Abstract
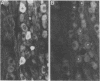
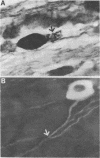
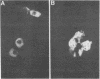
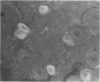
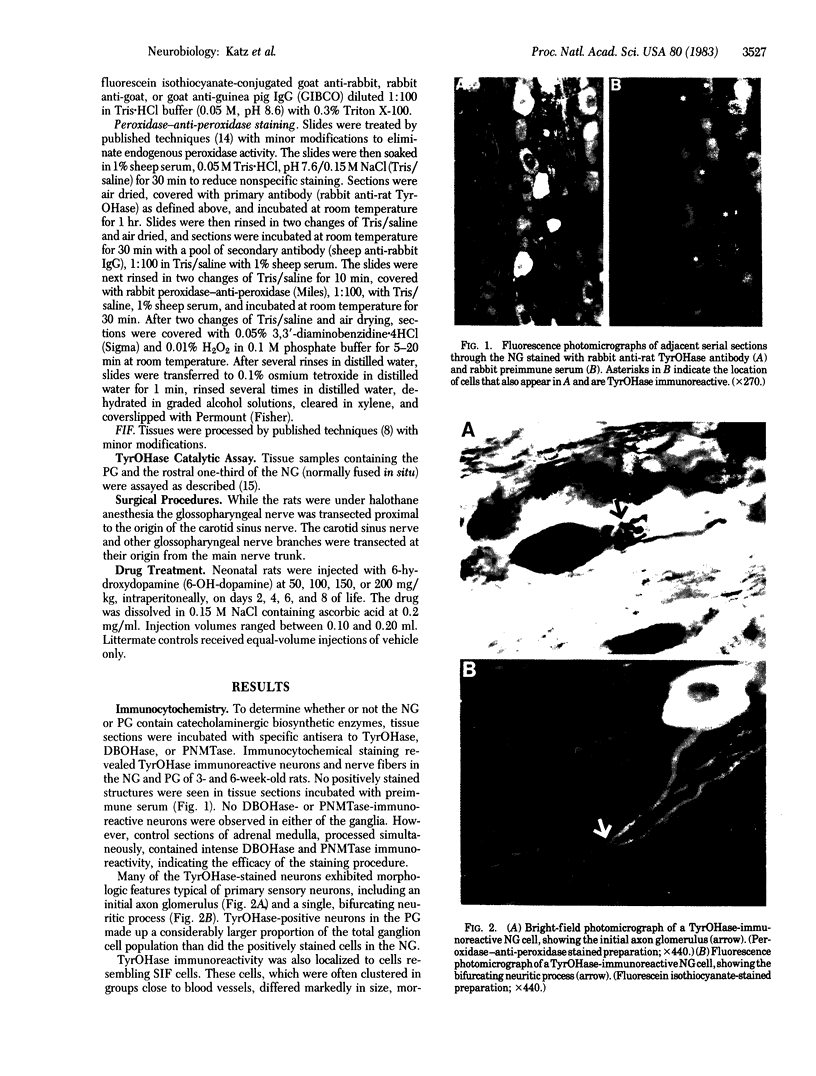
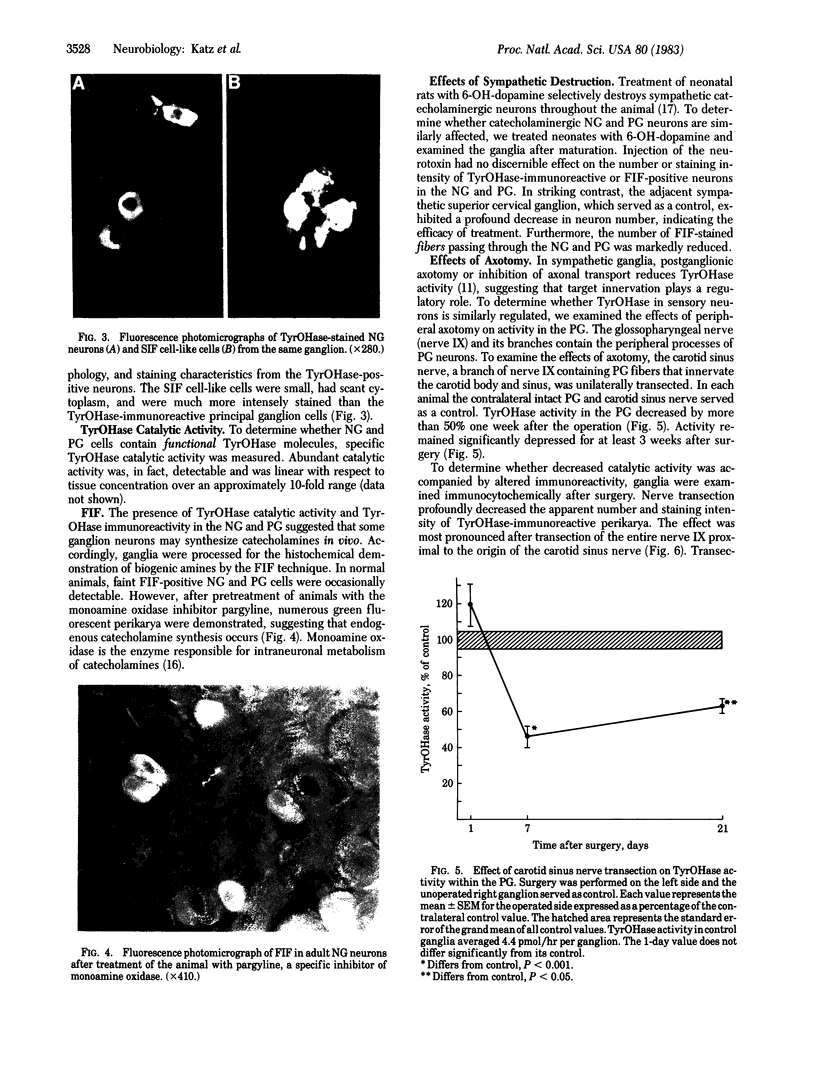
Expression of catecholaminergic characteristics by primary sensory neurons was examined in the vagal nodose and glossopharyngeal petrosal ganglia of the normal adult rat in vivo. Catecholaminergic phenotypic expression was documented by immunocytochemical localization of tyrosine hydroxylase (TyrOHase; EC 1.14.16.2), radiochemical assay of specific TyrOHase catalytic activity, and cytochemical localization of formaldehyde-induced catecholamine fluorescence (FIF) within principal ganglion cells. The TyrOHase-containing cells exhibited morphologic features typical of primary sensory neurons, such as an initial axon glomerulus and a single, bifurcating neurite process. These cells were distinguished from TyrOHase- and FIF-positive small intensely fluorescent cells by size, morphology, and staining intensity. TyrOHase-containing neurons appeared to be insensitive to neonatal treatment with 6-hydroxydopamine, thereby distinguishing them from sympathetic neurons. Nodose and petrosal ganglia of adult rats exhibited TyrOHase catalytic activity, linear with respect to tissue concentration over a 10-fold range, indicating that the immunoreactive enzyme was functional. Transection of specific ganglionic nerve roots depleted TyrOHase catalytic activity and neuronal immunoreactivity within the petrosal ganglion, suggesting that target organ innervation regulates enzyme levels within ganglion perikarya. Our study indicates that primary sensory neurons express catecholaminergic transmitter traits in the normal adult rat. Consequently, in the periphery, catecholaminergic characters are not restricted to the sympathoadrenal axis but are expressed by functionally and embryologically diverse populations of autonomic neurons.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angeletti P. U., Levi-Montalcini R. Sympathetic nerve cell destruction in newborn mammals by 6-hydroxydopamine. Proc Natl Acad Sci U S A. 1970 Jan;65(1):114–121. doi: 10.1073/pnas.65.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod A., Hartman B. K., Pujol J. F. Importance of fixation in immunohistochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981 Jul;29(7):844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Black I. B. Increased tyrosine hydroxylase activity in frontal cortex and cerebellum after reserpine. Brain Res. 1975 Sep 12;95(1):170–176. doi: 10.1016/0006-8993(75)90219-x. [DOI] [PubMed] [Google Scholar]
- Black I. B. Regulation of autonomic development. Annu Rev Neurosci. 1978;1:183–214. doi: 10.1146/annurev.ne.01.030178.001151. [DOI] [PubMed] [Google Scholar]
- Black I. B. Stages of neurotransmitter development in autonomic neurons. Science. 1982 Mar 5;215(4537):1198–1204. doi: 10.1126/science.215.4537.1198. [DOI] [PubMed] [Google Scholar]
- Bohn M. C., Goldstein M., Black I. B. Role of glucocorticoids in expression of the adrenergic phenotype in rat embryonic adrenal gland. Dev Biol. 1981 Feb;82(1):1–10. doi: 10.1016/0012-1606(81)90423-1. [DOI] [PubMed] [Google Scholar]
- Bunge R., Johnson M., Ross C. D. Nature and nurture in development of the autonomic neuron. Science. 1978 Mar 31;199(4336):1409–1416. doi: 10.1126/science.24273. [DOI] [PubMed] [Google Scholar]
- Cochard P., Goldstein M., Black I. B. Ontogenetic appearance and disappearance of tyrosine hydroxylase and catecholamines in the rat embryo. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2986–2990. doi: 10.1073/pnas.75.6.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., Gapp D. A., Kozak L. P. Immunohistochemical localization of sn-glycerol-3-phosphate dehydrogenase in Bergmann glia and oligodendroglia in the mouse cerebellum. Brain Res. 1981 Jun;227(3):341–354. doi: 10.1016/0165-3806(81)90072-9. [DOI] [PubMed] [Google Scholar]
- Grillo M. A., Jacobs L., Comroe J. H., Jr A combined fluorescence histochemical and electron microscopic method for studying special monoamine-containing cells (SIF cells). J Comp Neurol. 1974 Jan 1;153(1):1–14. doi: 10.1002/cne.901530102. [DOI] [PubMed] [Google Scholar]
- Jonakait G. M., Wolf J., Cochard P., Goldstein M., Black I. B. Selective loss of noradrenergic phenotypic characters in neuroblasts of the rat embryo. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4683–4686. doi: 10.1073/pnas.76.9.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson G., Sachs C. Effects of 6-hydroxydopamine on the uptake and storage of noradrenaline in sympathetic adrenergic neurons. Eur J Pharmacol. 1970 Feb;9(2):141–155. doi: 10.1016/0014-2999(70)90293-1. [DOI] [PubMed] [Google Scholar]
- KOPIN I. J. STORAGE AND METABOLISM OF CATECHOLAMINES: THE ROLE OF MONOAMINE OXIDASE. Pharmacol Rev. 1964 Jun;16:179–191. [PubMed] [Google Scholar]
- Katz D. M., Karten H. J. Substance P in the vagal sensory ganglia: localization in cell bodies and pericellular arborizations. J Comp Neurol. 1980 Sep 15;193(2):549–564. doi: 10.1002/cne.901930216. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Bohn M. C., Black I. B. Substance P in principal sympathetic neurons: regulation by impulse activity. Science. 1981 Oct 16;214(4518):335–336. doi: 10.1126/science.6169153. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Black I. B. Regulation of substance P in adult rat sympathetic ganglia. Brain Res. 1982 Feb 18;234(1):182–187. doi: 10.1016/0006-8993(82)90485-1. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Black I. B. The role of axonal transport in the regulation of enzyme activity in sympathetic ganglia of adult rats. Brain Res. 1979 Aug 10;171(3):415–424. doi: 10.1016/0006-8993(79)91046-1. [DOI] [PubMed] [Google Scholar]
- Kojima H., Suetake K., Yokoo H., Anraku S., Inanaga K., Higashi H., Nishi S., Yamamoto T., Ochi J. Dopamine-containing cells in rabbit nodose ganglia. Experientia. 1981 Dec 15;37(12):1332–1333. doi: 10.1007/BF01948393. [DOI] [PubMed] [Google Scholar]
- LEVITT M., SPECTOR S., SJOERDSMA A., UDENFRIEND S. ELUCIDATION OF THE RATE-LIMITING STEP IN NOREPINEPHRINE BIOSYNTHESIS IN THE PERFUSED GUINEA-PIG HEART. J Pharmacol Exp Ther. 1965 Apr;148:1–8. [PubMed] [Google Scholar]
- Le Douarin N. M., Smith J., Le Lièvre C. S. From the neural crest to the ganglia of the peripheral nervous system. Annu Rev Physiol. 1981;43:653–671. doi: 10.1146/annurev.ph.43.030181.003253. [DOI] [PubMed] [Google Scholar]
- Markey K. A., Kondo H., Shenkman L., Goldstein M. Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol. 1980 Jan;17(1):79–85. [PubMed] [Google Scholar]
- Otsuka M., Konishi S. Substance P and excitatory transmitter of primary sensory neurons. Cold Spring Harb Symp Quant Biol. 1976;40:135–143. doi: 10.1101/sqb.1976.040.01.015. [DOI] [PubMed] [Google Scholar]
- Patterson P. H. Environmental determination of autonomic neurotransmitter functions. Annu Rev Neurosci. 1978;1:1–17. doi: 10.1146/annurev.ne.01.030178.000245. [DOI] [PubMed] [Google Scholar]
- Purves D., Hume R. I. The relation of postsynaptic geometry to the number of presynaptic axons that innervate autonomic ganglion cells. J Neurosci. 1981 May;1(5):441–452. doi: 10.1523/JNEUROSCI.01-05-00441.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stensaas L. J., Fidone S. J. An ultrastructural study of cat petrosal ganglia: a search for autonomic ganglion cells. Brain Res. 1977 Mar 18;124(1):29–39. doi: 10.1016/0006-8993(77)90861-7. [DOI] [PubMed] [Google Scholar]
- Teitelman G., Baker H., Joh T. H., Reis D. J. Appearance of catecholamine-synthesizing enzymes during development of rat sympathetic nervous system: possible role of tissue environment. Proc Natl Acad Sci U S A. 1979 Jan;76(1):509–513. doi: 10.1073/pnas.76.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H., Tranzer J. P. Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-Hydroxydopamine. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;261(3):271–288. doi: 10.1007/BF00536990. [DOI] [PubMed] [Google Scholar]