Abstract
Background
The complex configuration of the thumb carpometacarpal (CMC-1) joint relies on musculotendinous and ligamentous support for precise circumduction. Ligament innervation contributes to joint stability and proprioception. Evidence suggests abnormal ligament innervation is associated with osteoarthritis (OA) in large joints; however, little is known about CMC-1 ligament innervation characteristics in patients with OA. We studied the dorsal radial ligament (DRL) and the anterior oblique ligament (AOL), ligaments with a reported divergent presence of mechanoreceptors in nonosteoarthritic joints.
Questions/purposes
This study’s purposes were (1) to examine the ultrastructural architecture of CMC-1 ligaments in surgical patients with OA; (2) to describe innervation, specifically looking at mechanoreceptors, of these ligaments using immunohistochemical techniques and compare the AOL and DRL in terms of innervation; and (3) to determine whether there is a correlation between age and mechanoreceptor density.
Methods
The AOL and DRL were harvested from 11 patients with OA during trapeziectomy (10 women, one man; mean age, 67 years). The 22 ligaments were sectioned in paraffin and analyzed using immunoflourescent triple staining microscopy.
Results
In contrast to the organized collagen bundles of the DRL, the AOL appeared to be composed of disorganized connective tissue with few collagen fibers and little innervation. Mechanoreceptors were identified in CMC-1 ligaments of all patients with OA. The DRL was significantly more innervated than the AOL. There was no significant correlation between innervation of the DRL and AOL and patient age.
Conclusions
The dense collagen structure and rich innervation of the DRL in patients with OA suggest that the DRL has an important proprioceptive and stabilizing role.
Clinical Relevance
Ligament innervation may correlate with proprioceptive and neuromuscular changes in OA pathophysiology and consequently support further investigation of innervation in disease prevention and treatment strategies.
Introduction
The first carpometacarpal (CMC-1) joint is often referred to as a “saddle joint” as proposed by Fick in 1884 in describing the concave-convex morphology of the thumb basal joint [10]. It possesses features of a universal joint allowing movement in a wide and complex range of movement (ROM) [6]. Besides its anatomical constraints, joint stability in the normal CMC-1 joint relies on neuromuscular control: refined integration of sensory input from muscles, ligaments, and skin.
Current evidence supports the concept of a joint as a synovial organ: a functioning unit with both neurosensory and neuromuscular impact on joint stability and control [20]. Consequently, neurosensory and neuromuscular impairment such as inadequate reflex control of periarticular muscles, impaired ligament function, or disturbed innervation may contribute to osteoarthritis (OA) [27, 34, 39]. Evidence in human and animal models [22, 36–38] supports the notion that joint denervation may cause an increase in cartilage degeneration, impairment of joint reflexes, and later onset or aggravation of OA. During the process of aging, there is a natural denervation process [37]; this may also contribute to an increasing prevalence of OA with age [22].
The purpose of this study was to examine the ultrastructural architecture and mechanoreceptors of CMC-1 ligaments in surgical patients with OA using immunohistochemical techniques. Previous studies of CMC-1 ligaments have demonstrated a connection between ligamentous instability and the development of CMC-1 OA [8, 31, 32]. However, a conflict exists in the literature as to which ligament has primary importance in CMC-1 stability: the volar anterior oblique (AOL) or dorsoradial (DRL) ligament. To our knowledge, no previous studies have outlined the relationship between the DRL and CMC-1 OA nor compared it with the AOL in surgical patients. We studied the DRL and the AOL because these are ligaments with the strongest mean difference in innervation density in nonosteoarthric joints [24].
To investigate these issues, we proposed the following research questions: (1) Are there histologic differences in the cellular and structural composition of the AOL and DRL in osteoarthritic subjects? This has been shown in normal cadaveric dissections [16, 24, 26] but the characteristics in surgical patients are unknown. (2) Are sensory nerve endings, ie, mechanoreceptors, present in the AOL and DRL of patients with CMC-1 OA, and, if so, is there a difference in mechanoreceptor types between the AOL and DRL, respectively? (3) Finally, is there a correlation between age and mechanoreceptor density? This has been reported in the literature in other joints [22, 36–38]. If a similar correlation is found, it may provide insight into the effect of age and severity progression of OA.
Materials and Methods
Specimens
The AOL and DRL ligaments were harvested from the hands of 11 patients undergoing trapeziectomy with ligament stabilization who had advanced clinical and radiographic disease (Eaton stage 2–4) [9]. The study cohort consisted of 10 women and one man and six right and five left hands (mean age, 67 years; age range, 51–83 years). All surgeries and ligament harvesting were performed by an experienced hand surgeon (ALL) with use of 3.5 loupe magnification and standard hand surgical instruments; ligament harvesting and identification were performed using techniques outlined by Bettinger et al. [1] and Ladd et al. [24]. A 5-mm length of each ligament (AOL and DRL) was harvested at the insertion into bone at both the trapezial and metacarpal sides and suture-marked at the distal insertion for orientation. The AOL is located deep to the insertion of the abductor pollicis longus, and the DRL is identified as the most radial of the dorsal ligaments [1, 24].
Approval for this project was granted through the local institutional review board, and the handling of human specimens was strictly according to ethical and practical protocols.
Slide Preparation
Harvested ligaments from all 11 surgical subjects (22 specimens total) were immediately fixed in 4% formaldehyde, embedded in paraffin, and then sectioned at a thickness of 5 μm before being mounted on glass slides. The sections used for architectural analysis were stained with Harris hematoxylin and eosin [41] before being mounted under coverslips.
Antibodies
Two primary antibodies were used: antinerve growth factor receptor p75 (p75) and antiprotein gene product 9.5 (PGP9.5) (Millipore, Billerica, MA, USA). Alexa Fluor 488 and 647 (Invitrogen, Carlsbad, CA, USA) were used for the secondary antibodies. ProLong Gold Anti-Fade Reagent with 49,69-diamidino-2-phenylindole (DAPI) (Invitrogen) was used concomitantly to highlight nuclear material in specimens. In conjunction, triple staining with p75, PGP9.5, and DAPI is a recently validated technique for visualizing mechanorecptors, nerves, and arteries/arterioles in contrast with collagen within ligaments [26].
Immunofluorescence Imaging
The immunohistochemical sections were imaged with use of a fluorescence microscope (Observer.Z1; Carl Zeiss MicroImaging, Thornwood, NY, USA). A multidimensional acquisition setting was used to analyze sensory nerve endings through the use of wavelength settings of 358, 488, and 596 nm.
Initial scanning for orientation was performed at a wavelength of 488 nm to visualize p75 staining and to scan for areas of interest. Epifascicular regions that envelope the dense collagen fibers were the main focus of study because nerves are most commonly found in these regions [15, 44]. Magnification was increased to x 20 and then x 40 to investigate regions with increased fluorescence relative to surrounding areas.
The light source was next switched to 350 nm to visualize DAPI staining to differentiate between nerve bundles in vascular structures versus in ligament tissue. If the structure of interest was found to be nervous, fluorescence of PGP9.5 was next visualized at 596 nm.
Lastly, similar regions were analyzed with multidimensional acquisition to compare the appearance of sensory nerve endings and evaluate for the presence of mechanoreceptors.
Outcome Measures
An ordinal grading system previously used for analysis of ligament innervation was used to quantify the degree of innervation and to assess for mechanoreceptor presence [15, 26]. The grading system ranged from (+++) to (−) with (+++) representing the presence of several nerve fascicles and mechanoreceptors, (++) representing the presence of a single nerve fascicle and receptor, (+) representing the presence of nerve fascicles but no receptors, and (−) representing the presence of no nerve fibers, fascicles, or mechanoreceptors.
Power Analysis
A prestudy power analysis was not performed because the sample size was fixed. However, a post hoc calculation revealed that group sample sizes of 11 (number of AOL samples) and 11 (number of DRL samples) achieve 80% power to detect a difference of 1.79 between the null hypothesis that both group means are 5.00 and the alternative hypothesis that the mean of AOL is 3.21. Therefore, approximately 35.8% of significant differences between the AOL and DRL would be missed using this sample size. This post hoc calculation was done with a two-sided two-sample t-test using known group SDs of 1.17 (DRL) and 1.77 (AOL) and a significance level (alpha) of 0.05. A clinically meaningful difference in innervation may be less than 35% depending on how dramatically the difference will prove to affect disease pathology in future studies.
Statistical Analysis
A Student’s paired t-test was used to estimate the differences in innervation grade between ligaments, obtaining two locations per ligament, in each of the two ligaments per specimen. From this model, pairwise comparisons were made between the ligaments producing p values and 95% confidence intervals for the mean differences between each ligament pair.
Standard linear regression analysis was used to determine whether a linear relationship existed between mean difference among the DRL and AOL grade innervation scores and patient age at the time of surgery. The Pearson correlation coefficient was used to determine the strength of that linear relationship.
Results
Ultrastructural Architecture of CMC-1 Ligaments
Hematoxylin and eosin staining presented reliable differences in the organization of the dorsal and volar ligaments of live patients with CMC OA. The DRL was comprised of organized collagen bundles with collinear orientation. The AOL contained disorganized connective tissue with few collagen fibers and an appearance similar to that of synovial tissue (Fig. 1). These differences were consistent with those demonstrated by a study examining dorsal and volar ligaments of cadavers without (or in one case with minimal) osteoarthritic change [24].
Fig. 1A–B.
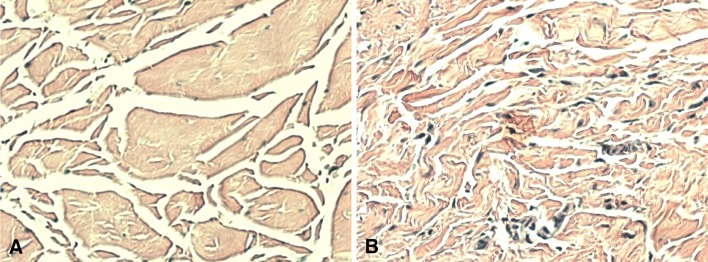
Hematoxylin and eosin stain of (A) DRL and (B) AOL. The dorsal radial oblique ligament reveals organized collagen bundles with collinear orientation. In contrast, the AOL contains disorganized connective tissue with no collagenous structure.
Also consistent was structural organization of both dorsal and volar ligaments using immunofluorescence with DAPI stain. DAPI stain revealed nuclei in the organized collagen bundles of the DRL but revealed a lack of strong nuclei presence in the disorganized connective of the AOL.
Identification of Mechanoreceptors
The following mechanoreceptor types were identified in the CMC-1 ligaments of patients who underwent surgery for CMC-OA: Ruffini endings, Pacini corpuscles, and unclassifiable corpuscles. These were readily distinguished from arterioles with triple staining of PGP9.5, p75, and DAPI.
Ruffini Ending
Immunofluorescent triple stain revealed several Ruffini endings in the ligaments of patients who underwent surgery for CMC OA. Ruffini endings are low-threshold, slowly adapting mechanoreceptors more common in nonweightbearing joints. As previously reported in cadavers, Ruffini endings were distinguished by their coiled dendritic endings that stain independently with p75 and PGP9.5 and also overlap, emitting yellow–orange fluorescence (Fig. 2) [26]. They range in size from 50 to 150 μm.
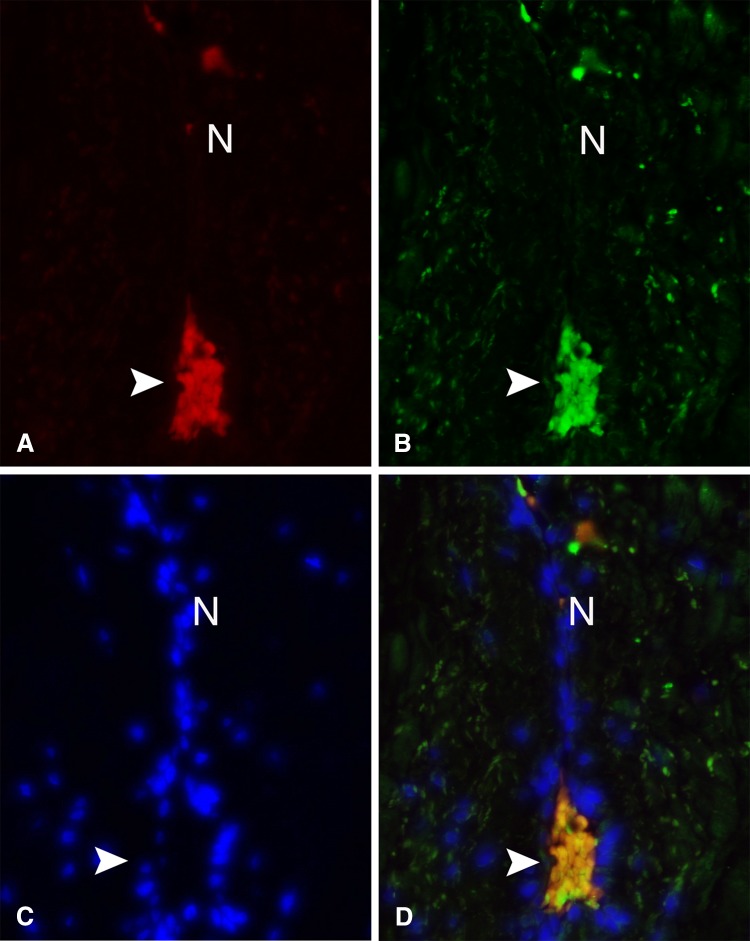
Fig. 2A–D.
Mechanoreceptor visualized in surgical patient with CMC-1 OA. Ruffini ending from a DRL stained with (A) p75 expressed on the cell membrane, (B) PGP9.5, a pan-neuronal marker present in neuronal cytoplasm, and (C) DAPI, which marks the nuclei of the surrounding fibrocytes and collagen. Triple staining allows for visualization of the terminal bulbous ending of the Ruffini (arrowhead) and afferent parent axon (N). (D) Areas of overlap on immunofluorescence.
Pacini Corpuscle
Pacini corpuscles were rarely found in the ligaments of patients who underwent surgery for CMC OA. Pacini corpuscles are low-threshold, rapidly adapting mechanoreceptors more common in weightbearing joints. As previously reported in cadavers, Pacini corpuscles were identified through their onion-like, lamellar capsule with p75 staining and lacking PGP9.5 and DAPI immunofluorescence (Fig. 3) [26].
Fig. 3.
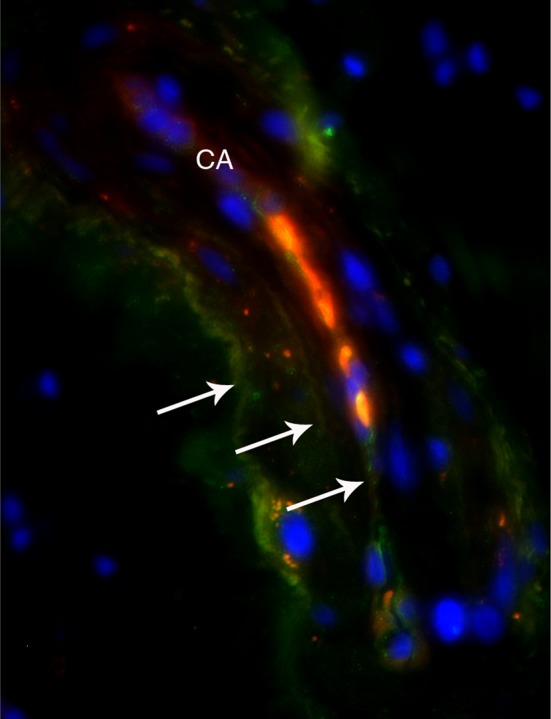
Mechanoreceptor visualized in a surgical patient with CMC-1 OA. Pacinian corpuscle from a DRL triple stained with p75, PGP9.5, and DAPI. Triple staining allows for visualization of the onion-like, lamellar capsule of the Pacini (arrows) and afferent central axon (CA).
Arteriole
The triple stain pattern was successful in distinguishing nerves from arterioles in patients with CMC OA just as in cadaveric specimens. p75 and PGP9.5 were found in the adventitia and between the media and adventitia. p75 was also found in the endothelial walls. Arterioles displayed linear (longitudinally cut) or circular (transversely cut) DAPI immunofluorescence (Fig. 4).
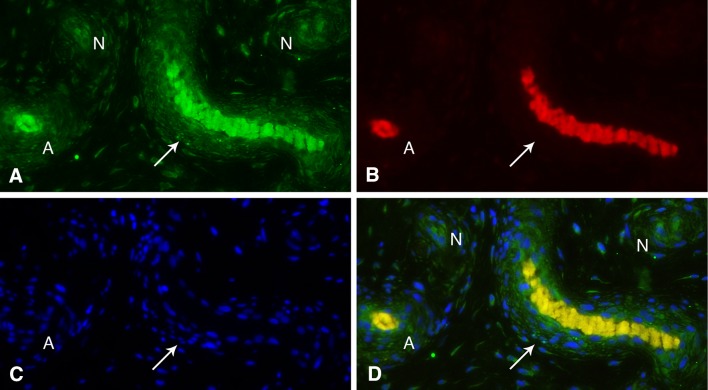
Fig. 4A–D.
Longitudinally (arrow) and transversely (A) cut arterioles and nerves (N) visualized in a surgical patient with CMC OA. Arterioles and nerves from a DRL stained with (A) p75 and (B) PGP9.5 in the adventitia and between the media and adventitia. P75 is also prominent in endothelial walls. (C) DAPI stains linear (longitudinally cut) or circular (transversely cut) arterioles. (D) Areas of overlap on immunofluorescence.
Unclassifiable Corpuscles
We also identified structures consistent with small, rounded sensory nerve corpuscles in the AOL not readily classifiable as either Pacini or Ruffini. These small corpuscles were labeled as unclassifiable and are being analyzed further as to type and importance.
Distribution of Innervation
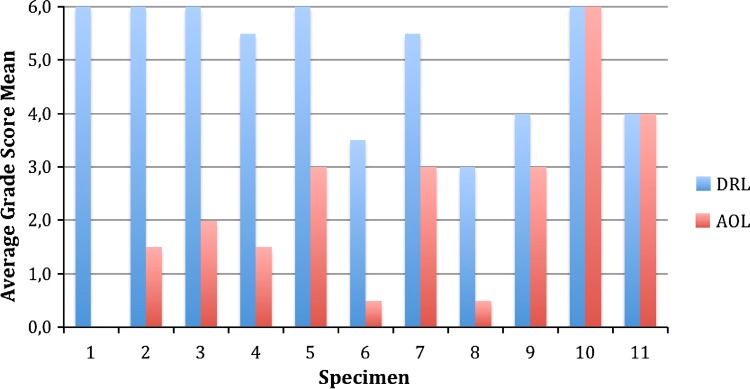
The DRL was significantly more innervated with mechanoreceptors and nerve endings than the AOL (Fig. 5). Mechanoreceptors and nerve fibers were most readily seen near arterioles, in the ligament epifascicular layers. A Student’s paired t-test revealed a statistically significant difference between innervation of the DRL (mean = 5.0455, SD = 1.17) and AOL (mean = 2.27, SD = 1.77) of surgical patients with CMC OA (t[10] = 4.903, p = 0.001, α = 0.05; 95% confidence interval, 1.513–4.033).
Fig. 5.
Bar graph displaying semiquantitative distribution of nerves and mechanoreceptors in the dorsal radial and anterior oblique ligaments from 11 surgical CMC OA patient specimens. The average grade score mean for each ligament is comprised of the sum of two representative ligament samples. For each sample, +++ (3.0) indicates richly innervated with several nerve fascicles and mechanoreceptors; ++ (2.0) indicates single nerve fascicle and mechanoreceptor; + (1.0) indicates nerve fascicle alone; and – (0.0) indicates no signs of innervation.
Age-related Differences in Mechanoreceptor Density
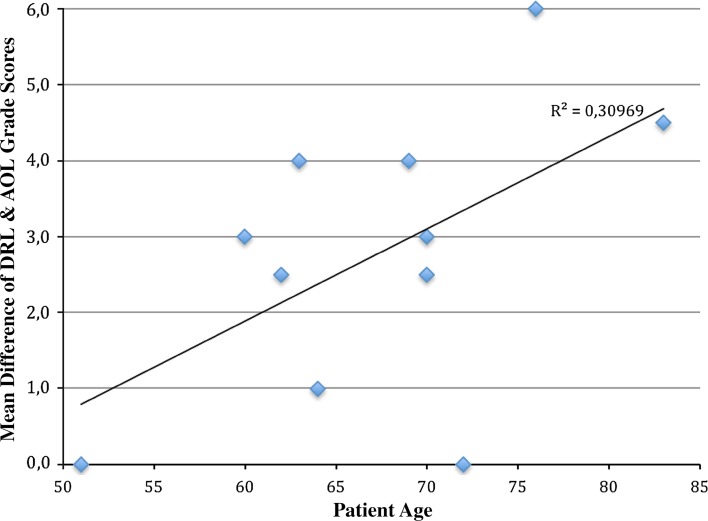
With the numbers available, there was no significant correlation between innervation of the DRL and AOL and patient age. The quantification of the linear relationship between mean innervation differences in each patient’s DRL and AOL and his or her age (Fig. 6) showed a Pearson’s r of 0.557 (p = 0.075).
Fig. 6.
Linear regression analysis comparing the mean difference between the DRL and AOL grade innervation scores and patient age at the time of surgery shows a Pearson’s r of 0.557. The data trends toward significance with a p value of 0.075.
Discussion
The CMC-1 is a complex joint with a wide ROM, providing stability in the opposite demands of both fine motor tasks and power grasp. Since 1958, when Palmer first revealed the mechanism of a reflex between muscles acting on a joint and its corresponding ligaments [30], many studies have proven the existence of ligamentomuscular reflexes in the knee, ankle, shoulder, elbow, and wrist [7, 11, 18, 33]. Afferent information from mechanoreceptors plays an important role in neuromuscular control and can directly affect muscle action [18, 27, 35]. Recent studies have suggested that ligamentous OA should be considered its own etiology in the development of joint OA [28]. This new term is based on the notion that a joint is a synovial organ in which any part of that organ such as the cartilage, subchondral bone, synovium, ligament, nerve, or periarticular muscle may contribute to the development of joint OA [4]. In other words, abnormal or impaired biomaterials or connective tissues such as ligaments normally stabilizing the joint may lead to changes in proprioception and neuromuscular control and thus contribute to the development of OA [20, 39, 46]. This form of ligamentous OA is believed to be especially frequent in the small joints of the hand [43]. In CMC-1 OA, changes in ligament structure such as increased laxity are frequent and may contribute to CMC-1 OA [19, 43]. In this study, we examined the two major ligaments of CMC-1: the DRL and AOL, and their structural and sensory properties in surgical patients with CMC-1 OA.
In addition to the known limitations of a descriptive study, our study is limited by the small number of subjects. Although our population reflects the known demographic high prevalence of CMC-1 OA in women, we would have preferred to have analyzed an equal number of female and male specimens. We did not include the other volar, dorsal, and intermetacarpal ligaments [1, 21, 29] described as important CMC-1 stabilizers. We did, however, choose the two most surgically accessible ligaments, which have demonstrated significant differences in structure and innervation in normal specimens [24]. A potential advantage of our study was the use of fresh paraffin-embedded tissue that had not previously been frozen as opposed to fresh-frozen cadavers. Our surgical sampling allowed for more precise analysis because there are less artifacts and better quality of nerve endings when compared with fresh-frozen specimens [16].
Our results confirm that there is a considerable structural difference between DRL and AOL in surgical patients with OA, as shown in previous cadaveric macro- and microscopic studies [16, 24, 26]. Where DRL is highly organized with dense collagenous bundles and an abundance of nuclei, AOL is mainly composed of loose connective tissue with sparse nuclei. Corresponding findings have been demonstrated in the dorsal wrist and scapholunate interosseous ligaments [14–17, 44], where these ligaments had ample innervation compared with volar ligaments. Our histologic observations also add support to the theory that the DRL is a primary stabilizer of CMC-1 [2, 3, 5, 42, 45]. In concordance with studies made on the scapholunate interosseous ligament [13], we support the idea that intact proprioceptive mechanisms, requiring the presence of functioning mechanoreceptors and ligamentomuscular reflexes, will preclude posttraumatic OA evolvement. In a clinical setting, this suggests the importance of early proprioceptive rehabilitation of patients with idiopathic CMC-1 OA as well as traumatic CMC-1 injuries. In addition, we propose that patients with ligamentous laxity will also benefit from proprioceptive rehabilitation, a population reported to possess decreased joint proprioception [25].
In addition to architectural differences, we also found significant differences in the innervation patterns of the AOL and DRL. Mechanoreceptors and nerve fibers were found in both the AOL and the DRL, mostly in their epifasicular regions and close to arterioles. The Ruffini ending was the most common mechanoreceptor found, as has been previously observed in the wrist [15] and CMC-1 joint from normal cadaveric specimens. These findings suggest that the DRL has greater proprioceptive potential in stabilizing CMC-1 in cases with OA compared with the AOL. Mechanoreceptors and nerve endings are more frequent in the DRL than AOL. The Pacinian corpuscle, a rapidly adapting receptor activated by changes in joint velocity and joint compression, was an uncommon finding in both ligaments. Subsequently, our current clinical findings concur with previous cadaveric studies, which have reported greater innervation of the dorsal deltoid ligaments, compared with the innervation of AOL and ulnar collateral ligaments [16]. The abundance of nerve endings and mechanoreceptors in the DRL may partially explain why patients with CMC-1 OA often experience dorsal joint pain.
We found no significant relationship between age and innervation, although this may have been the result of limitations of our study’s small sample size. Other studies have suggested an age-related decrease of mechanoreceptors and consequently impaired proprioceptive traits [23, 40, 44]. Previous studies have also established a correlation between age and mechanoreceptor density [22, 36–38]. To date, underlying pathophysiological mechanisms of age-related changes of proprioception have not been fully delineated. Further investigation in this area will provide us with a better understanding of the development of OA in the CMC-1 as well as ostheoarthritic changes occuring in other joints.
The DRL has previously been described as the most important ligament stabilizing CMC-1 [2, 3, 5, 42, 45] but ours is the first study to analyze this ligament from a proprioceptive and histological point of view. We conclude that the DRL is a ligament with greater innervation and thus a more significant proprioceptive role in stabilizing the CMC-1 as compared with the AOL in patients with CMC-1 OA. In addition to its proprioceptive qualities, the DRL has structural advantages with an increased organization of collagen tissue, which may provide the joint with better biomechanical support.
Although we have found the DRL to be structurally more resilient, we cannot, based on our present data, evaluate the condition of its proprioceptive functions or determine if its neuromuscular control has been disrupted as a result of OA. After joint trauma or in cases with OA, proprioceptive mechanisms from receptors in ligaments and tendons may be disrupted and this will subsequently disable proper neuromuscular control. This neuromuscular disruption can lead to disproportional joint load during certain tasks, causing pain and weakness [39], which are frequent in CMC-1 OA. To further define the proprioceptive properties, function, and significance of the mechanoreceptors of CMC-1 in cases with OA, studies of in vivo neuromuscular control would be required similar to proprioceptive studies performed about the wrist [12, 18]. Improved characterization of the innervation of the CMC-1 joint on a microscopic level may enhance current treatment options and delay progression of the very common form of OA. The clinical importance of this study, accordingly, may provide opportunities related to prevention and postoperative rehabilitation for patients with CMC-1 OA as well as proprioceptive rehabilitation for posttraumatic CMC-1 conditions.
Footnotes
One of the authors (NM) was supported by Karolinska Institute to cover travel expenses.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This investigation was performed at the Robert A. Chase Hand & Upper Limb Center, Department of Orthopedic Surgery and Division of Clinical Anatomy, Stanford University, Stanford, CA, USA.
References
- 1.Bettinger PC, Linscheid RL, Berger RA, Cooney WP, 3rd, An KN. An anatomic study of the stabilizing ligaments of the trapezium and trapeziometacarpal joint. J Hand Surg Am. 1999;24:786–798. doi: 10.1053/jhsu.1999.0786. [DOI] [PubMed] [Google Scholar]
- 2.Bettinger PC, Smutz WP, Linscheid RL, Cooney WP, 3rd, An KN. Material properties of the trapezial and trapeziometacarpal ligaments. J Hand Surg Am. 2000;25:1085–1095. doi: 10.1053/jhsu.2000.18487. [DOI] [PubMed] [Google Scholar]
- 3.Bosmans B, Verhofstad MH, Gosens T. Traumatic thumb carpometacarpal joint dislocations. J Hand Surg Am. 2008;33:438–441. doi: 10.1016/j.jhsa.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 4.Brandt KD, Dieppe P, Radin E. Etiopathogenesis of osteoarthritis. Med Clin North Am. 2009;93:1–24, xv. [DOI] [PubMed]
- 5.Colman M, Mass DP, Draganich LF. Effects of the deep anterior oblique and dorsoradial ligaments on trapeziometacarpal joint stability. J Hand Surg Am. 2007;32:310–317. doi: 10.1016/j.jhsa.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Cooney WP, 3rd, Lucca MJ, Chao EY, Linscheid RL. The kinesiology of the thumb trapeziometacarpal joint. J Bone Joint Surg Am. 1981;63:1371–1381. [PubMed] [Google Scholar]
- 7.Diederichsen LP, Norregaard J, Krogsgaard M, Fischer-Rasmussen T, Dyhre-Poulsen P. Reflexes in the shoulder muscles elicited from the human coracoacromial ligament. J Orthop Res. 2004;22:976–983. doi: 10.1016/j.orthres.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 8.Doerschuk SH, Hicks DG, Chinchilli VM, Pellegrini VD., Jr Histopathology of the palmar beak ligament in trapeziometacarpal osteoarthritis. J Hand Surg Am. 1999;24:496–504. doi: 10.1053/jhsu.1999.0496. [DOI] [PubMed] [Google Scholar]
- 9.Eaton RG, Glickel SZ. Trapeziometacarpal osteoarthritis. Staging as a rationale for treatment. Hand Clin. 1987;3:455–471. [PubMed] [Google Scholar]
- 10.Fick A. [The Joint With Saddle Shaped Surface] [in German]. Heidelberg, Germany: Adademische Verlagshandlung; 1854:314–321.
- 11.Freeman MA, Wyke B. Articular reflexes at the ankle joint: an electromyographic study of normal and abnormal influences of ankle-joint mechanoreceptors upon reflex activity in the leg muscles. Br J Surg. 1967;54:990–1001. doi: 10.1002/bjs.1800541204. [DOI] [PubMed] [Google Scholar]
- 12.Hagert E. Wrist Ligaments—Innervation Patterns and Ligamento-muscular Reflexes. PhD thesis, Department of Clinical Science and Education, Section of Hand Surgery. Stockholm, Sweden: Karolinska Institutet; 2008:1–51.
- 13.Hagert E. Proprioception of the wrist following posterior interosseous sensory neurectomy. J Hand Surg Am. 2010;35:690–691; author reply 691. [DOI] [PubMed]
- 14.Hagert E, Forsgren S, Ljung BO. Differences in the presence of mechanoreceptors and nerve structures between wrist ligaments may imply differential roles in wrist stabilization. J Orthop Res. 2005;23:757–763. doi: 10.1016/j.orthres.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Hagert E, Garcia-Elias M, Forsgren S, Ljung BO. Immunohistochemical analysis of wrist ligament innervation in relation to their structural composition. J Hand Surg Am. 2007;32:30–36. doi: 10.1016/j.jhsa.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Hagert E, Lee J, Ladd AL. Innervation patterns of thumb trapeziometacarpal joint ligaments. J Hand Surg Am. 2012;37(706.e701):714.e701. doi: 10.1016/j.jhsa.2011.12.038. [DOI] [PubMed] [Google Scholar]
- 17.Hagert E, Ljung BO, Forsgren S. General innervation pattern and sensory corpuscles in the scapholunate interosseous ligament. Cells Tissues Organs. 2004;177:47–54. doi: 10.1159/000078427. [DOI] [PubMed] [Google Scholar]
- 18.Hagert E, Persson JK, Werner M, Ljung BO. Evidence of wrist proprioceptive reflexes elicited after stimulation of the scapholunate interosseous ligament. J Hand Surg Am. 2009;34:642–651. doi: 10.1016/j.jhsa.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Hunter DJ, Zhang Y, Sokolove J, Niu J, Aliabadi P, Felson DT. Trapeziometacarpal subluxation predisposes to incident trapeziometacarpal osteoarthritis (OA): the Framingham Study. Osteoarthritis Cartilage. 2005;13:953–957. doi: 10.1016/j.joca.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Hurley MV. The role of muscle weakness in the pathogenesis of osteoarthritis. Rheum Dis Clin North Am. 1999;25:283–298, vi. [DOI] [PubMed]
- 21.Imaeda T, An KN, Cooney WP, 3rd, Linscheid R. Anatomy of trapeziometacarpal ligaments. J Hand Surg Am. 1993;18:226–231. doi: 10.1016/0363-5023(93)90352-4. [DOI] [PubMed] [Google Scholar]
- 22.Konttinen YT, Tiainen VM, Gomez-Barrena E, Hukkanen M, Salo J. Innervation of the joint and role of neuropeptides. Ann N Y Acad Sci. 2006;1069:149–154. doi: 10.1196/annals.1351.013. [DOI] [PubMed] [Google Scholar]
- 23.Kwan MM, Close JC, Wong AK, Lord SR. Falls incidence, risk factors, and consequences in Chinese older people: a systematic review. J Am Geriatr Soc. 2011;59:536–543. doi: 10.1111/j.1532-5415.2010.03286.x. [DOI] [PubMed] [Google Scholar]
- 24.Ladd AL, Lee J, Hagert E. Macroscopic and microscopic analysis of the thumb carpometacarpal ligaments: a cadaveric study of ligament anatomy and histology. J Bone Joint Surg Am. 2012;94:1468–1477. doi: 10.2106/JBJS.K.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HM, Cheng CK, Liau JJ. Correlation between proprioception, muscle strength, knee laxity, and dynamic standing balance in patients with chronic anterior cruciate ligament deficiency. Knee. 2009;16:387–391. doi: 10.1016/j.knee.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Ladd A, Hagert E. Immunofluorescent triple-staining technique to identify sensory nerve endings in human thumb ligaments. Cells Tissues Organs. 2012;195:456–464. doi: 10.1159/000327725. [DOI] [PubMed] [Google Scholar]
- 27.Lephart SM, Fu FH. Proprioception and Neuromuscular Control in Joint Stability; Introduction to the Sensorimotor System. Champaign, IL, USA: Human Kinetics; 2000. [Google Scholar]
- 28.McGonagle D, Tan AL, Carey J, Benjamin M. The anatomical basis for a novel classification of osteoarthritis and allied disorders. J Anat. 2010;216:279–291. doi: 10.1111/j.1469-7580.2009.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Napier JR. The form and function of the carpo-metacarpal joint of the thumb. J Anat. 1955;89:362–369. [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer I. Pathophysiology of the medical ligament of the knee joint. Acta Chir Scand. 1958;115:312–318. [PubMed] [Google Scholar]
- 31.Pellegrini VD., Jr Osteoarthritis of the trapeziometacarpal joint: the pathophysiology of articular cartilage degeneration. I. Anatomy and pathology of the aging joint. J Hand Surg Am. 1991;16:967–974. doi: 10.1016/S0363-5023(10)80054-1. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrini VD., Jr The ABJS 2005 Nicolas Andry Award: osteoarthritis and injury at the base of the human thumb: survival of the fittest? Clin Orthop Relat Res. 2005;438:266–276. doi: 10.1097/01.blo.0000176968.28247.5c. [DOI] [PubMed] [Google Scholar]
- 33.Phillips D, Petrie S, Solomonow M, Zhou BH, Guanche C, D’Ambrosia R. Ligamentomuscular protective reflex in the elbow. J Hand Surg Am. 1997;22:473–478. doi: 10.1016/S0363-5023(97)80015-9. [DOI] [PubMed] [Google Scholar]
- 34.Riemann BL, Lephart SM. The sensorimotor system, part I: the physiologic basis of functional joint stability. J Athl Train. 2002;37:71–79. [PMC free article] [PubMed] [Google Scholar]
- 35.Riemann BL, Lephart SM. The sensorimotor system, part II: the role of proprioception in motor control and functional joint stability. J Athl Train. 2002;37:80–84. [PMC free article] [PubMed] [Google Scholar]
- 36.Salo P. The role of joint innervation in the pathogenesis of arthritis. Can J Surg. 1999;42:91–100. [PMC free article] [PubMed] [Google Scholar]
- 37.Salo PT, Hogervorst T, Seerattan RA, Rucker D, Bray RC. Selective joint denervation promotes knee osteoarthritis in the aging rat. J Orthop Res. 2002;20:1256–1264. doi: 10.1016/S0736-0266(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 38.Salo PT, Seeratten RA, Erwin WM, Bray RC. Evidence for a neuropathic contribution to the development of spontaneous knee osteoarthrosis in a mouse model. Acta Orthop Scand. 2002;73:77–84. doi: 10.1080/000164702317281459. [DOI] [PubMed] [Google Scholar]
- 39.Sharma L. Proprioceptive impairment in knee osteoarthritis. Rheum Dis Clin North Am. 1999;25:299–314, vi. [DOI] [PubMed]
- 40.Skinner HB, Barrack RL, Cook SD. Age-related decline in proprioception. Clin Orthop Relat Res. 1984;184:208–211. [PubMed] [Google Scholar]
- 41.Slaoui M, Fiette L. Histopathology procedures: from tissue sampling to histopathological evaluation. Methods Mol Biol. 2011;691:69–82. doi: 10.1007/978-1-60761-849-2_4. [DOI] [PubMed] [Google Scholar]
- 42.Strauch RJ, Behrman MJ, Rosenwasser MP. Acute dislocation of the carpometacarpal joint of the thumb: an anatomic and cadaver study. J Hand Surg Am. 1994;19:93–98. doi: 10.1016/0363-5023(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 43.Tan AL, Toumi H, Benjamin M, Grainger AJ, Tanner SF, Emery P, McGonagle D. Combined high-resolution magnetic resonance imaging and histological examination to explore the role of ligaments and tendons in the phenotypic expression of early hand osteoarthritis. Ann Rheum Dis. 2006;65:1267–1272. doi: 10.1136/ard.2005.050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomita K, Berger EJ, Berger RA, Kraisarin J, An KN. Distribution of nerve endings in the human dorsal radiocarpal ligament. J Hand Surg Am. 2007;32:466–473. doi: 10.1016/j.jhsa.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 45.Van Brenk B, Richards RR, Mackay MB, Boynton EL. A biomechanical assessment of ligaments preventing dorsoradial subluxation of the trapeziometacarpal joint. J Hand Surg Am. 1998;23:607–611. doi: 10.1016/S0363-5023(98)80045-2. [DOI] [PubMed] [Google Scholar]
- 46.Weerakkody NS, Blouin JS, Taylor JL, Gandevia SC. Local subcutaneous and muscle pain impairs detection of passive movements at the human thumb. J Physiol. 2008;586:3183–3193. doi: 10.1113/jphysiol.2008.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]