Abstract
Purpose
Bone marrow stromal cells (BMSCs) have been proposed to complement the declining population of nucleus pulposus cells (NPCs) found in a degenerative intervertebral disc. Although able to stop degeneration, they could not produce enough matrix to restore a healthy state. Looking at development, when a large amount of matrix is produced, the disc also contains notochordal cells (NCs), potential progenitors or regulators of NPCs. The aim of the study was, therefore, to combine NCs to a BMSC/NPC mix and evaluate their effects on cell phenotype and matrix production, in long-term culture.
Methods
In a 3D hydrogel, NCs were co-cultured in different ratios with BMSCs and/or NPCs. Matrix production, cell morphology, and gene expression of disc markers were assessed after 4 weeks of culture.
Results
At day 28, BMSCs/NPCs highly expressed disc matrix markers (type II collagen and aggrecan) and produced disc matrix up to 30 % of values obtained for the positive control (BMSCs under TGFβ stimulation). The addition of NCs only slightly up-regulated marker expression (6–12× increase); an up-regulation not reflected at the matrix level. During the 4 weeks of culture, however, the NC phenotype changed drastically (morphology, disc marker expression).
Conclusion
In contrast to previously reported short-term studies, long-term co-cultures with NCs had no substantial effects on BMSCs and NPCs, most likely due to the loss of the NC native phenotype during culture. It, therefore, appears critical to maintain this specific phenotype for a long-term effect of the NCs.
Keywords: Notochordal cells, Bone marrow stromal cells, Nucleus pulposus cells, Intervertebral disc degeneration, Cell therapy
Introduction
Intervertebral disc degeneration is one of the main factors in low back pain, a common disorder with tremendous socioeconomic impact. Although mostly successful to alleviate pain, actual treatments are only symptom relieving and new therapies are needed to treat the underlying pathophysiology of disc degeneration.
The first observed degenerative signs occur in the nucleus pulposus and are characterized by an altered anabolic–catabolic balance due, in part, to a declining nucleus pulposus cell (NPC) number and/or a change of their phenotype. It has been, therefore, proposed to introduce exogenous cells to complement the native cell population and to help tissue maintenance.
Bone marrow stromal cells (BMSCs) are promising candidates. Treated with specific growth factors [1] or co-cultured with disc cells [2–4], they express several disc-related markers (aggrecan, types II and VI collagens, sox9, cytokeratin 19) [1–4] and produce proteoglycan-rich matrix at levels consistent with a NPC-like phenotype [1, 4]. Moreover, used in vivo, BMSCs have been able to stop the degenerative process induced by nucleotomy in rabbit [5], dog [6], and pig [7] models. In all these studies, however, BMSCs were not able to produce enough matrix to reverse the degenerative process and restore the disc to a healthy state [5–7]. Moreover, leakage of BMSCs from implantation site to adjacent vertebrae may give rise to osteophytes, as reported in a rabbit model [8].
Looking back at disc physiology, a large amount of matrix is produced during development, when the nucleus pulposus does not only contain NPCs, but also notochordal cells (NCs) that are remnants of the embryonic notochord entrapped within the forming annulus fibrosus and vertebral bodies. So far, the potential of these cells for disc regeneration has not been extensively explored, although they might be important for its homeostasis. Their premature disappearance in dog breeds having an early onset of disc degeneration [9] indicates that NC loss may be consistent with degeneration. Their exact role in the disc is still unclear, as in vitro studies demonstrated a stimulatory function on NPC matrix production and phenotype [10, 11] as well as a potential role as progenitor cells able to produce disc matrix and acquire a NPC-like phenotype [12, 13]. Moreover, NCs have also been shown to effectively promote type II collagen and sox9 expression as well as proteoglycan-rich matrix production in BMSCs [14].
We hypothesized that by re-introducing the NCs that may be involved, either as regulator or as progenitor cells, in large matrix production during development, we should further promote the regenerative potential of BMSCs and NPCs. The aim of this study was, therefore, to co-culture NCs with NPCs and BMSCs, representing a therapeutic scenario where BMSCs are used to complement the native NPC population, and evaluate their effects on matrix production and discogenic phenotype.
Materials and methods
Cell sources
All slaughterhouse materials were obtained in accordance with local regulations. Bovine BMSCs were obtained from bone marrow aspirates of calves (4–12 months-old; N = 4). BMSCs were isolated by adhesion [15] and expanded up to P1 in growth medium (high glucose Dulbecco’s modified Eagle’s medium (hgDMEM); Gibco Invitrogen, Carlsbad, CA, USA) supplemented with 10 % fetal bovine serum (FBS; Gibco Invitrogen; batch selected for BMSC growth and differentiation) and 1 % penicillin/streptomycin (P/S; Lonza, Basel, Switzerland) before freezing. A fresh batch of BMSCs was thawed and cultured up to P3 for each experiment.
Bovine NPCs were obtained from caudal intervertebral discs harvested from young cows (22–26 months-old; N = 12). Discs were dissected and the nucleus pulposus harvested under sterile conditions and digested following a sequential pronase (Calbiochem, Darmstadt, Germany)/collagenase type 2 (Worthington, Lakewood, NJ, USA) digestion [11]. Using a cell strainer, cells smaller than 100 μm were kept after digestion.
Porcine NCs were isolated from lumbar and thoracic intervertebral discs harvested from young pigs (<10 weeks-old; N = 8). Discs were dissected and the nucleus pulposus harvested under sterile conditions and digested following a sequential TrypLE™ Express/collagenase P digestion: 90 min in TrypLE™ Express (Gibco Invitrogen) at 37 °C in hgDMEM + 1 % P/S; 16 h in 0.05 % collagenase P (Roche, Basel, Switzerland) at 37 °C in hgDMEM + 1 % P/S + 5 % FBS. Using a cell strainer, cells smaller than 100 μm were kept after digestion.
Porcine skin fibroblasts (SFs), used as control for the specific effects of the NCs, were extracted from the ears of pigs (6–8 months-old; N = 8). Sterile skin biopsies were digested following a sequential trypsin (Fluka, Zwijndrecht, The Netherlands)/collagenase (Sigma, Zwijndrecht, the Netherlands) digestion [16]. Obtained cells were rinsed, seeded into flasks and expanded up to P1 in growth medium before freezing. Fresh batches were thawed and cultured up to P3 for each experiment.
Cell seeding and culture
To obtain enough cells for the groups described in Table 1, NPCs from three donors and NCs and SFs from two donors were pooled per repeat (N = 4). Prior to seeding, NCs and SFs were prestained with 10 μM Vybrant™ CFDA-SE (Molecular Probes, Carlsbad, CA, USA) and NPCs with 10 μM Cell Tracker™ CMTMR Orange (Molecular Probes) according to manufacturer’s instructions. BMSCs remained unstained. Cell suspensions were then mixed to produce the groups described in Table 1. A ratio of 1:1 was selected for the BMSC/NPC mixture (Mix) [3, 17]. NPCs alone were also cultured with the highest ratio of NCs to control for the benefits of adding BMSCs. Produced mixes were centrifuged and re-suspended into 1.2 % sodium alginate (Sigma) to the final concentrations indicated in Table 1. Beads were produced as previously described [18] and cultured for 4 weeks in growth medium, as described for BMSC expansion, under 2 % O2, 5 % CO2, and 93 % N2. To validate their differentiation potential, BMSCs alone were cultured under chondrogenic conditions [hgDMEM + 1 % penicillin/streptomycin + 0.1 μM dexamethasone (Sigma) + 1 % ITS + (BD Bioscience, Breda, The Netherlands) + 1.25 mg/ml bovine serum albumin (Roche) + 50 μg/ml ascorbic acid 2-phosphate (Sigma) + 40 μg/ml l-proline (Sigma) + 100 μg/ml sodium pyruvate (Gibco Invitrogen) + 10 ng/ml TGFβ3 (Peprotech, Rocky Hill, NJ, USA)].
Table 1.
Experimental groups
| Groups | BMSCs | NPCs | NCs | SFs |
|---|---|---|---|---|
| 1.5NC | – | – | 1.5 × 106 (100 %) | – |
| 1.5SF | – | – | – | 1.5 × 106 (100 %) |
| NPC +1.5NC | – | 3 × 106 (66.6 %) | 1.5 × 106 (33.3 %) | – |
| NPC +1.5SF | – | 3 × 106 (66.6 %) | – | 1.5 × 106 (33.3 %) |
| Mix | 1.5 × 106 (50 %) | 1.5 × 106 (50 %) | – | – |
| Mix +0.75NC | 1.5 × 106 (40 %) | 1.5 × 106 (40 %) | 0.75 × 106 (20 %) | – |
| Mix +0.75SF | 1.5 × 106 (40 %) | 1.5 × 106 (40 %) | – | 0.75 × 106 (20 %) |
| Mix +1.5NC | 1.5 × 106 (33.3 %) | 1.5 × 106 (33.3 %) | 1.5 × 106 (33.3 %) | – |
| Mix +1.5SF | 1.5 × 106 (33.3 %) | 1.5 × 106 (33.3 %) | – | 1.5 × 106 (33.3 %) |
Bovine bone marrow stromal cells (BMSCs), bovine nucleus pulposus cells (NPCs), and porcine notochordal cells (NCs) were mixed at different ratios in alginate beads: values are cell concentrations (cells/ml of alginate). Ratios relative to total cell number are given within bracket. For all combinations porcine skin fibroblasts (SFs) were used as negative controls for NCs
Cell viability
At day 1 and 28, 3 beads/repeat/group were washed and incubated in 10 μM calcein blue (Sigma)/10 μM propidium iodide (Invitrogen) solution for 1 h at 37 °C. Cells were then imaged in the center of the beads at a depth of 200 μm using a confocal microscope (CLSM 510 Meta; Zeiss, Sliedrecht, the Netherlands).
FACS
At day 1, alginate beads were dissociated [18] and the recovered cells were centrifuged, rinsed, and fixed with 3.7 % buffered formalin (Sigma) for 10 min at room temperature. Fixed cells were analyzed by fluorescence activated cell sorting (FACS) for yellow and green fluorescence to evaluate proportions of green-, orange-, and un-labeled cells. The instrument used for analysis was Guava® easyCyte™ (Millipore, Darmstadt, Germany).
Cartilaginous matrix formation and cell proliferation
At day 1 and 28, 5 beads/repeat/group were pooled and digested in papain solution at 60 °C for 16 h [150 mM NaCl (Merck, Darmstadt, Germany), 789 μg/ml l-cystein (Sigma), 5 mM Na2EDTA·2H2O (Sigma), 55 mM Na3citrate·2H2O (Sigma), 125 μg/mL papain (Sigma)]. Digested samples were then used to determine their content of: sulfated glycosaminoglycans (sGAG), as a measure of proteoglycans, hydroxyproline (HYP), as a measure for collagen content, and DNA. sGAG content was determined with the dimethyl methylene blue assay, adapted for alginate presence [19] with alginate concentration identical for references and experimental samples. HYP content was measured using the chloramine-T assay [20]. DNA content was measured using the Hoechst dye method [21].
Gene expression
At day 28, 9–12 beads/repeat/group were snap-frozen and stored at −80 °C until RNA isolation. Frozen beads were disrupted with a microdismembrator (Sartorius) [22] and RNA was extracted using TRIzol® (Invitrogen) and purified using RNeasy® mini-kit (Qiagen, Venlo, The Netherlands). Using the same method, RNA was extracted from native porcine nucleus pulposus tissue (N = 6) to evaluate the gene expression profile of in situ NCs. Quantity and purity of isolated RNA were measured by spectrophotometry (ND-1000; Isogen) and integrity by gel electrophoresis. The absence of genomic DNA was validated using primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). RNA was then reverse transcribed (Superscript®Vilo™; Invitrogen) and gene expression levels of type II collagen (COL2A1), aggrecan (ACAN), Brachyury(T), cytokeratin-8 (KRT8)/-18 (KRT18)/-19 (KRT19) were assessed with SYBR green qPCR (iCycler; Biorad, Hercules, CA, USA) (see Table 2 for primer list). 18S was selected as the reference gene for our experimental groups at day 28 as the most stable gene throughout our experimental conditions from three tested genes (HPRT1, GAPDH, and 18S). Similarly, GAPDH was selected as the reference gene for the NC phenotype. The expression of gene of interest is reported as relative to 18S or GAPDH expression (2−ΔCt method). When gene expression was not detected, the Ct value was set to 40 to conduct the statistical analysis.
Table 2.
Primer sequences for target and reference genes used in RT–qPCR assays
| Gene | Accession genea | Sequence 5′–3′ | Product size (bp) | Source |
|---|---|---|---|---|
| GAPDH | NM_001034034* | GGCGTGAACCACGAGAAGTATAA CCCTCCACGATGCCAAAGT |
119 | [25] |
| HPRT1 | AF176419* | TGCTGAGGATTTGGAGAAGG CAACAGGTCGGCAAAGAACT |
154 | [26] |
| 18S | NA* | NA | NA | PD |
| COL2A1 | NM_001113224* | TGGCTGACCTGACCTGAC GGGCGTTTGACTCACTCC |
187 | BD |
| ACAN | NM_173981* | CCAACGAAACCTATGACGTGTACT GCACTCGTTGGCTGCCTC |
107 | [27] |
| T | NM_001192985 | AAGAACGGCAGGAGGATGT CCAGCAGGAAGGAGTACATG |
82 | PD |
| KRT8 | NM_001159615* | TGCTGGAGGGAGAGGAGAG CTGTAGTTGAAGCCTGGAGTC |
118 | PD |
| KRT18 | NM_001192095* | AAGAACGGCAGGAGGATGT CCAGCAGGAAGGAGTACATG |
82 | PD |
| KRT19 | XM_003131437 | AACCAGGAGTACCAGCATCT CTTGATGATGGTCAGGTTGTTG |
117 | PD |
GAPDH glyceraldehyde-3-phosphate dehydrogenase, HPRT1 hypoxanthine phosphoribosyltransferase, COL2A1 collagen, type II, alpha 1, ACAN aggrecan, T Brachyury, KRT8 cytokeratin 8, KRT18 cytokeratin 18, KRT19 cytokeratin 19, NA Information not provided by the manufacturer, BD Primers designed with Beacon designer software (Premier Biosoft, Palo Alto, CA, USA) and ordered from Sigma, PD primers designed by and ordered from PrimerDesign Ltd (Southampton, UK); other primers ordered from Sigma
* Primers tested and validated for both bovine and porcine gene
aThe accession number is the GenBank™ accession number
Statistical analysis
General linear regression models based on ANOVAs were used to examine the effects of co-culture on the variables. In all analyses, full factorial models were fitted to the data, and then a backward-stepwise procedure was used to remove the nonsignificant effects. For each significant effect, a Tukey’s-HSD post hoc test was conducted. p < 0.05 was considered significant. All statistical analyses were performed with IBM® SPSS® (v20.0; IBM Corp., Armonk, NY, USA).
Results
Cell viability and proliferation
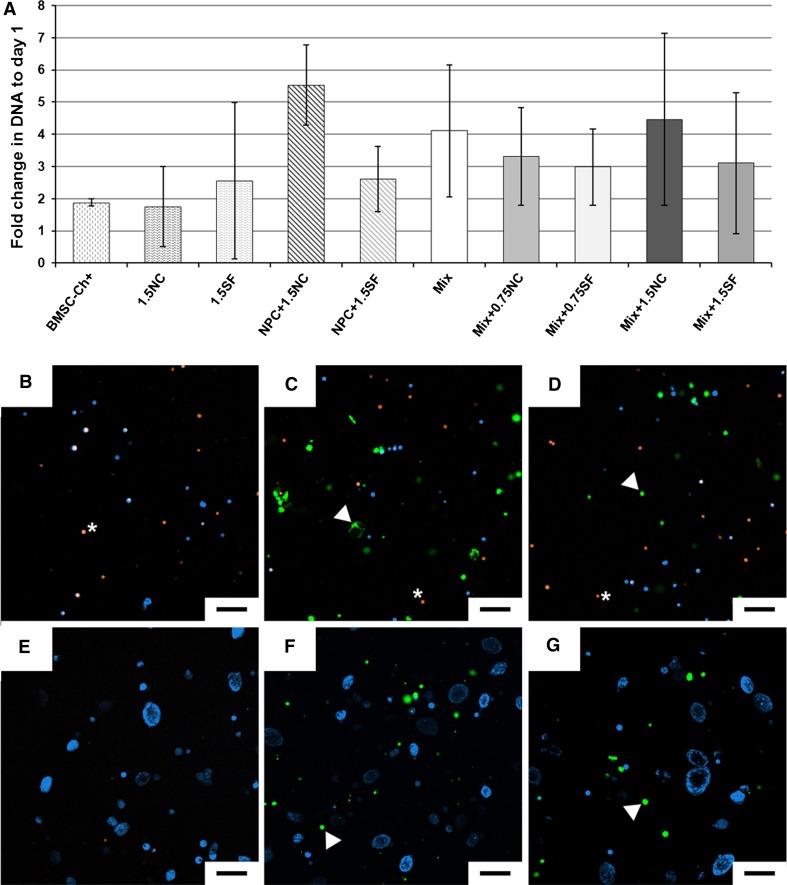
At day 1, all groups showed very good cell survival (Fig. 1b–d). Initial seeding ratios were confirmed by FACS analysis (Table 3). At day 28, cells survived in all groups and proliferated (Fig. 1a). The formation of aggregates could be observed (Fig. 1e–g) in all, but without differences between, Mix and NPC groups. Although NCs and SFs could still be distinguished from the other cell types due to their green prestaining (Fig. 1f–g), it was not possible to distinguish NPCs from BMSCs at day 28 as the orange prestaining of NPCs faded during culture, most likely due to extensive NPC proliferation.
Fig. 1.
Cell viability and proliferation assessment. a DNA fold change at day 28 relative to day 1. Values are mean ± standard deviation; N = 4 per group. b–g Cell viability and morphology at days 1 (b–d) and 28 (e–g). Cells were stained with calcein (blue fluorescence). Before seeding NPCs were prestained with orange CMTMR (orange fluorescence; star) and NCs/SFs prestained with Vybrant (green fluorescence; arrow head). b, e Mix; c, f Mix +1.5NC; d, g Mix +1.5SF. Representative of four repeats per group. Scale bar is 100 μm
Table 3.
Cell ratios at day 1
| Groups | Green (%) | Orange (%) | ||
|---|---|---|---|---|
| Theoretical | Experimental | Theoretical | Experimental | |
| BMSC-Ch+ | 0.0 | 1.4 (±1.1) | 0.0 | 0.5 (±0.3) |
| 1.5NC | 100.0 | 96.0 (±1.5) | 0.0 | 2.8 (±1.7) |
| 1.5SF | 100.0 | 97.1 (±1.6) | 0.0 | 1.0 (±0.8) |
| NPC +1.5NC | 33.3 | 27.9 (±6.0) | 66.6 | 71.5 (±7.8) |
| NPC +1.5SF | 33.3 | 29.0 (±6.5) | 66.6 | 77.4 (±10.2) |
| Mix | 0.0 | 2.9 (±1.8) | 50.0 | 63.9 (±6.3) |
| Mix +0.75NC | 20.0 | 19.8 (±3.2) | 40.0 | 60.6 (±7.7) |
| Mix +0.75SF | 20.0 | 22.3 (±4.0) | 40.0 | 57.9 (±7.4) |
| Mix +1.5NC | 33.3 | 32.5 (±7.9) | 33.3 | 55.3 (±10.5) |
| Mix +1.5SF | 33.3 | 32.0 (±8.3) | 33.3 | 49.4 (±10.0) |
Ratios of green and orange fluorescent cells as determined with FACS analysis at day 1 (experimental). Targeted values are indicated as theoretical. Values are mean (±standard deviation); N = 4
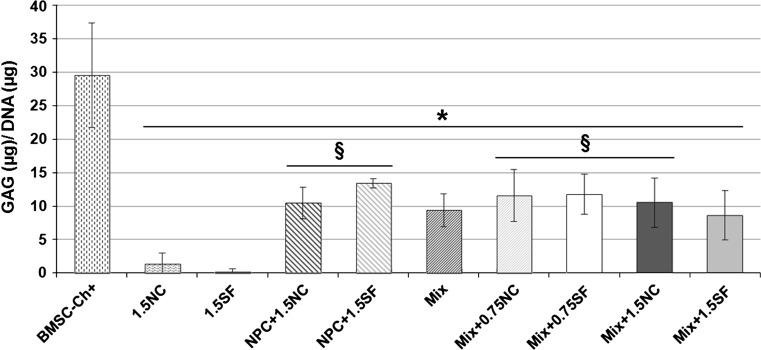
Matrix production
At day 28, sGAG were detected in all groups, but only in limited amounts for NC and SF groups (Fig. 2). Mix groups produced substantial amount of sGAG, reaching up to 30 % of values obtained for BMSC-Ch+, but adding NCs, or SFs, did not significantly promote this synthesis, regardless of NC/SF concentration (Fig. 2). Same trends were observed for the NPC groups (Fig. 2). High level of GAG production confirmed the chondrogenic potential of BMSCs under chondrogenic stimulation (BMSC-Ch+). HYP (for collagen content) was only detected in limited quantity in the BMSC-Ch+ group (data not shown).
Fig. 2.
Matrix production. At day 28, sGAG content (μg) was determined as a measure for proteoglycans and normalized to DNA content (μg). Values are mean ± standard deviation; N = 4 per group; *p < 0.05 as compared to BMSC-Ch+; § p < 0.05 as compared to 1.5SF or 1.5NC
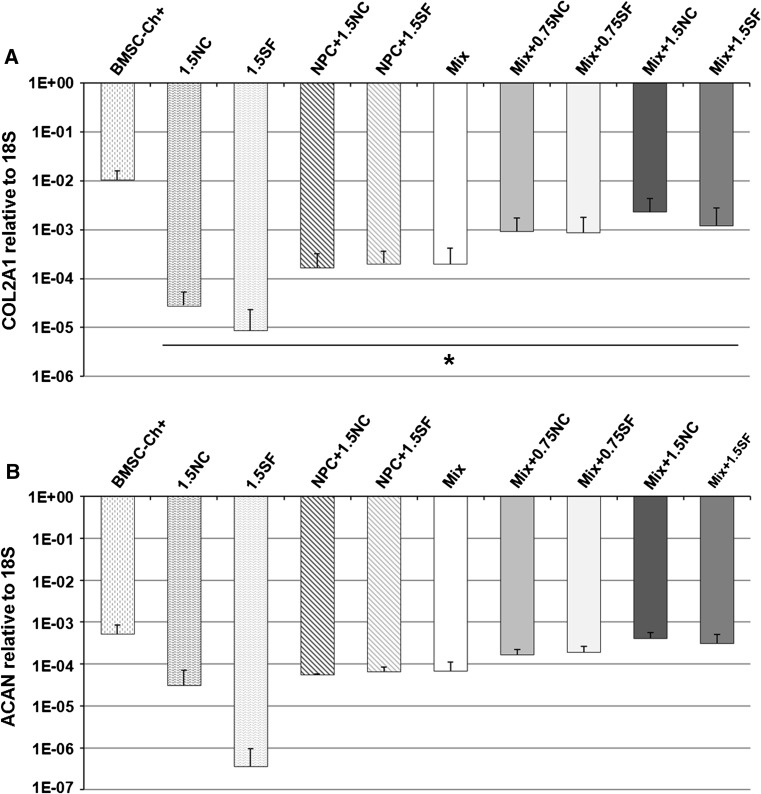
Gene expression
At day 28, NPC and Mix groups showed high expression levels for type II collagen (Fig. 3a) and aggrecan (Fig. 3b). Adding NCs to Mix increased these levels (6–12× expression levels of Mix), but no significant differences were found. Moreover, same effects were found with SFs. The highest levels of expression were observed in the BMSC-Ch+ group, supporting the chondrogenic differentiation of these cells.
Fig. 3.
Gene expression profiles. a Type II collagen (COL2A1) and b aggrecan (ACAN) gene expression relative to 18S (2−∆Ct method) was evaluated at day 28. Values are mean + standard deviation; N = 4 per group; *p < 0.05 as compared to BMSC-Ch+. Please note the logarithmic scale for both means and standard deviations
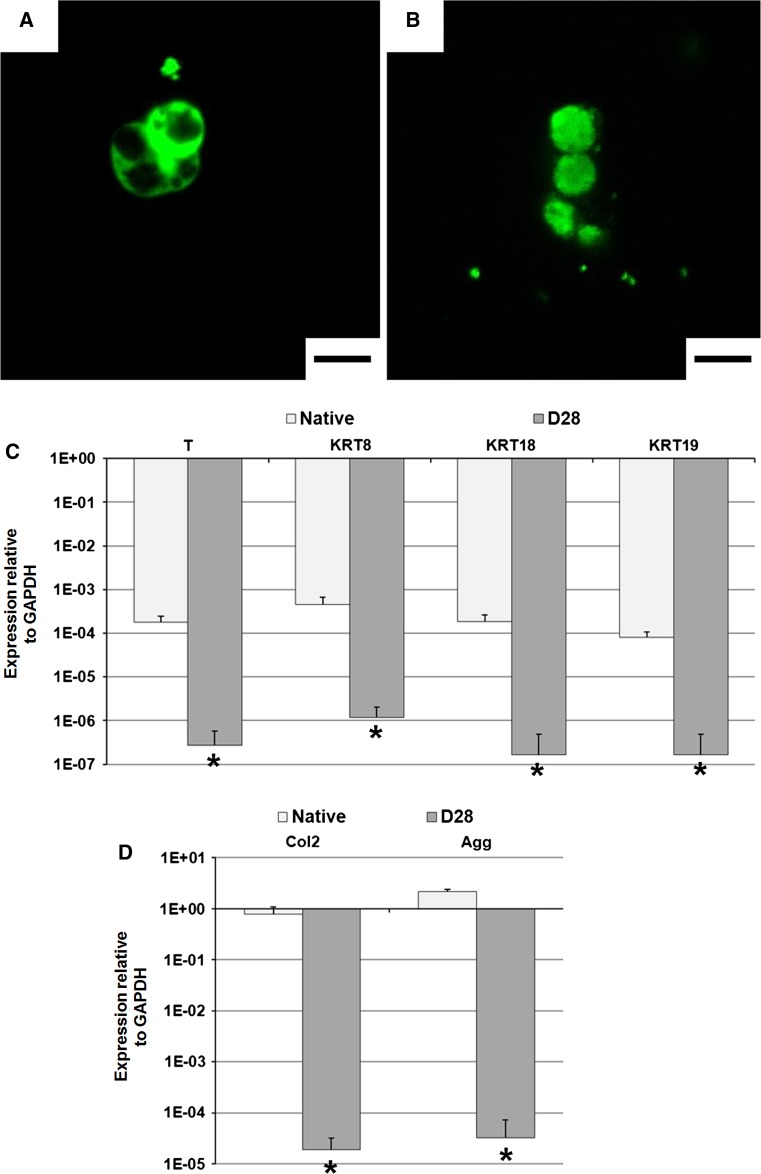
NC phenotype
At day 1, a large majority of the porcine NCs displayed their typical morphology of large, vacuolated cells (Fig. 4a). Only 10–20 % of the isolated cells were small, round cells (as previously reported [14]). At day 28, however, almost all vacuolated cells were replaced by small, round cells (Fig. 4b). The expression of putative notochordal markers (brachyury, cytokeratin-8/-18/-19) also decreased radically as compared to NCs in native tissue (Fig. 4c). Similarly, aggrecan and type II collagen expression dropped during the culture period (Fig. 4d).
Fig. 4.
NC phenotype. Cell morphology was assessed at days a 1 and b 28 using confocal imaging. NCs were prestained with Vybrant (green fluorescence) before seeding. Representative of 4 repeats per group. Scale bar is 20 μm. c Brachyury (T), cytokeratin-8/-18/-19 (KRT8/18/19) and d type II collagen (COL2A1) and aggrecan (ACAN) gene expression relative to GAPDH (2−∆Ct method) in native tissue and after 28 days of culture. Values are mean + standard deviation; N = 4 for day 28; N = 6 for native tissue. *p < 0.05 as compared to native tissue. Please note the logarithmic scale for both means and standard deviations
Discussion
In this study, we explored the ability of the NCs, either as progenitor or as regulator, to promote discogenic phenotype and proteoglycan-rich matrix production of NPCs/BMSCs. After 28 days of culture, NCs acquired a chondrocyte-like morphology (small, round cells; Fig. 4b). They also expressed type II collagen and aggrecan to levels consistent with the NPC groups (Fig. 3a–b). When compared with native values (Fig. 4d), however, these expression levels drastically decreased hinting toward NC de-differentiation rather than differentiation into NPCs. Furthermore, NCs did not produce proteoglycans (Fig. 2) or collagen (data not shown) indicating that, under the conditions used here, NCs did not differentiate into NPC-like cells, and therefore, did not act as progenitor cells. These findings are contrary to previous studies where dog [12] and rabbit [13] NCs seeded into alginate synthesized proteoglycans at a rate similar to NPCs. This discrepancy could be explained by species-specific difference, but also, and most likely, by variances in isolation protocols and/or in culture conditions.
In terms of regulatory functions, NCs also did not show an anabolic effect. A slight, but not significant, up-regulation in type II collagen and aggrecan gene expression was observed (Fig. 3a–b) when adding NCs to the Mix. This stimulation, though, was barely reflected at the matrix level (Fig. 2). Moreover, the same effects were observed when NCs were replaced with SFs (Fig. 3a–b).
This absence of effects could be explained by the cross-species model used here. Porcine NCs, however, have already been shown to stimulate bovine [10] or human [14, 23] cells, in short/mid-term cultures. Another explanation may be that the proportions of NCs added to Mix was too low. We selected a maximum of 33.3 % NCs to 66.6 % Mix to maintain the ratios of NCs to NPCs and NCs to BMSCs to 1:1. This ratio, indeed, has successfully been used to enhance matrix production and/or discogenic phenotype in NC/NPC [10, 11] and BMSC/NPC [3, 17] co-cultures. Unfortunately, at the time of the experiment, no data were available on direct NC/BMSC co-cultures. This targeted ratio of 1:1, however, was slightly shifted due to the method of isolation of dispersed NCs which led to the presence of 10–20 % more of NPCs in the NC cell suspension. The resulting mixtures may, therefore, contain more NPCs and less NCs than intended. For example for the Mix +1.5NC, beads may include up to 55 % NPCs and only 32 % NCs (for 33.3 % intended for both). This slight shift in NPC/NC proportion, however, should not substantially affect the results. Data from partial (N = 2) Mix +2.25NC group, indeed, suggest that augmenting NC proportion would not further elevate sGAG production (data not shown). The FACS analysis (Table 3) also revealed that all groups contained more NPCs, and less BMSCs, than intended. Although a ratio of 1:1 was selected for the present study, positive effects of BMSC and NPC co-culture have also been reported for a ratio of NPC:BMSC of 3:1 [2, 24].
Thus, it appears that the absence of stimulation by NCs is most likely the result of NCs changing phenotype during long-term culture rather than an insufficient ratio of NCs. During the 4 weeks of culture, indeed, NCs switched from their typical morphology (large, vacuolated cells) at day 1 (Fig. 4a) to chondrocyte-like cells (small, round cells) at day 28 (Fig. 4b). This change in morphology was accompanied by a drastic decrease in the expression of several genes used to characterize NCs (Fig. 4c). Cytokeratin-8/-18/-19 are highly expressed in fetal NC-rich nucleus pulposus, with all NCs strongly positive for these markers [25]. Their expression, then, decreases with aging, which corresponds to NC disappearance [25]. Although NPCs also express cytokeratin-8/-18/-19, as well as Brachyury, they do so in much lower expression levels than NCs [26]. Gene expression of cultured NCs was compared with fresh porcine NP tissue rich in NCs. Although the NP tissue still contains NPCs, these cells only represent 10–20 % of the cell population [14] and should, therefore, only slightly affect the overall gene expression levels. Moreover, as NPCs express Brachyury and cytokeratin -8/-18/-19 to much lower levels than NCs [24, 25], the difference observed here will be even higher if cultured NCs were compared to a pure fresh NC population, supporting the hypothesis of NC change of phenotype during culture. As vacuoles were still present after isolation and partial data on gene expression at day 1 (N = 2) showed that Brachyury and cytokeratin-8/-18/-19 gene expressions were not or only slightly affected, conditions of culture rather than cell isolation were responsible for this loss of phenotype. Although oxygen concentration was maintained at disc physiological level [27], the other characteristics of disc environment (low nutrition, acidic pH, high mechanical loading and osmolarity) were not reproduced in the in vitro system used. However, only low oxygen levels have been showed to have a beneficial influence on NC metabolic activity when low glucose and pH have a detrimental effect [28]. Similarly, mechanical loading leads to the loss of NC phenotype [29]. Although low osmolarity may still be involved, medium composition, and particularly the presence of FBS, is most likely responsible for NC dedifferentiation observed in the present study, as shown in another study where porcine NCs were cultured under different medium composition (our data, unpublished).
The hypothesis of NC dedifferentiation during long-term culture is supported by the fact that the positive effects reported for NCs are either in short-term (4 days) culture [11] or in systems using medium conditioned with NCs for 4 days [14]. One long-term (14 days) study reported a stimulative influence of NCs on NPCs [10], but the up-regulation was limited when compared to NPCs alone and might be lost at day 28. Moreover, the fact that we used NPC +1.5SF as control rather than NPCs alone makes difficult to directly compare the two studies.
Interestingly, levels of matrix production (Fig. 2) and gene expression (Fig. 3) were similar for the NPC (with either +1.5NC or +1.5SF) as compared to the Mix (with either +1.5NC or +1.5SF), which only contains 50 % of NPCs. These data confirm previous reports on the positive effects of BMSC/NPC co-culture [2, 17]. Histological analysis (data not shown) showed that proteoglycans were mainly deposited within the aggregates formed at day 28 (Fig. 1e–g). Unfortunately, due to the fading of the NPC prestaining, we could not distinguish the NPCs from the BMSCs. The observed up-regulation could therefore be caused either by the differentiation of the BMSCs induced by NPCs or by the stimulation of the NPCs by BMSCs.
In conclusion, this study shows that NCs did not substantially promote the discogenic phenotype of BMSCs/NPCs in long-term culture. The observation that NCs radically changed phenotype during the 4 weeks culture, combined with the fact that studies reporting any appreciable effects used the NCs for maximum 4 days, imply that the regulatory role of the NCs may be intrinsic to their native phenotype (presence of vacuoles, high expression of Brachyury, cytokeratin-8/-18/-19).
Acknowledgments
This work was supported by an AOSpine International Hans Joerg Wyss Foundation Research Award Grant No HJW2011-SU12. The authors would like to thank R. R. Delcher for his help with statistical analysis; I. Arkesteijn, B. van Dijk, and R. Dittmar for their help with cell extraction.
Conflict of interest
None.
Contributor Information
Esther Potier, Email: e.potier@tue.nl.
Keita Ito, Phone: +31-40-2473851, FAX: +31-40-2473744, Email: k.ito@tue.nl.
References
- 1.Stoyanov JV, et al. Role of hypoxia and growth and differentiation factor-5 on differentiation of human mesenchymal stem cells towards intervertebral nucleus pulposus-like cells. Eur Cell Mater. 2011;21:533–547. doi: 10.22203/ecm.v021a40. [DOI] [PubMed] [Google Scholar]
- 2.Richardson SM, et al. Intervertebral disc cell-mediated mesenchymal stem cell differentiation. Stem Cells. 2006;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 3.Vadala G et al (2008) Coculture of bone marrow mesenchymal stem cells and nucleus pulposus cells modulate gene expression profile without cell fusion. Spine (Phila Pa 1976) 33:870–876 [DOI] [PubMed]
- 4.Wei A, et al. Differentiation of rodent bone marrow mesenchymal stem cells into intervertebral disc-like cells following coculture with rat disc tissue. Tissue Eng Part A. 2009;15:2581–2595. doi: 10.1089/ten.tea.2008.0458. [DOI] [PubMed] [Google Scholar]
- 5.Sakai D, et al. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 6.Hiyama A, et al. Transplantation of mesenchymal stem cells in a canine disc degeneration model. J Orthop Res. 2008;26:589–600. doi: 10.1002/jor.20584. [DOI] [PubMed] [Google Scholar]
- 7.Henriksson HB et al (2009) Transplantation of human mesenchymal stems cells into intervertebral discs in a xenogeneic porcine model. Spine (Phila Pa 1976) 34:141–148 [DOI] [PubMed]
- 8.Vadala G, et al. Mesenchymal stem cells injection in degenerated intervertebral disc: cell leakage may induce osteophyte formation. J Tissue Eng Regen Med. 2012;6:348–355. doi: 10.1002/term.433. [DOI] [PubMed] [Google Scholar]
- 9.Smolders LA, et al. Intervertebral disc degeneration in the dog. Part 2: chondrodystrophic and non-chondrodystrophic breeds. Vet J. 2013;195:292–299. doi: 10.1016/j.tvjl.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Gantenbein-Ritter B, Chan SC. The evolutionary importance of cell ratio between notochordal and nucleus pulposus cells: an experimental 3-D co-culture study. Eur Spine J. 2012;21(Suppl 6):S819–S825. doi: 10.1007/s00586-011-2026-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: regulation of proteoglycan synthesis. Exp Cell Res. 1999;246:129–137. doi: 10.1006/excr.1998.4287. [DOI] [PubMed] [Google Scholar]
- 12.Cappello R, Bird JL, Pfeiffer D, Bayliss MT, Dudhia J (2006) Notochordal cell produce and assemble extracellular matrix in a distinct manner, which may be responsible for the maintenance of healthy nucleus pulposus. Spine (Phila Pa 1976) 31:873–882 [DOI] [PubMed]
- 13.Kim JH et al (2009) Differentiation of intervertebral notochordal cells through live automated cell imaging system in vitro. Spine (Phila Pa 1976) 34:2486–2493 [DOI] [PubMed]
- 14.Purmessur D, et al. Notochordal conditioned media from tissue increases proteoglycan accumulation and promotes a healthy nucleus pulposus phenotype in human mesenchymal stem cells. Arthr Res Ther. 2011;13:R81. doi: 10.1186/ar3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Potier E, et al. Hypoxia affects mesenchymal stromal cell osteogenic differentiation and angiogenic factor expression. Bone. 2007;40:1078–1087. doi: 10.1016/j.bone.2006.11.024. [DOI] [PubMed] [Google Scholar]
- 16.Rittie L, Fisher GJ. Isolation and culture of skin fibroblasts. Methods Mol Med. 2005;117:83–98. doi: 10.1385/1-59259-940-0:083. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, et al. Effect of reinsertion of activated nucleus pulposus on disc degeneration: an experimental study on various types of collagen in degenerative discs. Connect Tissue Res. 2003;44:104–108. doi: 10.1080/713713657. [DOI] [PubMed] [Google Scholar]
- 18.Guo JF, Jourdian GW, MacCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277–297. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 19.Enobakhare BO, Bader DL, Lee DA. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243:189–191. doi: 10.1006/abio.1996.0502. [DOI] [PubMed] [Google Scholar]
- 20.Huszar G, Maiocco J, Naftolin F. Monitoring of collagen and collagen fragments in chromatography of protein mixtures. Anal Biochem. 1980;105:424–429. doi: 10.1016/0003-2697(80)90481-9. [DOI] [PubMed] [Google Scholar]
- 21.Cesarone CF, Bolognesi C, Santi L. Improved microfluorometric DNA determination in biological material using 33258 Hoechst. Anal Biochem. 1979;100:188–197. doi: 10.1016/0003-2697(79)90131-3. [DOI] [PubMed] [Google Scholar]
- 22.van Dijk BGM, Potier E, Ito K. Culturing bovine nucleus pulposus explants by balancing medium osmolarity. Tissue Eng Part C Methods. 2011;17:1089–1096. doi: 10.1089/ten.tec.2011.0215. [DOI] [PubMed] [Google Scholar]
- 23.Korecki CL, Taboas JM, Tuan RS, Iatridis JC. Notochordal cell conditioned medium stimulates mesenchymal stem cell differentiation toward a young nucleus pulposus phenotype. Stem Cell Res Ther. 2010;1:18. doi: 10.1186/scrt18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sobajima S, et al. Feasibility of a stem cell therapy for intervertebral disc degeneration. Spine J. 2008;8:888–896. doi: 10.1016/j.spinee.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Weiler C, et al. Immunohistochemical identification of notochordal markers in cells in the aging human lumbar intervertebral disc. Eur Spine J. 2010;19:1761–1770. doi: 10.1007/s00586-010-1392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minogue BM, Richardson SM, Zeef LA, Freemont AJ, Hoyland JA. Transcriptional profiling of bovine intervertebral disc cells: implications for identification of normal and degenerate human intervertebral disc cell phenotypes. Arthr Res Ther. 2010;12:R22. doi: 10.1186/ar2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartels EM, Fairbank JC, Winlove CP, Urban JP (1998) Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine (Phila Pa 1976) 23:1–7 [DOI] [PubMed]
- 28.Guehring T, et al. Notochordal intervertebral disc cells: sensitivity to nutrient deprivation. Arthr Rheum. 2009;60:1026–1034. doi: 10.1002/art.24407. [DOI] [PubMed] [Google Scholar]
- 29.Guehring T, Nerlich A, Kroeber M, Richter W, Omlor GW. Sensitivity of notochordal disc cells to mechanical loading: an experimental animal study. Eur Spine J. 2010;19:113–121. doi: 10.1007/s00586-009-1217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]