Abstract
A brain polypeptide termed diazepam-binding inhibitor (DBI) and thought to be chemically and functionally related to the endogenous effector of the benzodiazepine recognition site was purified to homogeneity. This peptide gives a single band of protein on NaDodSO4 and acidic urea gel electrophoresis. A single UV-absorbing peak was obtained by HPLC using three different columns and solvent systems. DBI has a molecular mass of approximately equal to 11,000 daltons. Carboxyl-terminus analysis shows that tyrosine is the only residue while the amino-terminus was blocked. Cyanogen bromide treatment of DBI yields three polypeptide fragments, and the sequences of two of them have been determined for a total of 45 amino acids. DBI is a competitive inhibitor for the binding of [3H]diazepam, [3H]flunitrazepam, beta-[3H]carboline propyl esters, and 3H-labeled Ro 15-1788. The Ki for [3H]-diazepam and beta-[3H]carboline binding were 4 and 1 microM, respectively. Doses of DBI that inhibited [3H]diazepam binding by greater than 50% fail to change [3H]etorphine, gamma-amino[3H]butyric acid, [3H]-quinuclidinyl benzilate, [3H]dihydroalprenolol, [3H]adenosine, and [3H]imipramine binding tested at their respective Kd values. DBI injected intraventricularly at doses of 5-10 nmol completely reversed the anticonflict action of diazepam on unpunished drinking and, similar to the anxiety-inducing beta-carboline derivative FG 7142 (beta-carboline-3-carboxylic acid methyl ester), facilitated the shock-induced suppression of drinking by lowering the threshold for this response.
Full text
PDF

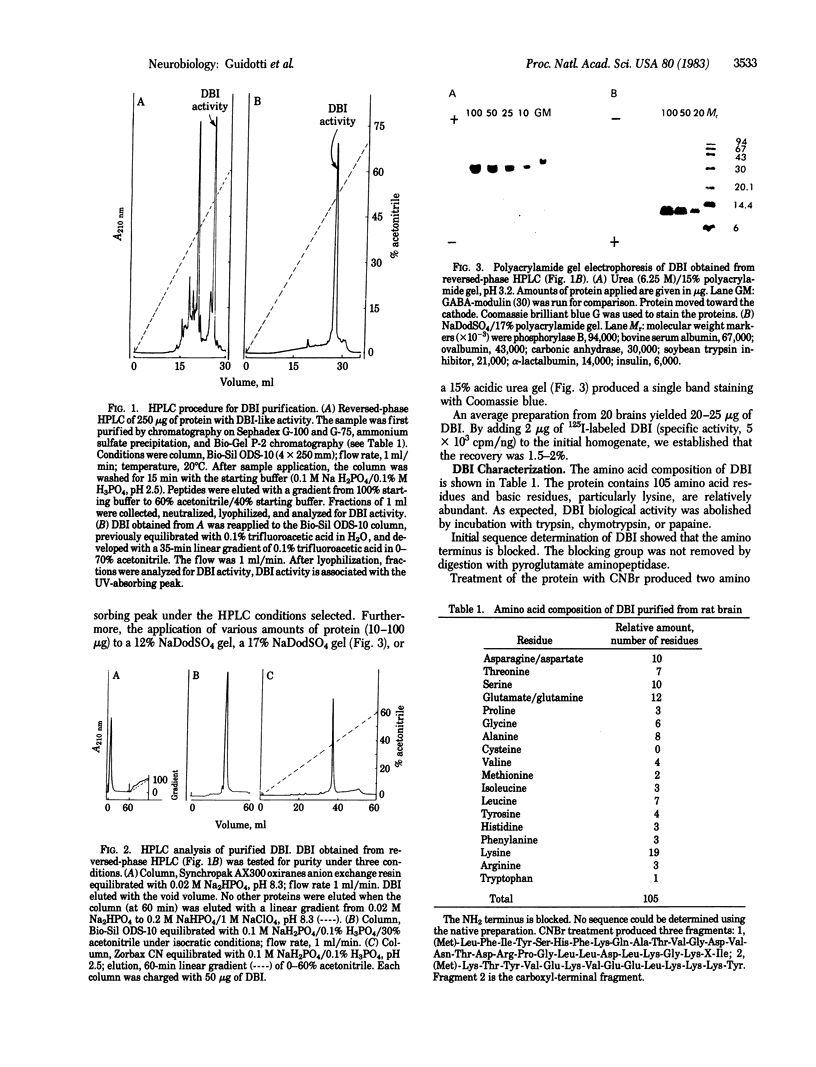
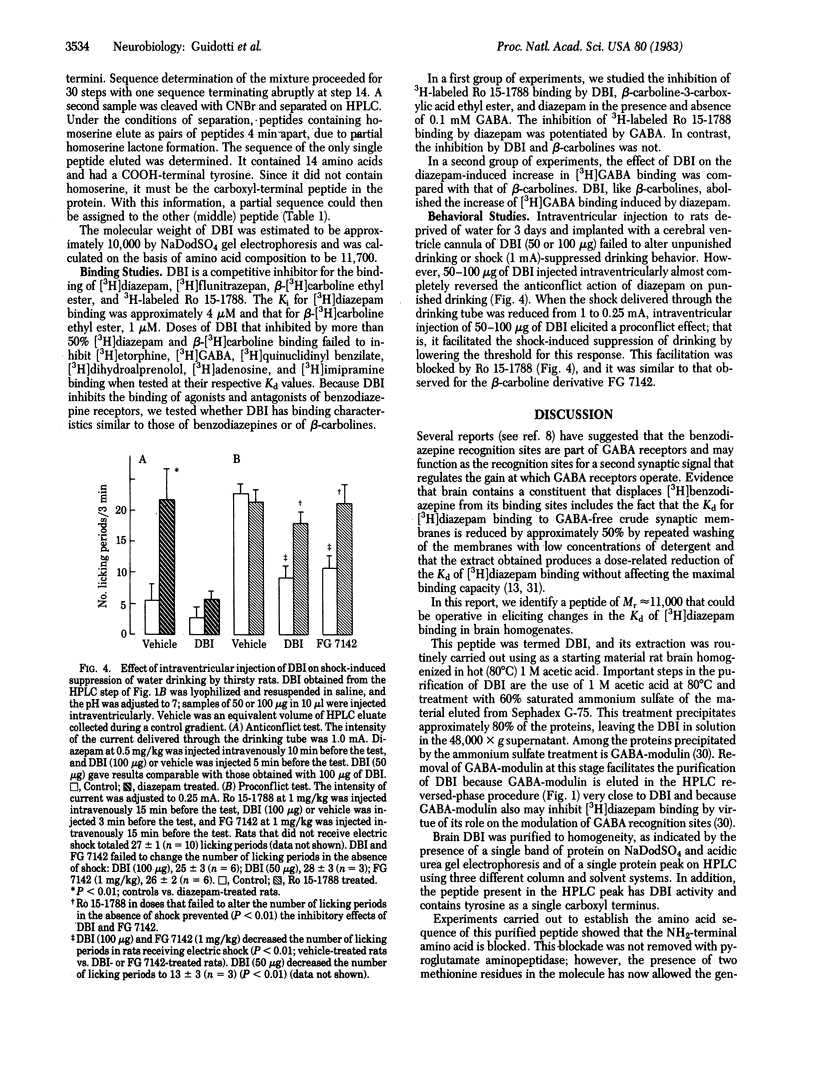

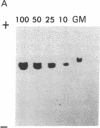
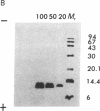
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano T., Spector S. Identification of inosine and hypoxanthine as endogenous ligands for the brain benzodiazepine-binding sites. Proc Natl Acad Sci U S A. 1979 Feb;76(2):977–981. doi: 10.1073/pnas.76.2.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett C. D., Rodkey J. A., Sondey J. M., Hirschmann R. Dihydrofolate reductase: the amino acid sequence of the enzyme from a methotrexate-resistant mutant of Escherichia coli. Biochemistry. 1978 Apr 4;17(7):1328–1337. doi: 10.1021/bi00600a030. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Schmiechen R., Neef G., Nielsen M., Petersen E. N. Interaction of convulsive ligands with benzodiazepine receptors. Science. 1982 Jun 11;216(4551):1241–1243. doi: 10.1126/science.6281892. [DOI] [PubMed] [Google Scholar]
- Braestrup C., Squires R. F. Specific benzodiazepine receptors in rat brain characterized by high-affinity (3H)diazepam binding. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3805–3809. doi: 10.1073/pnas.74.9.3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castel A., Kraal B., De Graaf J. M., Bosch L. The primary structure of the coat protein of alfalfa mosaic virus strain VRU. A hypothesis on the occurrence of two conformations in the assembly of the protein shell. Eur J Biochem. 1979 Dec;102(1):125–138. doi: 10.1111/j.1432-1033.1979.tb06272.x. [DOI] [PubMed] [Google Scholar]
- Chuang D. M., Costa E. Evidence for internalization of the recognition site of beta-adrenergic receptors during receptor subsensitivity induced by (-)-isoproterenol. Proc Natl Acad Sci U S A. 1979 Jun;76(6):3024–3028. doi: 10.1073/pnas.76.6.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colello G. D., Hockenbery D. M., Bosmann H. B., Fuchs S., Folkers K. Competitive inhibition of benzodiazepine binding by fractions from porcine brain. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6319–6323. doi: 10.1073/pnas.75.12.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corda M. G., Blaker W. D., Mendelson W. B., Guidotti A., Costa E. beta-Carbolines enhance shock-induced suppression of drinking in rats. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2072–2076. doi: 10.1073/pnas.80.7.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa E., Guidotti A. Molecular mechanisms in the receptor action of benzodiazepines. Annu Rev Pharmacol Toxicol. 1979;19:531–545. doi: 10.1146/annurev.pa.19.040179.002531. [DOI] [PubMed] [Google Scholar]
- Davis L. G., Cohen R. K. Identification of an endogenous peptide-ligand for the benzodiazepine receptor. Biochem Biophys Res Commun. 1980 Jan 15;92(1):141–148. doi: 10.1016/0006-291x(80)91531-4. [DOI] [PubMed] [Google Scholar]
- Guidotti A., Konkel D. R., Ebstein B., Corda M. G., Wise B. C., Krutzsch H., Meek J. L., Costa E. Isolation, characterization, and purification to homogeneity of a rat brain protein (GABA-modulin). Proc Natl Acad Sci U S A. 1982 Oct;79(19):6084–6088. doi: 10.1073/pnas.79.19.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkeler W., Möhler H., Pieri L., Polc P., Bonetti E. P., Cumin R., Schaffner R., Haefely W. Selective antagonists of benzodiazepines. Nature. 1981 Apr 9;290(5806):514–516. doi: 10.1038/290514a0. [DOI] [PubMed] [Google Scholar]
- Karobath M., Sperk G., Schönbeck G. Evidence for an endogenous factor interfering with 3H-diazepam binding to rat brain membranes. Eur J Pharmacol. 1978 Jun 1;49(3):323–326. doi: 10.1016/0014-2999(78)90111-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Massotti M., Guidotti A., Costa E. Characterization of benzodiazepine and gamma-aminobutyric recognition sites and their endogenous modulators. J Neurosci. 1981 Apr;1(4):409–418. doi: 10.1523/JNEUROSCI.01-04-00409.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massotti M., Guidotti A. Endogenous regulators of benzodiazepine recognition sites. Life Sci. 1980 Sep 8;27(10):847–854. doi: 10.1016/0024-3205(80)90079-x. [DOI] [PubMed] [Google Scholar]
- Möhler H., Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977 Nov 25;198(4319):849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- Möhler H., Okada T. The benzodiazepine receptor in normal and pathological human brain. Br J Psychiatry. 1978 Sep;133:261–268. doi: 10.1192/bjp.133.3.261. [DOI] [PubMed] [Google Scholar]
- Möhler H., Polc P., Cumin R., Pieri L., Kettler R. Nicotinamide is a brain constituent with benzodiazepine-like actions. Nature. 1979 Apr 5;278(5704):563–565. doi: 10.1038/278563a0. [DOI] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Raisman R., Briley M., Langer S. Z. Specific tricyclic antidepressant binding sites in rat brain. Nature. 1979 Sep 13;281(5727):148–150. doi: 10.1038/281148a0. [DOI] [PubMed] [Google Scholar]
- Simon E. J., Hiller J. M., Edelman I. Stereospecific binding of the potent narcotic analgesic (3H) Etorphine to rat-brain homogenate. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1947–1949. doi: 10.1073/pnas.70.7.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick P., Marangos P. J., Goodwin F. K., Edwards M., Paul S. Identification of inosine and hypoxanthine as endogenous inhibitors of [3H] diazepam binding in the central nervous system. Life Sci. 1978 Oct 9;23(14):1473–1480. doi: 10.1016/0024-3205(78)90128-5. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Katims J. J., Annau Z., Bruns R. F., Daly J. W. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981 May;78(5):3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess J., Rivier J. E., Rodkey J. A., Bennett C. D., Vale W. Isolation and characterization of somatostatin from pigeon pancreas. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2974–2978. doi: 10.1073/pnas.76.6.2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf J. H., Nixon J. C. Endogenous effector of the benzodiazepine binding site: purification and characterization. Biochemistry. 1981 Jul 21;20(15):4263–4269. doi: 10.1021/bi00518a005. [DOI] [PubMed] [Google Scholar]
- Yamamura H. I., Snyder S. H. Muscarinic cholinergic binding in rat brain. Proc Natl Acad Sci U S A. 1974 May;71(5):1725–1729. doi: 10.1073/pnas.71.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]