 10 μg ceftazidime/clavulanate and cefotaxime/clavulanate, obtained from the Mast Company [9].
The preparation of silver nanoparticles solutions
A solution of silver nanoparticles as a 4-liter vial was purchased from Nano Nasb Tehran Pars Company. Particles were 4 nm in size and were spherically shaped. Serial dilutions were prepared from the primary stock solution with a concentration of 500 ppm of silver nanoparticles. The obtained concentrations were 12.5, 25, 50, 100, 200, and 400 ppm. The blank disks were placed for 1 hour in 20 microliters of solution of colloidal silver nanoparticles with the above-mentioned concentrations. Finally, these disks were placed on Mueller-Hinton agar (MHA) containing bacterial suspension equivalent to half a McFarland unit of the beta-lactamase-positive bacteria isolated from clinical specimens from patients or the following standard bacteria: Enterobacter cloacae 1003, Enterobacter aerogenes 1221, Escherichia coli 1399, Escherichia coli 1551, Escherichia coli 1270, Acinetobacter baumannii 1318, Klebsiella oxytoca 1402, Klebsiella pneumoniae 1290, Klebsiella pneumoniae 1058, Pseudomonas aeroginosa 27853, Citrobacter freundii 1600, Proteus vulgaris 1079, and Serratia marcescens 1621.
In addition to the 7 listed concentrations of silver nanoparticle solutions, another disk impregnated with distilled water was placed in the above-mentioned environments as the control [10].
Determining MIC and MBC
The minimum inhibitory concentration (MIC) for bacterial growth and the minimum bactericidal concentration (MBC) using serial dilutions were prepared from the solutions of silver nanoparticles, TSB medium, and inoculated microbial suspension, equivalent to half a McFarland unit.
Preparation of Wistar rats
This empirical study was conducted on 40 adult male Wistar rats. The animals were purchased from the Pasteur Institute in Tehran. They were kept study for 1 month in the animal room of Falavarjan Azad University in order to be prepared for the study. The laboratory animals were housed at proper temperature conditions (22±2°C) and sufficient light (12 hours light and 12 hours dark). The laboratory animals had a mean weight of 25±2.5 grams and were divided into 8 groups. The first group, which was called the control group, received 1 cc of normal saline in order to obtain identical shock effect from the injection treatment in both groups. The second group received 1 cc of silver nanoparticles with concentrations of 50 and 100 ppm. The injections were repeated for 5 consecutive days. The method of injection was intraperitoneal in all groups. Blood sampling in rats was performed from the corner of the eyelid with a very thin tube at 3 and 8 days after the treatment. Serum ALT concentration was analyzed by using biochemical kits and spectrophotometer. Then, the mean of ALT serum concentrations were compared in the treated and control groups with each other. Finally, the effect of different solutions of silver nanoparticles was determined [11,12]. The significant difference between samples was considered to be 1%. Also, in order to compare the rate of changes of ALT activity in all treated groups after treatment, ANOVA was used in different concentrations of silver nanoparticles.
Results
We sampled 186 patients admitted to 3 hospitals (Gharazi, Sina, and Alzahra) in Isfahan during 8 months; 140 (75.3%) gram-negative bacilli with ESBL isolates and 46 (24.7%) gram-negative bacilli without ESBL isolates were isolated and identified. Among the patients examined in this study, 176 isolates (61.5%) were from Alzahra (SA) hospital, 76 isolates (26.6%) were from Gharazi hospital, and 34 isolates (11.9%) were from Sina Hospital. Out of a total of 276 patients only 140 patients with gram-negative bacilli with ESBL were isolated and identified (Table 1).
Table 1.
Frequency and percentage of gram-negative bacilli with ESBL, isolated from the patients of Gharazi, Sina, and Alzahra (SA) hospitals in Isfahan (p=0.01).
| Row | Isolate strain | Frequency | Percent |
|---|---|---|---|
| 1 | Klebsiella pneumoniae | 52 | 37/15 |
| 2 | Pseudomonas aeroginosa | 31 | 22/14 |
| 3 | Escherichia coli | 23 | 16/45 |
| 4 | Acinetobacter baumannii | 10 | 7/14 |
| 5 | Serratia marcescens | 7 | 5/00 |
| 6 | Enterobacter cloacae | 6 | 4/28 |
| 7 | Klebsiella oxytoca | 5 | 3/57 |
| 8 | Enterobacter aerogenes | 3 | 2/14 |
| 9 | Citrobacter freundii | 2 | 1/43 |
| 10 | Proteus vulgaris | 1 | 0/70 |
| 11 | Total | 140 | 100 |
The frequency of the studied isolates were: urinary tract infections, 107 samples (38.8%); blood, 40 samples (14.5%), stool, 27 samples (9.7%), sputum, 22 samples (7.9%), throat secretions, 21 samples (7.6%); cerebrospinal fluid and bone marrow, 16 samples (5.8%); catheter, 14 samples (5.0%); bronchial secretions, 13 (4.7%); wound secretions, 12 samples (4.3%); and abdominal cavity fluid, 4 samples (1.4%). Among the 3 studied hospitals, the one with the most clinical samples was the Specialized Alzahra (SA) hospital in the south of Isfahan. The most abundant was Klebsiella pneumoniae (37.2%) (Table 1). All clinical isolates were separated and investigated in terms of sensitivity and resistance to the following antibiotics: ceftazidime (CAZ: 30 μg), cefotaxime (GTX: 30 μg), ceftriaxone (GRO: 30 μg), cefixime (CFM: 5 μg), piperacillin (PRL: 30 μg), ticarcillin (TC: 30 μg), tobramycin (TN: 30 μg), amikacin (AN: 30 μg), ciprofloxacin (CIP: 30 μg), norfloxacin (NOR: 30 μg), ofloxacin (OFX: 30 μg), ceftizoxime (CT: 30 μg), cephalotin (CF: 30 μg), penicillin (P: 10 μg), ampicillin amplifier (AM: 30 μg), and amoxicillin (AMX: 10 μg). The highest resistance in isolated strains was to penicillin, ampicillin, and cefotaxime antibiotics. After reviewing the results of clinical isolates by the Bauer and Kirby method and using the antibiogram test, the production of ESBL (on disk) was determined in 140 isolates resistant to cephalosporins. ESBL discs showed broad-spectrum beta-lactamase in most of the clinical isolates. There was no growth inhibition zone around the cefotaxime disk, indicating the high resistance of the isolates. On the disk containing cefotaxime/ceftazidime + clavulanic acid, the inhibition from the growth (equal to sensitivity) was clearly observed. The clinical isolated samples along with the standard samples of bacteria were influenced by various solutions of silver nanoparticles with the concentrations of: 12.5, 25, 50, 100, 200, and 400 ppm. The inhibition zone diameter of each of the isolates was evaluated against the different concentrations of silver nanoparticle solutions (Table 2).
Table 2.
Results of susceptibility tests of isolates from patients in Gharazi, Sina, and Alzahra (SA) hospitals against different concentrations of silver nanoparticle solutions of (ppm) (p =0/01).
| Row | Average inhibition zone diameter (mm) | Various concentration solution of nanosilver (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | 200 | 400 | 500 | ||
| 1 | Klebsiella pneumoniae | 0 | 2.11±0.18 | 3.890.98 | 6.85±1.85 | 14.96±0.88 | 18.44±0.34 | 19.99±0.84 |
| 2 | Pseudomonas aeroginosa | 0 | 1.14±0.25 | 3.35±1.45 | 7.25±2.54 | 16.11±0.58 | 19.99±0.38 | 23.23±2.33 |
| 3 | Escherichia coli | 0 | 3.43±0.18 | 8.11±0.88 | 10.43±0.64 | 11.79±2.88 | 16.38±0.84 | 19.01±0.85 |
| 4 | Acinetobacter baumannii | 0 | 4.35±1.02 | 6.78±1.67 | 9.99±1.18 | 10.53±0.79 | 15.46±0.99 | 16.23±1.25 |
| 5 | Serratia marcescens | 0 | 0 | 5.16±0.48 | 8.25±0.81 | 9.75±1.85 | 10.76±1.45 | 11.85±1.78 |
| 6 | Enterobacter cloacae | 0 | 0 | 7.89±0.58 | 10.11±2.93 | 12.32±0.77 | 13.27±2.99 | 15.09±1.08 |
| 7 | Klebsiella oxytoca | 0 | 1.25±0.45 | 3.25±2.11 | 12.78±1.48 | 14.51±1.97 | 16.44±1.74 | 18.29±1.49 |
| 8 | Enterobacter aerogenes | 0 | 5.48±1.45 | 9.59±1.11 | 11.77±0.93 | 15.19±0.57 | 20.38±0.18 | 23.55±0.99 |
| 9 | Citrobacter freundii | 0 | 6.99±0.58 | 8.28±0.78 | 12.43±2.11 | 14.22±0.54 | 16.11±2.05 | 18.00±1.52 |
| 10 | Proteus vulgaris | 0 | 0 | 6.11±0.05 | 10.50±1.57 | 11.51±0.71 | 11.69±1.11 | 15.06±0.59 |
Three bacteria – Enterobacter aerogenes, Klebsiella oxytoca, and Citrobacter freundii – showed the highest growth inhibitions against silver nanoparticle solution with the concentration of 100ppm among the clinical samples (Table 2). All of the clinical isolates of ESBL were sensitive to silver nanoparticle solutions at concentrations of 200, 400, and 500 ppm. Finally, the results of susceptibility tests were studied for the standard bacteria samples against different concentrations of silver nanoparticle solutions (Table 3).
Table 3.
Results of standard susceptibility tests of the standard bacteria samples against different concentrations of silver nanoparticle solutions (ppm) (p=0.01).
| Row | Average inhibition zone diameter (mm) | Various concentration solution of nano-silver (ppm) | ||||||
|---|---|---|---|---|---|---|---|---|
| 12.5 | 25 | 50 | 100 | 200 | 400 | 500 | ||
| 1 | Klebsiella pneumonia 1290 | 0 | 0 | 0 | 8.85±0.28 | 9.89±0.89 | 10.95±0.19 | 12.55±0.55 |
|
| ||||||||
| 2 | Klebsiella pneumonia 1058 | 0 | 0 | 5.36±1.88 | 11.12±1.57 | 15.32±1.20 | 20.09±1.76 | 22.06±0.84 |
|
| ||||||||
| 3 | Pseudomonas aeroginosa 27853 | 0 | 0 | 0 | 8.44±1.63 | 10.46±2.66 | 11.88±1.25 | 13.81±1.29 |
|
| ||||||||
| 4 | Escherichia coli 1270 | 0 | 0 | 0 | 5.06±2.81 | 8.22±1.49 | 12.08±1.01 | 13.08±2.41 |
|
| ||||||||
| 5 | Escherichia coli 1399 | 5.54±0.11 | 11.11±2.64 | 10.52±1.86 | 12.45±1.53 | 17.75±1.33 | 20.21±0.99 | 20.20±0.80 |
|
| ||||||||
| 6 | Escherichia coli 1551 | 7 | 10.09±1.28 | 11.99±0.55 | 18.55±1.67 | 20.11±0.91 | 22.75±0.85 | 25.00±0.33 |
|
| ||||||||
| 7 | Acinetobacter baumannii 1318 | 0 | 2.75±1.45 | 8.08±1.36 | 10.44±0.39 | 11.69±1.58 | 12.00±1.66 | 15.18±0.98 |
|
| ||||||||
| 8 | Serratia marcescens 1621 | 0 | 8.46±0.89 | 10.43±1.65 | 9.97±1.36 | 12.33±0.85 | 13.05±1.25 | 16.34±2.01 |
|
| ||||||||
| 9 | Enterobacter cloacae 1003 | 0 | 0 | 0 | 0 | 8.08±0.85 | 10.09±0.77 | 11.99±1.99 |
|
| ||||||||
| 10 | Klebsiella oxytoca 1402 | 0 | 0 | 4.77±0.61 | 8.33±2.88 | 11.07±1.11 | 13.11±1.11 | 15.41±0.72 |
|
| ||||||||
| 11 | Enterobacter aerogenes 1221 | 0 | 0 | 0 | 5.23±1.58 | 7.88±1.18 | 9.66±0.44 | 12.33±1.77 |
|
| ||||||||
| 12 | Citrobacter freundii 1600 | 0 | 0 | 0 | 6.09±0.79 | 12.59±0.99 | 13.22±0.88 | 16.21±1.04 |
|
| ||||||||
| 13 | Proteus vulgaris 1079 | 0 | 0 | 3.98±0.16 | 10.11±o.65 | 9.79±1.35 | 15.01±1.13 | 15.00±1.22 |
Escherichia coli 1551 was the most susceptible to silver nanoparticle solutions at the concentration of 100 ppm, and Enterobacter cloacae 1003 showed the greatest resistance among the standard bacteria samples. After performing the above tests and finding the MIC of silver nanoparticle solutions on the growth of gram-negative bacteria with ESBL, this solution at 100 ppm was used on Wistar rats. The results of the weight changes in Wistar rats after the injection of silver nanoparticles are shown in Table 4.
Table 4.
The mean weight of Wistar rats, before and after the injection of silver nanoparticles with the concentration of 100ppm (p=0.01).
| Groups | Mean weight of Wistar rats, after the injection (gr) | Mean weight of Wistar rats, before the injection (gr) |
|---|---|---|
| Control | 210±3/52 | 220±3/19 |
| Placebo | 220/8±2/99 | 230±5/30 |
| Treatment 1 (2 days after of the injection) | 230/6±1/89 | 250/3±4/90 |
| Treatment 2 (10 days after the injection) | 270/3±1/79 | 260/9±4/80 |
| Treatment 3 (30 days after the injection) | 210/3±3/82 | 250/3±4/07 |
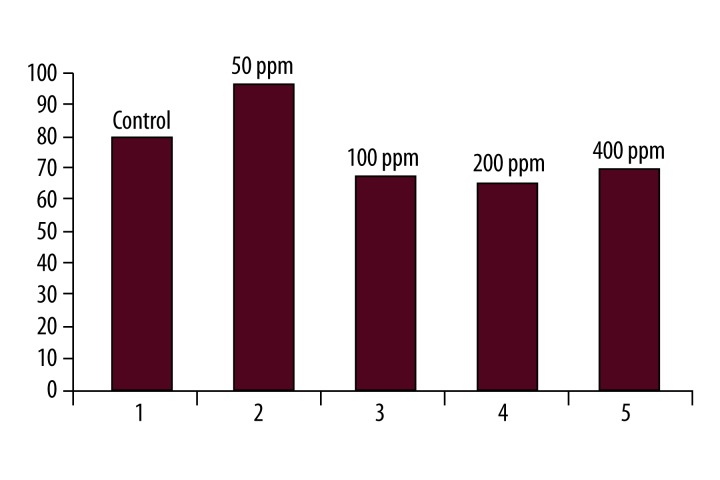
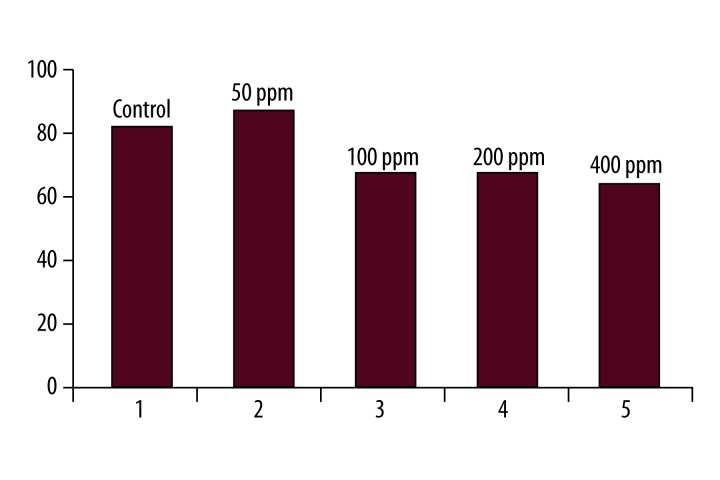
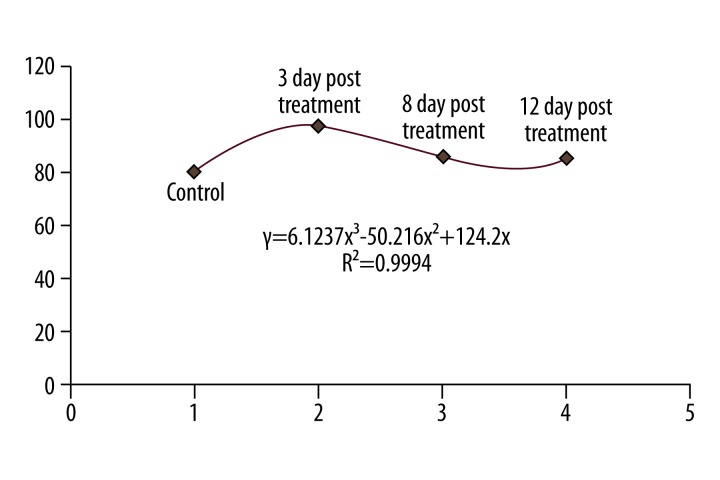
There were no significant differences in the mean weight of Wistar rats before and after the injection of silver nanoparticles with at the concentration of 100 ppm (Table 4). Finally, the results of the measurements of ALT hepatic factor in Wistar rats after the injection of silver nanoparticles at the concentrations of 50, 100, 200, and 400 ppm are shown in Figures 1 and 2. Figure 1 was shown that 3 days after treatment of rats with silver nanoparticles, the mean ALT activity in the control group was 80.25±23. This increased to 96.33±20 three days after the injection of silver nanoparticles at a concentration of 50 ppm. This difference was statistically significant (p=0.002). The mean ALT activity was 80.25±23 in the control group. The enzyme level fell to 73±38 at the concentration of 100 ppm after 3 days. This difference was not statistically significant compared to the control group. At the concentration of 50 ppm of silver nanoparticles, ALT activity on different days of treatment was compared. The Pearson’s correlation coefficient was (r2=0/9994), indicating a significant correlation between the different mean serum ALT between control and treatment groups (Figure 3).
Figure 1.
Comparison of mean serum ALT levels in the control and treatment groups, after three days of treatment with various concentrations of silver nanoparticles.
Figure 2.
Comparison of mean serum ALT levels in the control and treatment groups, after 8 days of injection in various concentrations of silver nanoparticles.
Figure 3.
Comparison of mean serum ALT levels in the control and treatment groups with various concentrations of silver nanoparticles.
The present study was conducted to evaluate the effects of time of injection and concentration of injected silver nanoparticles against the changes in serum ALT levels in male Wistar rats (at 50 ppm concentration of silver nanoparticles).
Discussion
We found that most of the clinical studied isolates from Gharazi, Sina, and Alzahra (SA) hospitals in Isfahan were associated with urinary tract infections (n=107, 38.8%), and discharge from the abdominal cavity was the lowest (n=4, 1.4%). In an epidemiology study in Norway during 2002 and 2003, most hospital infections were related to urinary tract infections (53%), and the least common were surgical infections (5–7%) [8]. The results of the present study demonstrate that the most frequent gram-negative bacilli with ESBL was K. pneumoniae bacteria (37.2%) and the least frequent was Proteus vulgaris bacteria (0.7%). However, in a survey in Turkey in 2001, the most common bacteria isolated from nosocomial infections with ESBL were E. coli and Klebsiella spp., with a prevalence of 22.1% and the lowest was Acintobacter spp. (3.5%) and Serratia spp. (2.9%) [1]. Among the family of Enterobacteriaceae isolated from the patients in our study, there was very high resistance to narrow-spectrum cephalosporins (cefixime), and broad-spectrum cephalosporins (ceftazidime, cefotaxime, and ceftriaxone), and there was sensitivity to cephamycin strain. On the other hand, the resistance to the antibiotics by many strains, especially to the third-generation cephalosporins, led to select these strains to examine the ability to produce ESBL. The selective pressure caused by ESBL-producing strains and the indiscriminate use of antibiotics has led to the emergence of resistant strains. The emergence and world-wide high prevalence of ESBL (and especially in Iran), and the fact that ESBL-producing strains have caused unresolved problems for clinical microbiologists and infection control clinicians [13], has focused attention on fighting these bacteria. Due to the dramatic antimicrobial effects of silver nanoparticles and their increasing use in various industries (e.g., health and beauty, catheters, antiseptic sprays, detergents, toothpaste), they were the most commonly used particles with daily utilization [10]. Spherical 4 nm silver nanoparticle solutions can produce the most growth inhibitory effects on gram-negative bacilli with ESBL at the concentrations of 100, 200, and 400 ppm (Table 2). Our results demonstrate that the effects of silver nanoparticle solutions are proportional to their dose. The same results have been achieved by Choi et al in 2008 [11]. There was no significant difference between the effects of silver nanoparticles on the antibacterial-resistant bacteria with ESBL (Table 2) and the bacteria that are sensitive to several antibiotics with no ESBL (Table 3). It appears that the drug-resistant proteins that enabled the bacteria to avoid antibiotics do not have any impact on the efficiency of silver nanoparticles. Ria et al. in 2009 arrived at the same conclusion on the capability of silver nanoparticles on bacteria resistant to multiple drugs with ESBL and drug-sensitive bacteria [7].
Two of the most important hepatic enzymes are alanine-amino-transferase (ALT) and serum glutamic pyruvic transaminase (SGPT) [15]. With injuries to the liver, the concentrations of these enzymes change in the blood serum. By proving the harmful effects of silver nanoparticles to the liver, it could be possible to demonstrate their indirect effects by this serum enzyme on the liver. There was no significant difference between the mean values of ALT between different groups of Wistar rats (Figures 1 and 2). However, at the 50 ppm concentration of silver nanoparticles, the liver injury in rats induced by intraperitoneal injection causes severe irritation in the oxidant system of these cells. The most damage to the liver cells at low concentrations (such as 50 ppm) was caused 3 days after the injection (Figure 1). Eight days after the injection of silver nanoparticles, the liver damage returned (Figure 3). The other liver enzymes activated and neutralized the effects of silver nanoparticles after 3 days. One of these enzymes was metalothioneine. The above-mentioned enzymes combined with silver nanoparticles and caused their deactivation. The oxidative damage due to silver nanoparticles with these concentrations was not investigated. The balance of oxidant-antioxidant system of hepatocytes were gone towards the oxidant properties after 3 days. According to these findings, it can be concluded that the injection of silver nanoparticles in the adult male Wistar rats with a concentration of 100 ppm did not cause liver toxicity. However, the exact determination in this case requires several other experiments with different shapes and concentrations of silver nanoparticles on these rats in order to decide if silver nanoparticle solutions can be used in humans. In our study there was no significant change in the weight of the liver or liver factors of the rats after intraperitoneal injection of 100 ppm of silver nanoparticles. The changes that developed in ALT levels after the injection returned to normal condition after 8 days.
Various reports have demonstrated that significant amounts of nanoparticles injected to the body are absorbed by the liver. The reticuloendothelial system in this organ can gradually remove the accumulated nanoparticles from the body [15,16]. In the present investigation, the silver nanoparticle with 4 nm diameter and spherical shape was used for study of aminoteransferase pathways in hepatocytes. In fact, free radicals from the silver nanoparticle particles have attacked hepatocytes and released ALT into the serum. Therefore, the probable reason for slight changes in some liver factors is the accumulation of nanoparticles in the liver. The levels of these accumulations decreased and the liver function returned to normal after 12 days. Susan et al demonstrated that with the changes in diameter and concentration of nanoparticles, their distribution in body tissues caused different effects. Smaller particles more easily penetrate the cells and the effects are more harmful [17]. Hepatocyte damage induced by intraperitoneal injection of silver nanoparticles in rats has possibly caused severe irritation of oxidant pathway in these cells. In 1989, Machiedo showed that free radicals induced by nanoparticles can cause cell membrane destruction [18]. On the other hand, world-wide use of different silver nanoparticles, especially in Iran, requires more accurate studies on the effects of these nanoparticles on hepatocytes. The use of laboratory rats as animal models, and various treatment methods and nanoparticles with different concentrations and shapes, presents new horizons for further research to investigate applications of nanotechnology [19]. In 2010 Sriram et al proved that silver nanoparticles can activate caspase mitochondrial enzymes, especially Caspase 3 in lymphoid cancerous cells, and could cause apoptosis in different cells in long-term treatment with different nanoparticles [20]; the mean diameter of the silver nanoparticles was 4 nm. Probably (only at the concentration of 50 ppm) the hepatocyte cell membranes were damaged and aminotransferase released to the serum. Thus, the destructive effects of silver nanoparticles on these cells can be attributed to the diameter of the silver ions.
Conclusions
We conclude that use of silver nanoparticles in vitro in small amounts did not cause any toxicity or special disorders in in vivo conditions in Wistar rats. However, it is necessary to conduct further investigations on the molecular effects of silver nanoparticles with different concentration and shapes in hepatocyte of Wistar rats before use on humans.
Footnotes
Source of support: Departmental sources
References
- 1.Koseoglu O, Kocaguz S, Gur D, Akova M. Nosocomial bloodstream infection in Turkish university hospital: Study of Gram-negative bacilli and their sensitivity patterns. Int J Antimicrob Agents. 2001;17:477–81. doi: 10.1016/s0924-8579(01)00325-9. [DOI] [PubMed] [Google Scholar]
- 2.Helfand MS, Bonomo RA. β-lactamase: A survey of protein Diversity. Curr Drug Targets Infect Discord. 2003;3:9–23. doi: 10.2174/1568005033342181. [DOI] [PubMed] [Google Scholar]
- 3.Shahverdi AR, Fakhimi A, Shahverdi HR, Minaian S. Synthesis and effect of silver nanoparticles on the antibacterial activiry of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanotechnol Biol Med. 2007;2:168–71. doi: 10.1016/j.nano.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Medeiros AA. Evolution and dissemination of beta-lactamases accelerated by generations of beta-lactam antibiotics. Clin Infect Dis. 1999;24:19–45. doi: 10.1093/clinids/24.supplement_1.s19. [DOI] [PubMed] [Google Scholar]
- 5.NCCLS: Performance Standard for Antimicrobial Susceptibility testing. Eight Information Supplement. NCCLS document M100-S8.NCCLS, Wayne, A 1998
- 6.Jung W, Koo H, Kim KW, et al. Antibacterial activity and mechanism of action for the silver ion in Staphylococcus aureus and Esherichia coli. Applied And Enviromental Microbiology. 2008:2171–78. doi: 10.1128/AEM.02001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rai M, Yadav A, Gade A. Silver nanoparticle as a new generation of antimicrobials. J Biotechnology Advances. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 8.Forbes AB, Sahm FD, Weissfeld SA. Bailey and Scott’s Diagnostic Microbiology. 11th ed. 2002. pp. 229–51.pp. 68–70. [Google Scholar]
- 9.Sougakoff W, Goussard S, Courvalin P. TEM-3 β-lactamases, which hydrolyzes broad-spectrum cephalosporins, is derived from the TEM-2 penicillinase by two amino acid substitutions. FEMS Microb Lett. 1988;56:343–48. [Google Scholar]
- 10.Ahari H, Peykan R, Dastmalchi F, et al. Nanotechnology in medicine and veterinary medicine. Tehran: Jahad Daneshgahi of Tehran Branch publication Co; 2008. pp. 15–25. [Google Scholar]
- 11.Choi O, Deng KK, Kim NJ, et al. The inhibitory effects of silver nnaoparticles, silver ions, and silver chloride colloids on microbial growth. Water Res. 2008;12:3066–74. doi: 10.1016/j.watres.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Schluesener HJ. Nanosilve: A nanoproduct in medical application. Toxicol Lett. 2008;1:1–12. doi: 10.1016/j.toxlet.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Kiratisin P, Apisarnthanarak A, Laesripe C, Saifon P. Molecular characterization and epidemiology of Extended-Spectrum β-Lactamase-Producing Escherichia coli and Klebsiella pneumonia Isolates Causing Health Care-Associated infection in Thailand, Where the CTX-M Family Is Endemic. Antimicrob Agents Chemother. 2008;8:2818–24. doi: 10.1128/AAC.00171-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lioyd JR. Microbial reduction of Metals and radionuclides. FEMS Microbial Rev. 2003;27:412–25. doi: 10.1016/S0168-6445(03)00044-5. [DOI] [PubMed] [Google Scholar]
- 15.Chaves SB, Silva LP, Lacava ZGM, et al. Interleukin-1 and interleukin-6 production in mice,s lungs induced by 2,3 meso-dimercaptosuccinic-coated magnetic nanoparticles. J Applied Physics. 2005;1:51–59. [Google Scholar]
- 16.Crystal YU. A thesis submitted to Oregon state University in partial fulfillment of the requirements for the degree of Master of Science Presented May 29, 2007 Commencement June 2008. In vivo assessment of nanomaterial-induced toxicity using embryonic zebrafish. [Google Scholar]
- 17.Susan WP, Williw GM, Maaike Van J. Nanosilver – a review of avelable data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;2:109–38. [Google Scholar]
- 18.Machiedo GW, Powell RJ, Rush BF, Jr, et al. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple system organ failure. Arch Surg. 1989;124(12):1386–89. doi: 10.1001/archsurg.1989.01410120032007. [DOI] [PubMed] [Google Scholar]
- 19.Hussain SM, Hess KL, Gearhart JM, et al. In-vitro toxicity of nanoparticles in BRL 3A rat liver cells. Toxicol In Vitro. 2005;19(7):975–83. doi: 10.1016/j.tiv.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 20.Sriram MI, Kanth SB, Kalishwaralal K, Gurunathan S. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int J Nanomedicine. 2010;5:753–62. doi: 10.2147/IJN.S11727. [DOI] [PMC free article] [PubMed] [Google Scholar]