Abstract
Background
Abnormal biomechanical loading has been identified as an associated risk factor of osteoarthritis in the wrist and hand. Empirical data to date are insufficient to describe the role of altered biomechanics in thumb carpometacarpal (CMC) arthritis.
Questions/purposes
This is a pilot study to evaluate motion analysis of the upper extremity while performing functional tasks. We wished to describe the in vivo kinematics of the thumb and hand in relation to the larger joints of the upper extremity in subjects without arthritis in functional positions at rest and while loading the CMC joint. If reproducible, we then planned to compare kinematics between these subjects and a subject with advanced thumb CMC arthritis.
Methods
In vivo kinematics of the hand and upper extremity during the functional tasks of grasp, jar opening, and pinch with and without loading of the CMC joint were evaluated using cameras and a motion-capture system in four asymptomatic female subjects and one female subject with advanced radiographic (Eaton Stage IV) osteoarthritis.
Results
Kinematics of the hand and upper extremity can be reliably quantified. Loading of the CMC joint did not alter the hand and forearm kinematics in control subjects. In the subject with osteoarthritis, the adduction-extension deformity at the CMC joint resulted in kinematic alterations as compared with the four control subjects.
Conclusions
This study represents preliminary steps in defining thumb CMC position, motion, and loading associated with activities of daily living. These findings enhance our understanding of motion at the CMC joint and how it differs in arthritic patients.
Level of Evidence
Level II, prognostic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
One of the defining human features is the opposable thumb capable of precision pinch and grasp. The thumb is critical to hand function with nearly all of the basic activities of daily living requiring painless positioning of the thumb against resistance to accomplish manipulative tasks [13, 14]. Loss of thumb function imparts a 40% to 50% impairment to the upper extremity as a result of its central role in nearly all grasp and handling maneuvers [1]. The role of compensatory motion of adjacent and contiguous joints is well known in musculoskeletal impairment [2, 3, 16, 20] but the interdependence has not been quantified in diseases and arthritis of the hand.
Thumb carpometacarpal (CMC) osteoarthritis (OA) is a common and disabling degenerative disease. Radiographically visible arthrosis has been reported in approximately 15% of adults over the age of 30 years and 25% of women over the age of 55 years [5, 10, 25]. CMC OA is most prevalent among middle-aged and postmenopausal women [20], who often seek medical and surgical treatment for loss of dexterity and strength [17]. With both pinch and grasp, thumb use imparts high forces directly to the joint surface as a result of the small moment arm of the flexor and extensor tendons and to the relatively long length of the thumb [4].
Abnormal biomechanical loading has been identified as a risk factor for the development of OA in the wrist and hand [1]. The data implicating altered biomechanics with CMC arthritis, however, are largely empirical based on associations between CMC osteoarthritis and sex, trauma (acute and repetitive), and ligamentous hypermobility [15]. The interdependence of joints throughout the upper extremity as they relate to both normal and abnormal thumb CMC function has not been studied. Although numerous in vitro cadaver studies have evaluated the anatomy and kinematics of the CMC joint, in vivo kinematic data that correlate altered kinematics and abnormal joint loading to joint degeneration are lacking [21].
The nonphasic, three-dimensional nature of upper extremity function that requires fine manipulation for targeted activity proves a challenge to quantify. To date, motion analysis of the hand and wrist has constrained larger joints or simplified activity to relatively uniplanar or phasic [3, 8, 9]. Because studies have previously demonstrated alterations in static ROM testing of the thumb in patients with OA [9], our first objective was to determine whether in vivo kinematics of the thumb and hand could be quantified in subjects without arthritis during functional tasks Specifically, we evaluated the effect of carpometacarpal joint loading on upper limb position during three functional tasks: grasp, jar opening, and key pinch. These activities occupy several planes of motion, are nonphasic, and are constrained only by the hand placed on the targeted objects equipped with load cells. Our second objective was to evaluate these patients performing these activities while loading the CMC joint. Our final aim was to determine whether a difference could be observed during motion analysis in a subject with advanced thumb CMC arthritis in whom adaptive or compensatory movements of the entire upper extremity would be anticipated.
Patients and Methods
We recruited four right hand-dominant women with a mean age of 26 years old (range, 25–27 years old) to serve as control subjects. These patients reported no history of thumb or wrist pathology and denied CMC pain. We also recruited one 52-year-old subject with advanced radiographic (Eaton Stage IV) CMC arthritis to serve as an initial comparison, anticipating divergent kinematics [7]. Institutional review board approval and informed consent were obtained for this study.
Prior studies have demonstrated the accuracy of skin surface markers [11] as well as static alterations in motion in patients with OA [21]. Twenty-five skin markers were used during the motion analysis. Fifteen markers size 4 mm were dedicated to right hand and wrist motion, whereas the other size 5-mm markers with a resolution just under 1.0 ± 0.5 mm were used to analyze motion throughout the rest of the upper extremity (Fig. 1). Six markers were placed dorsally on the proximal and distal aspects of the right thumb phalanges and metacarpal and one was placed on the radial border of the distal proximal thumb phalanx to allow for a three-dimensional understanding of thumb motion. Thumb kinematics were referenced to the third metacarpal coordinate system [11]. Markers were also placed on the dorsal distal tip of the distal phalanx and the proximal and distal aspects of the metacarpal of the index and long fingers on the right side along with markers on the right radial and ulnar styloid, left ulnar styloid, bilateral lateral epicondyles, and three markers outlining the scapulae to accurately record wrist, elbow, and shoulder motion. Small markers were used on the hand, whereas medium-sized ones were used for the rest of the upper extremity. This marker set allowed for segmental coordinate systems to be defined for the first metacarpal, third metacarpal, proximal phalanges, distal phalanges, forearm, and humerus.
Fig. 1.
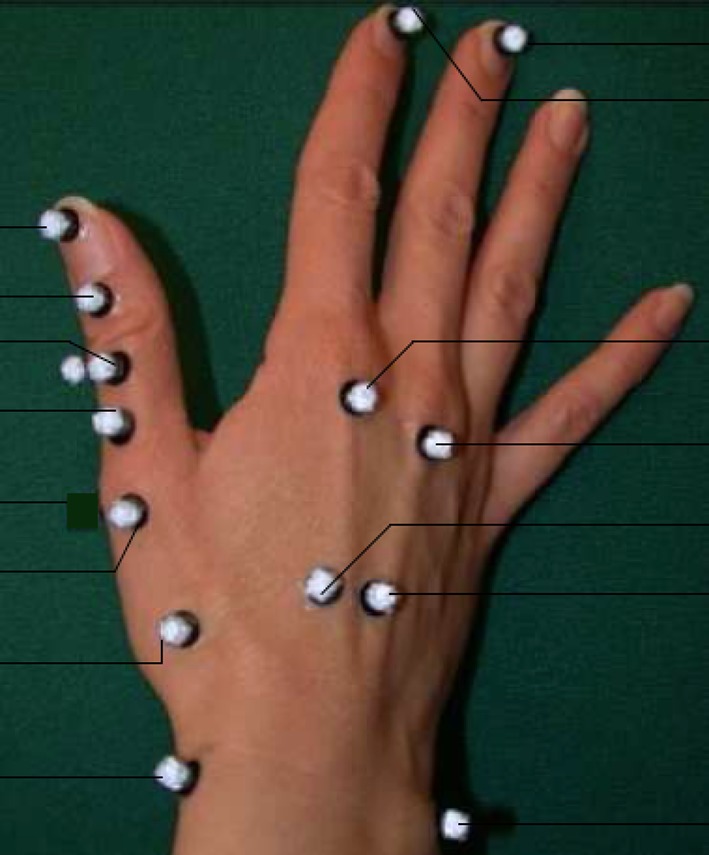
This is a schematic of finger markers for motion analysis.
Using eight cameras and a motion capture program (Cortex; Motion Analysis Corp, Santa Rosa, CA, USA) with a sampling frequency of 60 Hz with an average three-dimensional residual error for the motion capture system of 1.2 ± 0.6 mm, kinematics of the hand and upper extremity were quantified during ROM, lateral key pinch, object grasp, and jar opening (Fig. 2). Custom-made positioning jigs with embedded load cells (Orthopaedic Bioengineering Laboratories, Brown University/Rhode Island Hospital, Providence, RI, USA) permitted spatial registration and effort measurement, both digitally captured and visually represented to the subject. Subjects performed activities twice without loading and then again twice with loading to 80% maximal effort. In the unloaded scenario, subjects placed their hand on each of the three positioning jigs. Maximal loading was measured using a force sensor embedded in the jigs. The subjects exerted maximum force, as observed on the computer screen, and were then instructed to load to 80% of that maximal force. We examined motion through the CMC joint, interphalangeal joints, wrist, elbow, and shoulder as well as time to complete the task and force exerted. If compensatory motion was used by any party during the three trials, they were asked to reperform the activity while constraining their upper extremity motion so the shoulder, elbow, and wrist were all in the same coronal plane. Instruments were recalibrated before each new subject to ensure accuracy of measurements.
Fig. 2.

These are the tasks performed by each subject using positioning jigs with embedded load cells.
All kinematic data were processed using upper extremity motion software (UETrak; Motion Analysis Corp), a software program specifically designed for upper extremity motion analysis [3]. We measured shoulder elevation and horizontal abduction, elbow flexion/extension, forearm supination/pronation, wrist flexion/extension, wrist deviation, thumb CMC abduction/adduction, thumb metacarpal flexion/extension, and thumb interphalangeal flexion/extension.
Results
Control subjects demonstrated intratrial consistency in ROM while performing each task (Fig. 3; Table 1). However, the intertrial variability between subjects fluctuated depending on activity. During pinch, the average ROM of CMC flexion and adduction was 11.04° (SD 1.20) and 10.38° (SD 2.59), respectively, with ROM occurring between 11.85° and 26.79° of flexion and an adduction range of 4.51° to 24.47°. The average ROM at the metacarpophalangeal and interphalangeal joints was 10.39° (SD 2.12) and 35.88° (SD 6.65), respectively. In contrast, while performing grip and twist, which required more upper extremity motion, two subjects altered upper extremity kinematics by increasing their shoulder abduction and elbow flexion to increase force of hand extrinsic flexor musculature through flexion synergy, where shoulder abduction couples to assist in elbow, wrist, and finger flexion [6, 12]. Two subjects without arthritis maintained a relatively neutral shoulder abduction arc of motion during with a mean range of 13.24° (SD 1.58) during grip and 10.01° (SD 3.52) during jar twist. The other two subjects used flexion synergy and had shoulder abduction arcs of motion averaging 27.82° (SD 2.10) and 30.00° (SD 3.77) during grip and jar twist, respectively. The difference between these two sets of subjects was statistically significant with a p < 0.001. The subjects with increased shoulder abduction also used greater elbow flexion with an average maximal flexion of 96.81° and 100.24° during grip and jar twist, whereas the other two subjects obtained an average maximal flexion of 77.43° and 72.56° during these activities.
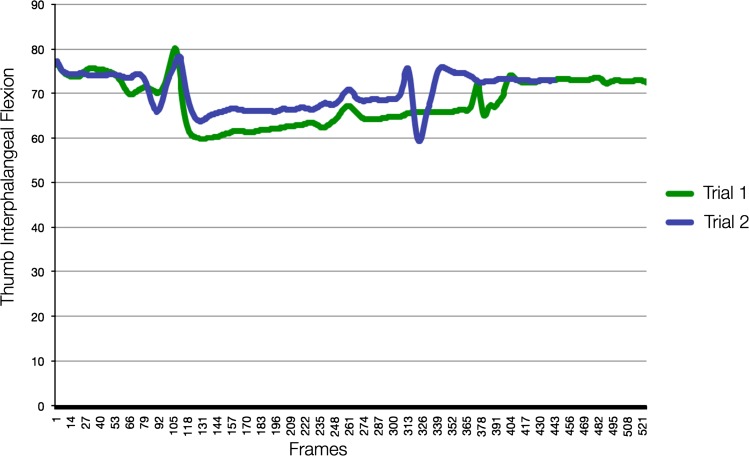
Fig. 3.
This is the motion analysis of a control subject’s thumb interphalangeal flexion during jar opening with force. This is an example of how control subjects demonstrated consistency throughout trials.
Table 1.
The angles measured during our functional activity motion analysis*
| Angle | Shoulder abduction | Shoulder elevation | Elbow flexion | FA pronation | Wrist flexion | Wrist deviation | CMC abduction | MCP flexion | IP flexion |
|---|---|---|---|---|---|---|---|---|---|
| S1 Tr1 | |||||||||
| Minimum | −71.4 | 90 | 47.5 | −48.79 | −38.77 | −11.9 | −17.64 | −27.49 | 42.48 |
| Maximum | −66.9 | 101.6 | 71.3 | −13.53 | 51.93 | −2.09 | 7.3 | −1.65 | 80.16 |
| Range | 4.5 | 11.6 | 23.8 | 35.26 | 90.7 | 9.81 | 24.93 | 25.84 | 37.68 |
| S1 Tr2 | |||||||||
| Minimum | −71.7 | 91 | 53.2 | −53.36 | −41.06 | −11.06 | −15.51 | −24.11 | 43.13 |
| Maximum | −66.5 | 98.4 | 70.8 | −18.16 | 53.5 | −2.69 | 6.54 | −6.78 | 78.44 |
| Range | 5.2 | 7.4 | 17.6 | 35.2 | 94.57 | 8.36 | 22.05 | 17.32 | 35.31 |
| S2 Tr1 | |||||||||
| Minimum | −77.8 | 67.3 | 68.5 | −68.27 | −20.34 | −4.38 | −21.78 | −18.76 | 46.6 |
| Maximum | −47.4 | 78.6 | 95.2 | −20.04 | 35.03 | 4.59 | −2.68 | −1.28 | 86.4 |
| Range | 30.4 | 11.3 | 26.7 | 48.23 | 55.37 | 8.97 | 19.09 | 17.47 | 39.81 |
| S2 Tr2 | |||||||||
| Minimum | −78 | 66.1 | 68.1 | −67.23 | −16.75 | −4.77 | −22.96 | −24.13 | 44.22 |
| Maximum | −48.5 | 78.8 | 92.4 | −26.58 | 36.86 | 4.36 | 16.32 | −2.19 | 83.55 |
| Range | 29.5 | 12.7 | 24.3 | 40.66 | 53.61 | 9.13 | 39.28 | 21.94 | 39.33 |
| S3 Tr1 | |||||||||
| Minimum | −64 | 63.3 | 65.3 | −65.93 | −8.38 | 10.57 | −20.61 | −21.66 | 48.41 |
| Maximum | −38.4 | 75.3 | 107.9 | −14.13 | 28.71 | 0.59 | 86.72 | 3.2 | 82.88 |
| Range | 25.6 | 12 | 42.6 | 51.8 | 37.09 | 11.17 | 107.33 | 24.86 | 34.47 |
| S3 Tr2 | |||||||||
| Minimum | −65.8 | 61.7 | 67.1 | −63.05 | −7.74 | −10.27 | −28.81 | −36.55 | 45.84 |
| Maximum | −38.2 | 74.9 | 108.3 | −25.44 | 32.09 | 2.27 | −5.77 | 5.49 | 82.98 |
| Range | 27.6 | 13.2 | 41.2 | 37.61 | 39.82 | 12.54 | 23.04 | 42.04 | 37.14 |
| S4 Tr1 | |||||||||
| Minimum | −74.1 | 88.9 | 32.7 | −47.99 | −8.49 | −8.76 | −30.43 | −40.49 | −84.04† |
| Maximum | −60.7 | 100.6 | 73.4 | 18.47 | 77.54 | 0.36 | 47.7 | 2.83 | 86.42 |
| Range | 13.4 | 11.7 | 40.7 | 66.46 | 86.04 | 9.13 | 78.13 | 43.32 | 170.47 |
| S4 Tr2 | |||||||||
| Minimum | −74.1 | 90.5 | 31 | −44.22 | −6.87 | −8.24 | −23.24 | −26.59 | 35.99 |
| Maximum | −61.3 | 101.8 | 64.5 | 14.22 | 75.74 | 0.36 | −4.32 | −7.68 | 81.19 |
| Range | 12.8 | 11.3 | 33.5 | 58.45 | 82.61 | 8.6 | 18.93 | 18.91 | 45.2 |
| Arth Tr1 | |||||||||
| Minimum | −71.5 | 82.3 | 52.7 | −51.45 | −39.22 | −3 | −40.59 | −12.27 | 18.87 |
| Maximum | −51.4 | 109.2 | 97.5 | −19.1 | 63.93 | 11.03 | −20.03 | 8.95 | 79.24 |
| Range | 20.1 | 26.9 | 44.8 | 32.35 | 103.15 | 14.03 | 20.57 | 21.22 | 60.37 |
| Arth Tr2 | |||||||||
| Minimum | −69.7 | 83.5 | 52.2 | −51.86 | −41.44 | −0.83 | −38.94 | −15.31 | 36.47 |
| Maximum | −57.2 | 107.4 | 90.3 | −17.3 | 74.54 | 10.31 | −21.65 | 9.17 | 82.34 |
| Range | 12.5 | 23.9 | 38.1 | 34.57 | 115.98 | 11.14 | 17.29 | 24.48 | 45.86 |
* Includes the data for jar twist with strength for all subjects. Angles measured include shoulder horizontal abduction, shoulder forward elevation, elbow flexion, forearm pronation, wrist flexion, wrist deviation, thumb CMC abduction, thumb MCP flexion, and thumb IP flexion; †anomalous reading as a result of marker tracking error; FA = forearm; CMC = carpometacarpal; MCP = metacarpophalangeal; IP = interphalangeal.
The subject with arthritis demonstrated similar ROM to the control subjects when performing a task without the requirement of force. However, she required a greater amount of time to shift from the starting position to the proper jig hand positioning as a result of subjective reports of pain during this positioning. She required a mean of 11.44 seconds (SD 1.18) during grip and 10.35 seconds (SD 1.33) during jar twist to complete the ascribed activity, whereas the subjects without arthritis required only 7.6 seconds (SD 1.21) and 8.44 seconds (SD 0.79), respectively (p < 0.001 for both activities).
Once obtaining the proper position, this subject demonstrated minimal movement at the CMC joint (Fig. 4) and instead exhibited increased motion at the wrist, thumb metacarpophalangeal joint, and thumb interphalangeal joint. During jar twist, her mean ROM in wrist flexion was 111.15° (SD 0.16), wrist deviation 14.9° (SD 1.60), thumb metacarpophalangeal joint flexion 36.25° (SD 5.2), and thumb interphalangeal flexion 45.55° (SD 1.35), whereas the mean ROM in the subjects without arthritis during this activity was 75.17° (SD 14.65) for wrist flexion (p = 0.02), 8.65° (SD 1.73) for wrist deviation (p = 0.005), 18.20° (SD 5.25) for thumb metacarpophalangeal flexion (p = 0.005), and 37.04° (SD 1.79) for thumb interphalangeal flexion (p < 0.001).
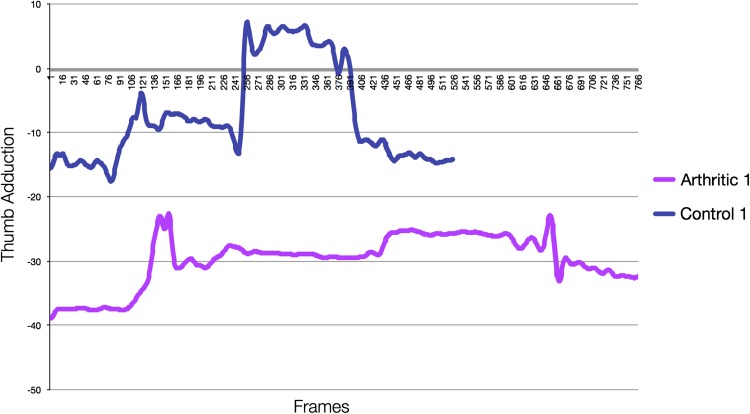
Fig. 4.
This is a comparison of the adduction/abduction motion of the thumb of an arthritic versus control subject during jar opening. Our subject without arthritis had the ability to adduct the thumb during loading. As compared with control subjects, our subject with arthritis demonstrated the need for overall increased abduction of the thumb phalanges resulting from her CMC adduction deformity. She also demonstrated minimal motion as compared with the subject without arthritis once reaching final position and required increased time to completion of the task and returning to the resting position.
Furthermore, the fixed adduction-extension deformity of the arthritic CMC joint was present throughout all motion as compared with control subjects. For example, during pinch her mean maximum and minimum CMC adduction was 37.91° (SD 0.04) and 19.62° (SD 0.61), whereas the mean maximum and minimum for subjects without arthritis was 19.10° (SD 5.56) and 8.72° (SD 3.10), respectively. She also demonstrated an extended posture at the thumb metacarpophalangeal joint throughout this activity with a mean maximal extension of 40.38° (SD 0.81) and mean minimal extension of 15.12° (SD 2.41). In contrast, all subjects without arthritis maintained a flexed position at this joint throughout this activity with a mean minimum flexion of 1.98° (SD 1.10) and mean maximum flexion of 12.37° (SD 2.97) (p < 0.001). Similar findings were present during grip and jar twist; during grip, her mean CMC adduction ranged from 19.36° to 34.80°, whereas the range for subjects without arthritis was a mean of 5.23° to 23.44°. This adduction deformity was accentuated during the application of force when her mean CMC adduction arc of motion increased to 21.11° to 45.78°, whereas subjects without arthritis maintained a relatively stable mean arc of motion from 5.69° to 25.97°.
Discussion
Abnormal biomechanical loading has been implicated as an associated risk factor of OA in the wrist and hand [8, 10]. Empirical data to date are insufficient to implicate the specific role of altered biomechanics in developing thumb CMC arthritis. We developed a protocol to perform reproducible kinematic analysis of both control and osteoarthritic patients in future biomechanical studies.
This study has several limitations. It is a pilot study to test the feasibility and capabilities of motion analysis in the upper extremity. Motion analysis has been traditionally used for phasic, relatively uniplanar gait analysis of larger joints of the lower extremity. Upper extremity functional activity, in contrast, typically represents nonphasic activity with multiplanar precision targeting. Thus, this feasibility study poses many challenges. Accordingly, the study possesses a small subject population with intertrial variability present. Because there were only four control subjects and one osteoarthritic subject, normal curves could not be created and statistics were difficult to perform. Subjects demonstrated intratrial reliability with minimal deviation throughout their trials, thus confirming the viability of this large joint/small joint analysis. However, as a result of intertrial variability between control subjects, further studies with increased numbers of subjects are required to better establish a representative curve for these functional activities in control subjects.
We demonstrated that in vivo kinematics of the thumb and hand could be quantified in subjects without arthritis. We have demonstrated that our protocol reproducibly examines motion of both the small and large joints of the upper extremity in combination. Prior studies, including our own, have demonstrated the ability to accurately perform motion analysis on the larger joints of the upper extremity with good intertrial reliability [3, 19, 23], whereas others have validated performing motion analysis on the hand alone [9, 18, 22].
In this study, we were able to appropriately address our primary aim by demonstrating that motion analysis of the entire hand and upper extremity may be performed concurrently and that we can reproducibly perform motion analysis during functional activities. Because upper extremity motion occurs in several planes of motion, is nonphasic, and unconstrained unlike motion of the lower extremity, our aim was to examine the position of the upper extremity as it relates to functional activities. With this study, we have created a protocol that reproducibly analyzes the motion of control subjects during activities of daily living including grasp, jar turn, and pinch.
We also effectively met our secondary aim of expanding our understanding of how motion of the upper extremity is altered with applied force. Prior studies have examined static ROM of the digits; however, they do not examine motion during functional activities [9, 18, 22]. Through our motion analysis, we have demonstrated that even healthy subjects tend to use flexion synergy to increase force generated similar to hemiparetic patients [6].
Subsequently, we were able to examine the kinematic alterations in a patient with OA compared with those without arthritis. Greater amounts of time were required to accomplish the same tasks as attributed to subjective pain, and her kinematic deviations from normal increased with increasing requirement of force transmission. Furthermore, as a result of the adduction-extension deformity at the CMC joint, alterations in her biomechanics throughout the rest of the thumb and upper extremity were required to appropriately perform the specified tasks [24]. She demonstrated increased radial deviation of the wrist, flexion of the elbow, and abduction of the shoulder during functional activities as compared with control subjects, especially with force transmission. Prior studies have demonstrated an asymmetric motion deficit but have not correlated these data with functional activities and examined how it affects the rest of the upper extremity [9].
Understanding the in vivo kinematics of these activities of daily living allows clinicians to understand how healthy subjects perform these activities and what movements are required to complete these tasks. Motion analysis grants the ability to objectively measure how subjects without arthritis perform these functions. Subsequently evaluating patients with functional limitations and comparing their motion with that of the control subjects allows us to understand why one subject is able to perform an activity, whereas the other is not [2]. Defining the carpometacarpal position and motion associated with functional activities known to cause symptoms and load the base of the thumb will augment current fundamental information of this complex joint [10]. Understanding compensatory motion of other joints creates an indirect measurement of advantageous force coupling. We seek to understand these kinematic alterations in patients with pathology of the hand and upper extremity to tailor our nonoperative and operative treatments while improving joint stabilization to minimize abnormal motion and joint loading.
This study has demonstrated that we can reproducibly evaluate fine motion of the thumb and gross motion of the entire upper extremity concurrently. We can use skin surface markers to understand motion of the hand and upper extremity in a three-dimensional fashion during functional activities. This protocol may also be applied to subjects with altered kinematics of the hand to evaluate the change in biomechanics throughout the entire upper extremity. Through further studies, we hope to be able to arrive at normative data, which may then allow us to better tailor our treatments for upper extremity pathologies using normal biomechanics of the entire upper extremity as a guide.
Acknowledgments
The authors wish to acknowledge Dr. Raymond Tse for his work in the development of the optimal marker placement for our functional task analysis.
Footnotes
One or more of the authors (ALL) received funding from the OREF/RJOS/DePuy Career Development Award and Ronald & Ann Williams Charitable Trust.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Each author certifies that Stanford University approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Delp Motion Laboratory, Stanford University, Stanford, CA, USA.
References
- 1.Acheson RM, Chan YK, Clemett AR. New Haven survey of joint diseases. XII. Distribution and symptoms of osteoarthrosis in the hands with reference to handedness. Ann Rheum Dis. 1970;29:275–286. doi: 10.1136/ard.29.3.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adams BD, Grosland NM, Murphy DM, McCullough M. Impact of impaired wrist motion on hand and upper-extremity performance. J Hand Surg Am. 2003;28:898–903. doi: 10.1016/S0363-5023(03)00424-6. [DOI] [PubMed] [Google Scholar]
- 3.Butler EE, Ladd Al, Louise SA, Wong W, Rose J. Three-dimensional kinematics of the upper limb during a reach and grasp cycle for children. Gait Posture. 2010;32:72–77. doi: 10.1016/j.gaitpost.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Cooney WP, III, Chao EY. Biomechanical analysis of static forces in the thumb during hand function. J Bone Joint Surg Am. 1977;59:27–36. [PubMed] [Google Scholar]
- 5.Dahaghin S, Bierma-Zeinstra SM, Ginai AZ, Pols HA, Hazes JM, Koes BW. Prevalence and pattern of radiographic hand osteoarthritis and association with pain and disability (the Rotterdam study) Ann Rheum Dis. 2005;64:682–687. doi: 10.1136/ard.2004.023564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dewald JPA, Pope PS, Given JD, Buchanan TS, Rymer WZ. Abnormal muscle coactivation patterns during isometric torque generation at the elbow and shoulder in hemiparetic subjects. Brain. 1995;118:495–510. doi: 10.1093/brain/118.2.495. [DOI] [PubMed] [Google Scholar]
- 7.Eaton RG, Glickel SZ. Trapeziometacarpal osteoarthritis. Staging as a rational for treatment. Hand Clin. 1987;3:455–471. [PubMed] [Google Scholar]
- 8.Gamble JG, Mochizuki C, Rinsky LA. Trapeziometacarpal abnormalities in Ehlers-Danlos syndrome. J Hand Surg Am. 1989;141:89–94. doi: 10.1016/0363-5023(89)90064-6. [DOI] [PubMed] [Google Scholar]
- 9.Gehrmann SV, Tang J, Li ZM, Goitz RJ, Windolf J, Kaufmann RA. Motion deficit of the thumb in CMC joint arthritis. J Hand Surg Am. 2010;35:1449–1453. doi: 10.1016/j.jhsa.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 10.Haara MM, Heliovaara M, Kroger H, Arokoski JP, Manninen P, Karkkainen A, Knekt P, Impivaara O, Aromaa A. Osteoarthritis in the carpometacarpal joint of the thumb. Prevalence and associations with disability and mortality. J Bone Joint Surg Am. 2004;86:1452–1457. doi: 10.2106/00004623-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Kuo LC, Cooney WP, 3rd, Oyama M, Kaufman KR, Su FC, An KN. Feasibility of using surface markers for assessing motion of the thumb trapeziometacarpal joint. Clin Biomech. 2003;18:558–563. doi: 10.1016/S0268-0033(03)00074-3. [DOI] [PubMed] [Google Scholar]
- 12.Miller LC, Dewald JPA. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012;123:1216–1225. doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Napier J. The evolution of the hand. Sci Am. 1962;207:56–62. doi: 10.1038/scientificamerican1262-56. [DOI] [PubMed] [Google Scholar]
- 14.Napier JR. The prehensile movements of the human hand. J Bone Joint Surg Br. 1956;38:902–913. doi: 10.1302/0301-620X.38B4.902. [DOI] [PubMed] [Google Scholar]
- 15.Musculoskeletal Disorders and the Workplace: Low Back and Upper Extremities. Washington, DC, USA: National Academy Press; 2001. [PubMed] [Google Scholar]
- 16.O’Neill OR, Morrey BF, Tanaka S, An KN. Compensatory motion in the upper extremity after elbow arthrodesis. Clin Orthop Relat Res. 1992;281:89–96. [PubMed] [Google Scholar]
- 17.Pellegrini VD., Jr The ABJS 2005 Nicolas Andry Award: osteoarthritis and injury at the base of the human thumb: survival of the fittest? Clin Orthop Relat Res. 2005;438:266–276. doi: 10.1097/01.blo.0000176968.28247.5c. [DOI] [PubMed] [Google Scholar]
- 18.Rash GS, Belliappa PP, Wachowiak MP, Somia NN, Gupta A. A demonstration of validity of 3-D video motion analysis method for measuring finger flexion and extension. J Biomech. 1999;32:1337–1341. doi: 10.1016/S0021-9290(99)00140-2. [DOI] [PubMed] [Google Scholar]
- 19.Reid S, Elliott C, Alderson J, Lloyd D, Elliott B. Repeatability of upper limb kinematics for children with and without cerebral palsy. Gait Posture. 2010;32:10–17. doi: 10.1016/j.gaitpost.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 20.Sonne-Holm S, Jacobsen S. Osteoarthritis of the first carpometacarpal joint: a study of radiology and clinical epidemiology. Results from the Copenhagen Osteoarthritis Study. Osteoarthritis Cartilage. 2006;14:496–500. doi: 10.1016/j.joca.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Steif F, Bohm H, Schwirtz A, Dussa CU, Doderlein L. Dynamic loading of the knee and hip joint and compensatory strategies in children and adolescents with varus malalignment. Gait Posture. 2001;33:490–495. doi: 10.1016/j.gaitpost.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Su FC, Kuo LC, Chiu HY, Hsu HY. The validity of using a video-based motion analysis system for measuring maximal area of fingertip motion and angular variation. Proc Inst Mech Eng H. 2002;216:257–263. doi: 10.1243/09544110260138745. [DOI] [PubMed] [Google Scholar]
- 23.Van Andel CJ, Wolterbeek N, Doorenbosch CA, Veeger DH, Harlaar J. Complete 3D kinematics of upper extremity functional tasks. Gait Posture. 2008;27:120–127. doi: 10.1016/j.gaitpost.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 24.Van Heest AE, Kallemeier P. Thumb carpal metacarpal arthritis. JAAOS. 2008;16:140–151. doi: 10.5435/00124635-200803000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Wilder FV, Barrett JP, Farina EJ. Joint-specific prevalence of osteoarthritis of the hand. Osteoarthritis Cartilage. 2006;14:953–957. doi: 10.1016/j.joca.2006.04.013. [DOI] [PubMed] [Google Scholar]