Abstract
Background
To examine the effect of carnosine on liver function and histological findings in experimental septic shock model, 24 Sprague-Dawley rats were used.
Material/Methods
Rats were divided into control, septic shock, and carnosine-treated septic shock groups. Femoral vein and artery catheterization were performed on all rats. Rats in the control group underwent laparotomy and catheterization; in the test groups, cecal ligation-perforation and bladder cannulation were added. Rats in the treatment group received a single intraperitoneal (IP) injection of 250 mg/kg carnosine 60 minutes after cecal ligation-perforation. Rats were monitored for blood pressure, heart rate, and body temperature to assess the postoperative septic response, and body fluids were replaced as necessary. At the end of 24 hours, rats were sacrificed and liver samples were collected.
Results
Statistically significant improvements were observed in liver function, tissue and serum MDA levels, and histological findings in rats treated with carnosine, compared to rats with untreated sepsis. HB and HCT values did not change significantly during the course of the experiment. Rats exposed to septic shock and treated with carnosine exhibited decreased sinusoidal dilatation and cellular inflammation into the portal region, compared to the sepsis group; the livers of rats in this group had near-normal histological structure.
Conclusions
We conclude that carnosine may be an effective treatment for oxidative damage due to liver tissue perfusion defects in cases of septic shock.
Keywords: carnosine, septic shock, rat, liver
Background
Sepsis is a systemic response that develops against infection and inflammation. Aggressive treatment with specific antibiotics and other pharmacological agents are currently used to treat septic shock and multiple organ dysfunction syndrome due to sepsis. However, these disorders remain the most important cause of mortality in intensive care units. In addition, septic shock is one of the most common reasons for intensive care unit admissions [1–3].
Carnosine is a dipeptide that has been proven to scavenge reactive oxygen species and α,β unsaturated aldehydes formed from the peroxidation of cell membrane fatty acids during oxidative stress [4–6]. Many studies have investigated the oxidant effects of carnosine, but no research showing the effects of carnosine on sepsis and septic shock has been published to date. In the present study, we used an experimental model of sepsis and septic shock in rats to investigate the effects of carnosine on oxidative stress and liver histology due to hypoperfusion.
Material and Methods
These animal studies were approved by the Eskisehir Osmangazi University Medical Faculty Ethics Committee (approval date and number: 19.10.2010-176/2010)
Rats and diet
Male and female Sprague-Dawley rats weighing an average of 200–300 grams were purchased from Eskisehir Osmangazi University, Experimental Research Centre for Medical and Surgical Investigation. Animals were housed at room temperature under controlled laboratory conditions during the experiment and were fed standard rat chow and tap water.
Experimental protocol
Rats were anaesthetized using 60 mg/kg ketamine hydrochloride (Parke Davis, insert location of company) and 5 mg/kg Xylazine (Bayer, location) via intraperitoneal injection. Response to the painful stimulus (screwed forceps onto abdominal skin) was used to ensure depth of anaesthesia 5 minutes after injection. Under sterilized conditions, inguinal incision was performed following 10% povidone iodine cleaning; all rats were catheterized, isolating the femoral artery and vein by pig tail 60 (polyethylene) catheters. The femoral vein catheter was advanced to the vena cava. Cannulae were washed with saline containing 100 IU heparin before and after cannulation, and blockage of cannula was prevented. Closed catheters were removed from the back of the neck through the subcutaneous tunnel. All surgeries were performed on a surgical table heated to 36.6°C to avoid hypothermia during surgery and follow-up.
Experimental groups consisting of 8 rats underwent laparotomy and cecal ligation to induce septic shock, or received bladder cannulation alone (control group), under anaesthesia. Urine follow-up, invasive blood pressure measurement, and ECG recordings were started in the 14th postoperative hour and recorded every 5 minutes. At first indication of septic shock – defined as invasive blood pressure measurements of <90 mmHg, or a decrease of at least 40 mmHg from the initial blood pressure measurement – rats received 1.25 gr carnosine [6], in saline, injected at a rate of 250 mg/kg/dose (group sepsis and treated) and 10 mg/min of saline (group sepsis). Baseline and outcome body temperatures were measured by rectal probe. After 24 hours, rats were sacrificed using excessive ketamine, and liver specimens were removed for histopathological investigation.
Blood samples (5 ml) were obtained for arterial blood gas, venous blood gas, hemogram, and biochemical measurements (AST and ALT) before catheterization and at the end of the experiment. Blood samples were also obtained for MDA measurements from blood and liver tissue. Arterial pressure was measured by pressure transducer (Transpac IV, USA). Invasive arterial pressure and heart rate were monitored by Data Equationsystem (MP 100 Biopac, USA). Hemogram and biochemical measurements were performed using standard methods in our hematology and biochemical departments.
MDA measurements in serum and liver tissue
Tissue samples were homogenized into 0.1 M KCL solution at a ratio of 1:10 (w/v). Homogenates were centrifuged at 4000 rpm at 4°C and supernatants were harvested. For 0.4 ml sample volume, 1.5 ml TBA (0.08%, pH 5.5), 1.5 ml acetic acid (20%, pH 3.5) and 0.2 ml sodium dodecyl sulfate were added. Fresh MDA standards were used for each experiment. Samples and standards were boiled at 100°C for 1 hour, cooled using cold water, combined with 5 ml n-butanol, and centrifuged at 4000 rpm for 10 minutes. Identical procedures were performed on plasma samples.
Histological methods
Liver specimens were fixed in 10% formalin for 48 hours, washed with tap water for 3–4 hours, and dehydrated in increasing concentrations of alcohol (70%, 80% and 90%). To make pellucid, specimens were incubated in xylol twice for 20 minutes and embedded in 3 paraffin incubators. Tissue was sectioned by microtome, and incubated 1 hour in a 37°C water bath prior to staining with hematoxylin-eosin dual stain for 2 and 10 minutes, respectively. Sections were washed, dehydrated in increasing alcohol concentrations, washed twice in xylol for 30 minutes, and mounted on slides using entellan. Sections were imaged on an Olympus BH-2 microscope and an Olympus DP-70 digital camera.
Statistical analysis
Data were evaluated with SPSS 13.0 and Sigma Stat 3.1 software One-way analysis of variance was performed for normally distributed variables with nonparametric post-hoc Tukey HSD and Fisher LSD tests. The paired t test was used for binary comparisons of before and after variables (Pairt Samples Statistics). Non-normally distributed variables were analyzed by Kruskal-Wallis one-way analysis of variance on ranks; binary comparisons of before and after variables were analyzed by using Wilcoxon signed rank test.
Results
Table 1 shows mean weight, hemoglobin (HB1), and hematocrit (HCT1) values at the start of the experiment, and hemoglobin (HB2) and hematocrit (HCT2) values at the end of experiment.
Table 1.
Comparison of haematologic parameters and weights of rats.
| Control group | Group sepsis + carnosine | Group sepsis | p | |
|---|---|---|---|---|
| HB1 (g/dL) | 11.2625±1.11 | 11.6350±1.32 | 11.724±2.27 | p≥0.05 |
| HCT1 | 34.6500±3.56 | 34.4235±2.87 | 34.7206±3.14 | p≥0.05 |
| HB2 | 10. 812±0.789 | 10.912±0.780 | 10.898±0.987 | p≥0.05 |
| HCT2 | 32.21±1.28 | 31.56±2.26 | 32.13±1.06 | p≥0.05 |
| Weight (gram) | 252,134 | 261,165 | 258,373 | p≥0.05 |
HB and HCT values did not change significantly during the course of the experiment. The mean weight of rats was 252.134 (±14) grams for the control group, 261.165 (±16) grams for CLP + carnosine, and 258.373 (±13) grams for cecal ligation perforation group. All biochemical values tested were the same among the groups at the start of the experiment. At the end of the experiment ALT, AST, MDAS, and MDAT values were significantly different between groups sepsis and treated groups (p<0,001; Table 2).
Table 2.
Biochemical findings.
| Group | Median (25–75) | Multiple comparison results | |||
|---|---|---|---|---|---|
| Sepsis | Sepsis + carnosine | Control | |||
| AST1 serum (U/l) baseline | Sepsis | 35.12±6.87 (1204–8913) | ns | ns | ns |
| Sepsis + carnosine | 34.62±2.5 (50–5357) | ns | ns | ns | |
| Control | 37.5±5.63 (183–336) | ns | ns | ns | |
| H=0.995 DF=2 (P=0.608) | |||||
| AST2 (U/l) | Sepsis | 383.87±17.5 (420–3543) | * | ** | |
| Sepsis + carnosine | 119.5±11.3 (309–1575) | * | * | ||
| Control | 50.37±9.55 (50–118) | ** | * | ||
| H=14.256 DF=2 | |||||
| ALT1 (U/l) baseline | Sepsis | 20.37±6.9 (3.20–7.66) | ns | ns | ns |
| Sepsis + carnosine | 20.62±6.25 (1.89–3.76) | ns | ns | ns | |
| Control | 21.5±4.1 (1.02–3.85) | ns | ns | ns | |
| H=1.540 D=2 (P=0.463) | |||||
| ALT2 (U/l) | Sepsis | 168.5±52.05 (10.8–26.7) | ** | ** | |
| Sepsis + carnosine | 17.92±3.12 (6.98–8.34) | ** | |||
| Control | 6.78±1.92 (5.2–11.9) | ** | |||
| H=12.128 DF=2 | |||||
| MDAT nmol/g.pr | Sepsis | 20.23±1.94 (43.8–75.6) | * | ** | |
| Sepsis + carnosine | 12.28±0.75 (28.6–42.2) | * | |||
| Control | 11.45±1.64 (10.2–11.9) | ** | |||
| H=11.752 DF=2 | |||||
| MDAS pg/ml | Sepsis | 7.78±1.42 (43.8–75.6) | * | ** | |
| Sepsis + carnosine | 3.8±0.74 (28.6–42.2) | * | |||
| Control | 2.41±0.66 (10.2–11.9) | ** | |||
| H=17.956 DF=2 | |||||
ns – p>0.05;
p<0.05;
p<0.001;
H – Kruskal-Wallis.
Histological findings
Hepatocytes, vena centralis structures, the portal region, and sinusoid capillary structures were histologically normal in control group rats (Figure 1). Sinusoidal dilatation, portal cellular inflammation, and hepatocyte nuclear hypertrophy were observed in the livers of rats in the untreated septic shock group (Figure 2). Rats exposed to septic shock and treated with carnosine exhibited decreased sinusoidal dilatation and cellular inflammation into the portal region, compared to the sepsis group; the livers of rats in this group had near-normal histological structure (Figure 3). Statistically significant improvements were observed in histological findings in rats treated with carnosine, compared to rats with untreated sepsis (Tables 3 and 4).
Figure 1.
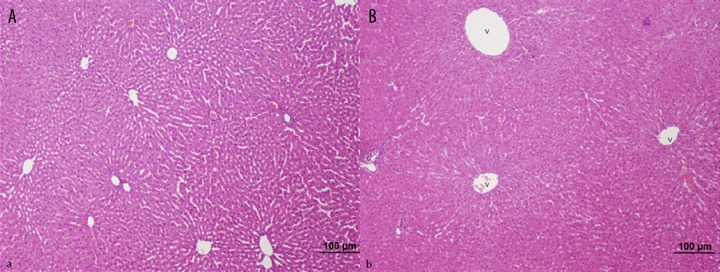
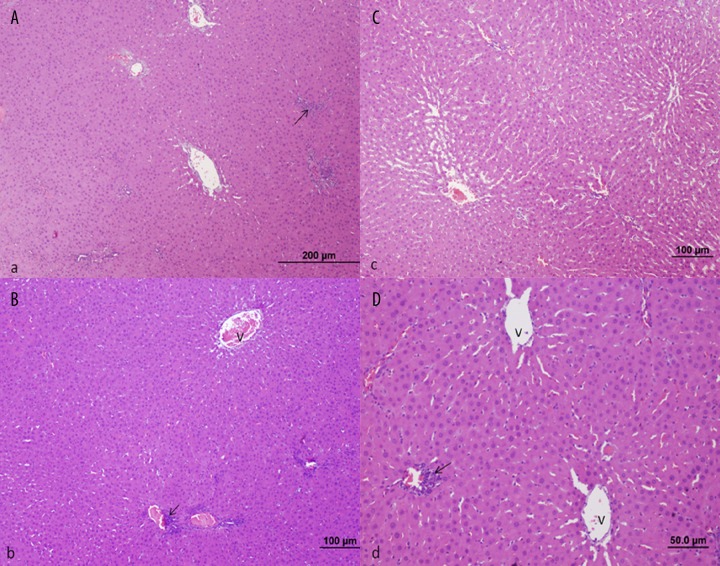
Control Group; Liver histology was normal in control animals (bar: 100 μm, HE).
Figure 2.
Treatment Group (septic shock + carnosine); Rats treated with carnosine exhibit a marked decrease in sinusoidal dilatation and cellular inflammation of the portal region, compared to rats with untreated septic shock (bar: 200 μm, bar: 100 μm, HE).
Figure 3.
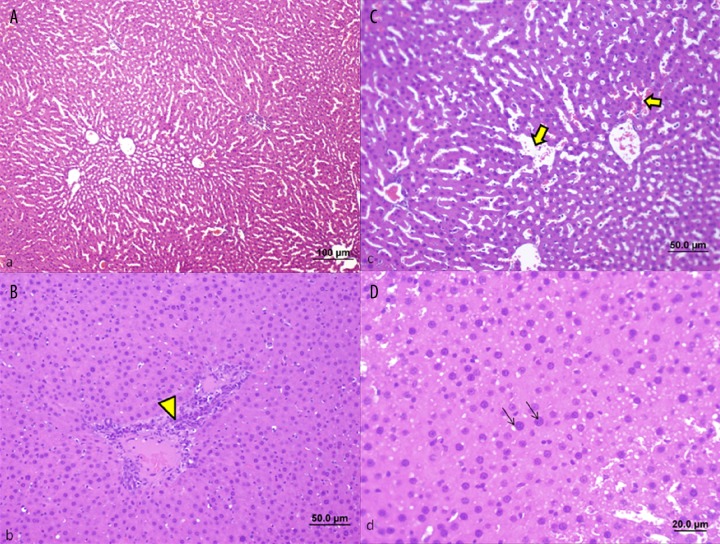
Sepsis Group; Sinusoidal dilatations (dense arrow) (A, C), excessive cellular inflammation in portal region (arrow head) (B) and necrotic cell structures and hypertrophy in hepatocyte cell nucleus (arrow) (D) were remarkable rats exposed to septic shock by cecal ligation (bar: 100 μm, bar: 50.0 μm, bar: 20.0 μm, HE).
Table 3.
Histological evaluation findings of all groups at the end of the study.
| Liver | Necrosis | Sinusoidal Dilatation | Inflammation | Congestion |
|---|---|---|---|---|
| Control 1 | − | − | − | − |
| Control 2 | − | − | − | − |
| Control 3 | − | − | − | − |
| Control 4 | + | − | − | − |
| Control 5 | − | − | − | − |
| Control 6 | − | − | − | − |
| Control 7 | − | − | − | − |
| Control 8 | − | − | − | − |
| Sepsis 1 | +++ | +++ | ++ | − |
| Sepsis 2 | + | ++ | − | − |
| Sepsis 3 | − | ++ | − | + |
| Sepsis 4 | ++ | − | +++ | − |
| Sepsis 5 | ++ | + | − | + |
| Sepsis 6 | − | + | ++ | − |
| Sepsis 7 | + | − | + | − |
| Sepsis 8 | +− | +++ | − | ++ |
| Sepsis + carnosine 1 | − | − | − | − |
| Sepsis + carnosine 2 | − | − | + | + |
| Sepsis + carnosine 3 | − | − | + | − |
| Sepsis + carnosine 4 | − | + | + | − |
| Sepsis + carnosine 5 | − | − | + | − |
| Sepsis + carnosine 6 | − | − | − | − |
| Sepsis + carnosine 7 | − | − | + | − |
| Sepsis + carnosine 8 | − | − | − | − |
Histological findings: absent (−); mild (+); moderate (++); severe (+++).
Table 4.
Histological results.
| Groups | Median (25–75) | Multiple Comparison Results | |||
|---|---|---|---|---|---|
| Sepsis | Sepsis + carnosine | Control | |||
| Necrosis | Sepsis | 3.000 (1.5–3.0) | * | * | |
| Sepsis + carnosine | 1.500 (1.0–2.0) | * | ns | ||
| Control | 0.00 (0.0-0.0) | * | ns | ||
| H=12.806 DF=2 P=0.012 | |||||
| Congestion | Sepsis | 2.500 (1.5–3.0) | * | * | |
| Sepsis + carnosine | 1.000 (1.0–2.0) | * | * | ||
| Control | 0.000 (0.0-0.0) | * | * | ||
| H=12.845 DF=2 P=0.059 | |||||
| Sinusoidal dilatation | Sepsis | 3.000 (3.0-3.0) | * | * | |
| Sepsis + carnosine | 2.000 (2.0-3.0) | * | * | ||
| Control | 0.000 (0.0-0.0) | * | * | ||
| H=14.62 DF=2 P=0.018 | |||||
| Inflammation (PNL) | Sepsis | 3.000 (3.0-3.0) | * | * | |
| Sepsis + carnosine | 1.000 (2.0-2.0) | * | * | ||
| Control | 0.000 (0.0-0.0) | * | * | ||
| H=14.82 DF=2 P=0.036 | |||||
n – p>0.05;
p<0.05;
p<0.001;
H – Kruskal-Wallis.
Discussion
Septic shock remains a significant health problem, with a high mortality rate and urgent need for improved treatment methods. Impaired tissue perfusion in cases of septic shock leads to oxidative damage [7]. Accordingly, numerous antioxidants have been studied as candidates for treatment of septic shock; to date, however, no experimental study has been performed to investigate the effectiveness of carnosine. In the present study we aimed to investigate the effects of carnosine on liver histology and oxidative damage due to septic shock, using a cecal ligation perforation (CLP) experimental rat model. This easy-to-use method leads to development of shock status and invasion of various microorganisms, resulting in a situation similar to clinical shock status [8–10].
Carnosine (β-alanyl-L-histidine) was isolated by V. S. Gulewitsch as a component of compounds extracted from muscle tissue at the beginning of the 20th century [11]. It has been established that this natural dipeptide performs important biological functions; in particular, it exhibits antioxidative properties directed at suppression of free-radical reactions [12,13].
A previous study of the antioxidative action of carnosine has shown that its effects are not only due to binding of lipid oxidation products in the course of free-radical reactions, but also interaction with active oxygen species. Carnosine may also serve as a scavenger of peroxyl and hydroxyl radicals and singlet oxygen and superoxide anion oxygen, and neutralize hypochlorite anions by forming stable chloramine complexes [14]. The antioxidative properties of carnosine make it an effective treatment in a variety of conditions, including cataracts, superficial burns of the epidermis, diabetes, neuropathy, renal dysfunction, Down syndrome, seizures, autistic spectrum disorders, ethanol intoxication, cardiomyopathy, and various inflammatory processes related to cellular membrane damage. The anti-oxidant, free radical and metal ion-scavenging activities of carnosine cannot adequately explain its effectiveness in all of these disorders. Previous studies have shown that carnosine reacts with small carbonyl compounds (aldehydes and ketones) and protects macromolecules against their cross-linking actions. The accumulation of carbonyl groups on proteins is associated with aging [15].
The antioxidative activity of carnosine and a number of related compounds, both synthetic and natural, has been previously investigated [16]. These researchers estimated the antioxidative effect of each dipeptide by its ability to prevent MDA (malondialdehyde) accumulation in response to lipid peroxidation (LPO) induced in rabbit sarcoplasmic reticulum membranes by the Fe2+ ascorbate system. They found antioxidative effects comparable to carnosine with water-soluble (cyclo-L-histidyl-L-proline) and alcohol-soluble (cyclo-L-histidyl-L-phenylalanine) dipeptides as well as by the histidine-free cyclodipeptides (cyclo-L-tyrosyl-L-proline). In contrast to its synthetic analogues, carnosine not only inhibited LPO but also decreased accumulation of peroxidative byproducts [17]. Oxidative stress plays an important role in the pathogenesis of alcohol-induced liver injury. Research in ethanol-treated rats indicates that carnosine prevents increased serum transaminase and lipid peroxide activity in the liver without any change in steatosis [18]. The protective effects of carnosine and histidine against acetaminophen-induced hepatotoxicity were shown in another study [19]. In our study, a statistically significant difference was observed in AST and ALT between the carnosine treatment and sepsis groups at the end of the study. In other words, liver function was protected in rats treated with carnosine, compared to those with untreated septic shock.
Stvolinsky et al. and Gallant et al. reported decreased mortality after ischemic attack in rats treated with carnosine (from 55% to 17%), with most learning parameters maintained at pre-ischemic levels [20,21]. One of the underlying mechanism of ischemic attack is thought to be oxidative damage due to the generation of free radicals. In previous studies, Fouad et al and Baykara et al investigated the putative protective role of carnosine against oxidative organ damage due to ischemia and reperfusion and found that carnosine can be useful as a prophylactic treatment to protect the liver against hypoxia-reoxygenation damage [22,23]. In another study, Aydin et al found that carnosine is beneficial in decreasing age-related oxidative stress and lipid peroxidation, and improves antioxidant status of liver, heart, and brain in young and in aged male rats [24].
Sepsis causes organ damage and loss of function, resulting in oxidative stress. Because lipid peroxidation is a well known mechanism of cellular damage, it is commonly used as a marker of oxidative stress in cells and tissues [25]. In our study, serum and tissue MDA levels were significantly decreased in the group treated with carnosine, compared to the untreated sepsis group. As has been seen in other studies, our findings demonstrate that carnosine has antioxidant effects in sepsis. In the present study we also evaluated the efficiency of carnosine by assessing the histopathology of liver tissue. In another study by Fouad, decreased hepatotoxicity due to cadmium exposure was observed in animals treated with carnosine [26]. In our study, histopathological investigation of liver tissue from rats treated with carnosine showed markedly decreased sinusoidal dilatation and cellular inflammation in the portal region, especially when compared to the control group.
Conclusions
In conclusion, we generated an experimental rat model of septic shock, with symptoms similar to those seen in clinical practice, and treated the animals with carnosine, which has wide-spectrum efficiency for the treatment of ischemia perfusion injury and inflammation. The treatment was effective, as assessed by clinical and histopathological parameters. These data suggest that carnosine may be effective for the treatment of sepsis, as well as the liver damage resulting from sepsis. Our results should prompt future clinical studies by other investigators. Since carnosine administration reversed oxidant responses, it seems likely that carnosine protects the organs against sepsis-induced oxidative organ injury.
Abbreviations
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- MDA
malondialdehyde
- MDAS
malondialdehyde serum
- MDAT
malondialdehyde tissue
- HB
haemoglobin
- HCT
haematocrit
Footnotes
Source of support: Departmental sources
References
- 1.Hines DW, Bone RC. In: Septic Shock İnfectious Diseases. 2nd Edition. Gorbach SL, Bartlett JG, Blacklow NR, editors. WB Saunders Co; Philadelphia: 1992. pp. 544–48. [Google Scholar]
- 2.Pavoa P. C Reactive protein: A valuable marker of sepsis. Intensive Care Med. 2002;28:235–43. doi: 10.1007/s00134-002-1209-6. [DOI] [PubMed] [Google Scholar]
- 3.Matot I, Sprung CL. Definition of sepsis. Intensive Care Med. 2001;27:3–9. doi: 10.1007/pl00003795. [DOI] [PubMed] [Google Scholar]
- 4.Guliaeva NV, Dupin AM, Levshina IP, et al. Carnosine prevents the activation of free-radical lipid oxidation during stress. Biull Eksp Biol Med. 1989;107:144–47. [PubMed] [Google Scholar]
- 5.Boldyrev AA, Stvolinsky SL, Fedorova TN, Suslina ZA. Carnosine as a natural antioxidant and geroprotector: from molecular mechanisms to clinical trials. Rejuvenation Res. 2010;13(2–3):156–58. doi: 10.1089/rej.2009.0923. [DOI] [PubMed] [Google Scholar]
- 6.Nikolic J, Stojanovic I, Pavlovic R, et al. The role of L-arginine in toxic liver failure: interrelation of arginase, polyamine catabolic enzymes and nitric oxide synthase. Amino Acids. 2007;32(1):127–31. doi: 10.1007/s00726-006-0309-y. [DOI] [PubMed] [Google Scholar]
- 7.Vincent JL, Atalan HK. Epidemiology of severe sepsis the intensive care unit. Br J Hosp Med (Lond) 2008;69(8):442–43. doi: 10.12968/hmed.2008.69.8.30739. [DOI] [PubMed] [Google Scholar]
- 8.Alici O, Kavakli HS, Koca C, Altintas ND. Treatment of Nigella sativa in experimental sepsis model in rats. Pak J Pharm Sci. 2011;24(2):227–31. [PubMed] [Google Scholar]
- 9.Sener G, Toklu H, Ercan F, Erkanli G. Protective effect of beta-glucan against oxidative organ injury in a rat model of sepsis. Int Immunopharmacol. 2005;5(9):1387–96. doi: 10.1016/j.intimp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Portolés MT, Ainaga MJ, Pagani R. The induction of lipid peroxidation by E. coli lipopolysaccharide on rat hepatocytes as an important factor in the etiology of endotoxic liver damage. Biochim Biophys Acta. 1993;1158(3):287–92. doi: 10.1016/0304-4165(93)90027-6. [DOI] [PubMed] [Google Scholar]
- 11.Kim MY, Kim EJ, Kim YN, et al. Effects of α-lipoic acid and L-carnosine supplementation on antioxidant activities and lipid profiles in rats. Nutr Res Pract. 2011;5(5):421–28. doi: 10.4162/nrp.2011.5.5.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boldyrev AA, Stvolinsky SL, Fedorova TN, Suslina ZA. Carnosine as a natural antioxidant and geroprotector: from molecular mechanisms to clinical trials. Rejuvenation Res. 2010;13(2–3):156–58. doi: 10.1089/rej.2009.0923. [DOI] [PubMed] [Google Scholar]
- 13.Nikolic J, Stojanovic I, Pavlovic R, et al. The role of L-arginine in toxic liver failure: interrelation of arginase, polyamine catabolic enzymes and nitric oxide synthase. Amino Acids. 2007;32(1):127–31. doi: 10.1007/s00726-006-0309-y. [DOI] [PubMed] [Google Scholar]
- 14.Severina IS, Bussygina OG, Pyatakova NV. Carnosine as a Regulator of Soluble Guanylate Cyclase. Biochemistry. 2000;65(7):783–88. [PubMed] [Google Scholar]
- 15.Zaloga GP, Roberts PR, Black KW, et al. Carnosine is a novel peptide modulator of intracellular calcium and contractility in cardiac cells. Am J Physiol. 1997;272(1):462–68. doi: 10.1152/ajpheart.1997.272.1.H462. [DOI] [PubMed] [Google Scholar]
- 16.Babizhayev MA, Deyev AI, Yermakova VN, et al. Lipid peroxidation and cataracts: N-acetylcarnosine as a therapeutic tool to manage age-related cataracts in human and in canine eyes. Drugs R. 2004;5:125–39. doi: 10.2165/00126839-200405030-00001. [DOI] [PubMed] [Google Scholar]
- 17.Kang JH, Kim KS. Enhanced oligomerization of the alpha-synuclein mutant by the Cu, Zn-superoxide dismutase and hydrogen peroxide system. Mol Cells. 2003;15:87–93. [PubMed] [Google Scholar]
- 18.Artun BC, Küskü-Kiraz Z, Güllüoğlu M, et al. The effect of carnosine pretreatment on oxidative stress and hepatotoxicity in binge ethanol administered rats. Hum Exp Toxicol. 2010;29(8):659–65. doi: 10.1177/0960327109359460. [DOI] [PubMed] [Google Scholar]
- 19.Yan SL, Wu ST, Yin MC, et al. Protective effects from carnosine and histidine on acetaminophen-induced liver injury. J Food Sci. 2009;74(8):259–65. doi: 10.1111/j.1750-3841.2009.01330.x. [DOI] [PubMed] [Google Scholar]
- 20.Gallant S, Kukley M, Stvolinsky S, et al. Effect of carnosine on rats under experimental brain ischemia. Tohoku J Exp Med. 2000;1991:85–99. doi: 10.1620/tjem.191.85. [DOI] [PubMed] [Google Scholar]
- 21.Stvolinsky SL, Kukley ML, Dobrota D, et al. Carnosine: an endogenous neuroprotector in the ischemic brain. Cell Mol Neurobiol. 1999;19:45–56. doi: 10.1023/A:1006960407008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fouad AA, El-Rehany MA, Maghraby HK. The hepatoprotective effect of carnosine against ischemia/reperfusion liver injury in rat. Eur J Pharmacol. 2007;572(1):61–68. doi: 10.1016/j.ejphar.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 23.Baykara B, Tekmen I, Pekcetin C, et al. The protective effects of carnosine and melatonin in ischemia-reperfusion injury in the rat liver. Acta Histochem. 2009;111(1):42–51. doi: 10.1016/j.acthis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Aydin AF, Küçükgergin C, Ozdemirler-Erata G, et al. The effect of carnosine treatment on prooxidant-antioxidant balance in liver, heart and brain tissues of male aged rats. Biogerontology. 2010;11(1):103–9. doi: 10.1007/s10522-009-9232-4. [DOI] [PubMed] [Google Scholar]
- 25.Otero-Antón E, González-Quintela A, López-Soto A, et al. Cecal ligation and puncture as amodel of sepsis in the rat: influence of the puncture size onmortality, bacteremia, endotoxemia and tumor necrosis factoralpha levels. Eur Surg Res. 2001;33:77–79. doi: 10.1159/000049698. [DOI] [PubMed] [Google Scholar]
- 26.Fouad AA, Qureshi HA, Yacoubi MT, Al-Melhim WN. Protective role of carnosine in mice with cadmium-induced acute hepatotoxicity. Food Chem Toxicol. 2009;47(11):2863–70. doi: 10.1016/j.fct.2009.09.009. [DOI] [PubMed] [Google Scholar]