Abstract
Objectives:
Sphingosine kinase 1 (SphK1) phosphorylates the membrane sphingolipid, sphingosine, to sphingosine-1-phosphate (S1P), an oncogenic mediator, which drives tumor cell growth and survival. Although SphK1 has gained increasing prominence as an oncogenic determinant in several cancers, its potential as a therapeutic target in colon cancer remains uncertain. We investigated the clinical relevance of SphK1 expression in colon cancer as well as its inhibitory effects in vitro.
Methods:
SphK1 expression in human colon tumor tissues was determined by immunohistochemistry and its clinicopathological significance was ascertained in 303 colon cancer cases. The effects of SphK1 inhibition on colon cancer cell viability and the phosphoinositide 3-kinase (PI3K)/Akt cell survival pathway were investigated using a SphK1-selective inhibitor—compound 5c (5c). The cytotoxicity of a novel combination using SphK1 inhibition with the chemotherapeutic drug, 5-fluorouracil (5-FU), was also determined.
Results:
High SphK1 expression correlated with advanced tumor stages (AJCC classification). Using a competing risk analysis model to take into account disease recurrence, we found that SphK1 is a significant independent predictor for mortality in colon cancer patients. In vitro, the inhibition of SphK1 induced cell death in colon cancer cell lines and attenuated the serum-dependent PI3K/Akt signaling. Inhibition of SphK1 also enhanced the sensitivity of colon cancer cells to 5-FU.
Conclusion:
Our findings highlight the impact of SphK1 in colon cancer progression and patient survival, and provide evidence supportive of further development in combination strategies that incorporate SphK1 inhibition with current chemotherapeutic agents to improve colon cancer outcomes.
INTRODUCTION
Colon cancer is the third most common cancer in the world and the fourth most common cause of cancer-related mortality.1 Current cytotoxic drugs that are used to treat colon cancer include 5-fluorouracil (5-FU), oxaliplatin, leucovorin, and irinotecan. Although 5-FU has been used as a first-line treatment for advanced colon cancer and improves the survival of patients, up to 85–90% of patients respond poorly.2 More recently, monoclonal antibodies against growth factors have been developed and tested in clinical trials for advanced colon cancer.3 These include bevacizumab, an antibody against the vascular endothelial growth factor, as well as cetuximab and panitumumab, antibodies against the epidermal growth factor.3 These antibodies are more effective when used in combination with 5-FU, compared to use as single agents.3 However, a significant percentage of patients may not benefit from treatment with these drugs. For example, cetuximab is ineffective in cancers with KRAS mutations, which are found in approximately 38% of colon cancers.3 Drugs that target novel oncogenic pathways associated with advanced colon cancer are therefore promising strategies for improving patient outcome.
Sphingolipids are major components of the membrane bilayer and have critical roles in regulating cancer development. The major bioactive sphingolipids, ceramide and sphingosine-1-phosphate (S1P), regulate very distinct cellular functions. Increased levels of ceramide have been shown to promote apoptosis,4 whereas increased levels of S1P promote cell proliferation and survival.5 The “sphingolipid rheostat”, determined by the relative levels of ceramide to S1P, is proposed as a cell fate determinant in cancer6 and is regulated by sphingosine kinases (SphKs), which catalyze the phosphorylation of sphingosine to S1P. Although there are two known mammalian SphK isoforms, SphK1 and SphK2, SphK1 appears to be the predominant oncogenic isoform in human cancers.7 SphK1 is upregulated in various forms of cancer including colon cancer,8, 9 and is involved in oncogenesis and tumor progression. Importantly, SphK1 expression correlates with poor prognosis in breast10 and gastric11 cancer patients, although its prognostic value in colon cancer has not been previously reported.
There is increasing evidence that ceramide and S1P are important opposing factors in colon cancer development. Several studies have implicated ceramide in pro-apoptotic roles,12, 13 while S1P has emerged as a major oncogenic factor in colon cancer. In normal intestinal epithelial cells, S1P is maintained at low levels14 and is protective against apoptosis.15 However, in neoplastic tissues, S1P accumulates and contributes to polyp enlargement and tumor progression.14 This suggests that neoplastic transformation in the colonic epithelium arises from loss of regulation of S1P levels. Consistent with this, S1P lyase, which catalyzes S1P catabolism, is downregulated in colon cancer.16 A previous study of 61 mixed human colonic tumors showed that SphK1 expression was higher in tumors compared with normal mucosa, as well as malignant adenocarcinomas compared with adenomas, suggesting that SphK1 is an important tumorigenic determinant in colon cancer.9 SphK1 is the predominant isoform that generates S1P in the colon, and it contributes to colon adenoma progression by enhancing colon cancer cell proliferation,17 as well as promoting the expression and activity of pro-inflammatory proteins involved in colon carcinogenesis, including cyclooxgenase-2 and prostaglandin E2.8, 9
In this study, we seek to evaluate the prognostic significance of SphK1 in colon cancer. We carried out immunohistochemical staining of SphK1 in 303 colon adenocarcinoma cases and performed competing risk analysis to estimate the impact of SphK1 expression on patient mortality and disease recurrence. Using a SphK1-specific inhibitor, we also investigated the cytotoxicity of SphK1 inhibition in colon cancer cells in vitro.
METHODS
All reagents and chemicals unless stated otherwise were bought from Sigma-Aldrich (St Louis, MO, USA).
Immunohistochemistry
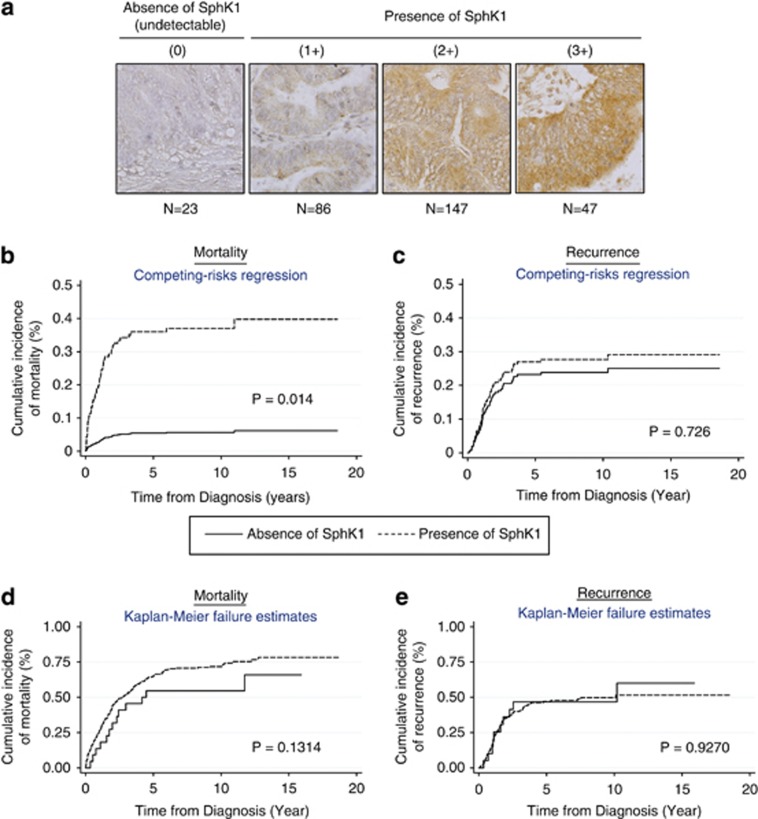
Colon tumors used were obtained with patient consent and approved by the institutional review board at the National University Health System in Singapore. Immunohistochemical staining of SphK1 expression was performed on tissue microarrays containing 303 colon adenocarcinomas. Tissue microarrays were constructed according to previously published methods.18 Tissue microarray slides were deparaffinized with xylene, and rehydrated in a graded alcohol series. Slides were then heated with the antigen unmasking solution (Vector Laboratories, Peterborough, UK) for 10 min, and stained with the DakoCytomation EnVision+ System–HRP (AEC) kit (Dakocytomation, Singapore, Singapore). After blocking endogenous peroxidase with hydrogen peroxide, slides were incubated with the primary rabbit anti-SPHK1 antibody (Abcam, Cambridge, UK) and secondary anti-rabbit antibody. Tissue sections were counterstained with hematoxylin. A four-tier scoring system was used, whereby the intensity of SphK1 expression was classified on an increasing scale of 0–3, with “0” being undetectable and “3” being the most intensely stained. Figure 1a shows representative images of each staining intensity, and Table 1 shows the distribution of SphK1 staining expression in the patient cohort.
Figure 1.
The expression of sphingosine kinase 1 (SphK1) in human colon adenocarcinomas correlates with advanced disease and poor survival. (a) Immunohistochemistry staining of SphK1 was carried out in 303 tumor tissues. SphK1 expression was tabulated using the four-tier scoring system (0, 1+, 2+, 3+). SphK1 expression was classified as absent/undetectable (0) or present (1+, 2+, 3+). (b) Competing risk regression model taking into account disease recurrence. The probability of mortality within 5 years was 37% in patients with detectable SphK1 expression, and the probability of mortality within 5 years was 7% in patients with undetectable SphK1 expression. Both probabilities take into account the probability that recurrence status could occur instead. (c) Competing risk regression model taking into account death due to colon cancer. The probability of having a recurrence within 5 years was 23% in patients with detectable SphK1 expression, and the probability of mortality within 5 years was 28% in patients with undetectable SphK1 expression. Both probabilities take into account the probability that the death could occur instead. (d, e) Kaplan–Meier cumulative incidence estimates of mortality and recurrence, respectively.
Table 1. Distribution of SphK1 expression in the patient cohort.
| SphK1 expression | Frequency | Percentage (%) | Cumulative percentage (%) |
|---|---|---|---|
| 0 | 23 | 7.6 | 7.6 |
| 1+ | 86 | 28.4 | 36.0 |
| 2+ | 147 | 48.5 | 84.5 |
| 3+ | 47 | 15.5 | 100 |
| Total | 303 | 100 | — |
SphK1, sphingosine kinase 1.
Statistical analysis for clinicopathological significance
The mortality and recurrence status of patients were captured using in-house hospital database as well as the registry of birth and death. The median duration of follow-up was 6.8 years (95% confidence interval (CI): 4.46–8.92). Survival analysis was used to assess mortality and disease-free survival (recurrence) among patients with and without SphK1 expression using Kaplan–Meier survival curves and a Cox regression model. Standard Cox proportional hazards models, which assumes that the hazard ratio is constant over time, were applied initially. The proportionality assumption of the Cox regression model was assessed graphically and with the use of Schoenfeld residuals19 using “estat phtest” STATA command after fitting a model with stcox. All analysis were carried out using the scoring system of SphK1 expression (0 vs. 1–2–3+). Finally, the competing risk regression method was used to estimate the impact of SphK1 expression (main covariate of interest) on the probability of mortality due to colon cancer, in which disease recurrence was treated as a competing event. The results (effect sizes) were expressed as sub-hazard ratios(SHR). The estimated SHRs were also taken into account for the presence of other competing risk events such as death due to colon cancer. We also adjusted for various demographic, clinical and pathological factors, including tumor staging. The crude cumulative probability of mortality while accounting for the dependence of the cumulative probability function on the hazards of other competing event (i.e., disease recurrence) was calculated. Similarly, the crude cumulative probability of recurrences while accounting for the dependence of the cumulative probability function on the hazards of other competing event (i.e., mortality) was calculated. We then compared the resulting curves between two groups (absence vs. presence of SphK1 expression). The percentage of local disease recurrence was 4% (12 patients; out of which 0 patients had negative SphK1 expression), and distant disease recurrence was 23.72% (73 patients; out of which 8 patients had negative SphK1 expression). All statistical analyses were performed using Stata statistical software (version 11.1; StataCorp LP, College Station, TX).
Cell lines and cell viability measurements
Human colon tumor cell lines HCT116, SW480, SW620, and RKO were purchased from American Type Culture Collection (ATCC, Manassas, VI, USA). HCT116 cells were cultured in McCoy 5A Modified Medium containing 10% fetal bovine serum (FBS; Gibco, Life Technologies, Singapore, Singapore). SW480, SW620, and RKO cells were cultured in the Dulbecco's Modified Eagle Medium containing 10% FBS. For cell viability measurements, cells (2 × 104 per well) in 96-well plates were synchronized with 1% FBS for 18 h. Subsequently, respective drugs were added and cells were incubated for another 48 h in 10% FBS. Dimethylsulfoxide was added into the cultures as a vehicle control. Cell viability was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) dye reduction assay as previously described.20 Results are expressed as a percentage of live cells relative to the untreated controls at the indicated time.
For serum stimulation, cells (1 × 106 per well) in six-well plates were synchronized with 1% FBS for 18 h. Subsequently, cells were pre-treated with inhibitors for 1 h before stimulation with serum (10% FBS). Samples were lysed in RIPA buffer (50 mM Tris-HCl, pH 7.4; 1% NP-40; 0.25% Na-deoxycholate; 150 mM NaCl; 1 mM EDTA) together with protease and phosphatase inhibitors (Roche, Singapore, Singapore). Protein concentration was measured using the Bradford reagent (Bio-Rad, Berkeley, CA, USA). Lysates were used for immunoblotting and measurement of SphK activity.
Immunoblotting
In all, 20 μg protein aliquots were electrophoresed in 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transblotted to polyvinylidene difluoride membranes (Millipore, Billerica, MA, USA). Blots were incubated overnight with primary antibodies directed against SphK1, phosphorylated-AktSer473, Thr308, AKT (Cell Signaling Technology, Beverly, MA, USA), glyceraldehyde 3-phosphate dehydrogenase (Santa Cruz Biotechnology) followed by appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies—anti-rabbit IgG HRP (Thermo Scientific, Waltham, MA, USA); anti-mouse IgG HRP (Santa Cruz Biotechnology, Dallas, TX, USA). Immunocomplexes were visualized by enhanced chemiluminescence detection on X-ray film (Thermo Scientific).
Measurement of SphK activity (fluorometric method)
The protocol has been previously established.21 In all, 80 μg of protein was incubated with 20 μM of 15-NBD-Sph (prepared as a complex with bovine serum albumin) and 1 mM ATP in SphK buffer (50 mM 4-(2-hydroxyethyl)piperazine-1-ethanesulfonic acid, pH 7.4, containing 15 mM MgCl2, 0.005% Triton X-100, 1 0 mM KCl). After incubation for 30 min at 37 °C, 100 μl 1 M potassium phosphate buffer (pH 8.5) was added followed by 500 μl CHCl3/MeOH 2:1. After brief mixing, phases were separated by centrifugation (5 min, 3,000 g). An aliquot (75 μl) of the upper aqueous layer was removed and placed into each well of a 96-well polystyrene microplate (Greiner Bio-One, Monroe, NC, USA), followed by 75 μl of dimethylformamide (Merck, Singapore, Singapore). Fluorescence intensity was measured at 485 nm excitation and 535 nm emission.
Cell cycle analysis
Cells (2 × 105 per well) were synchronized with 1% FBS for 18 h. Subsequently, cells were incubated with 10–100 μM 5c for 24 h in the presence of 10% FBS. All samples were harvested and fixed with 70% ethanol, followed by suspension in propidium iodide (Invitrogen, Life Technologies)/RNase staining buffer solution. Cell cycle analysis was performed using cell flow cytometer (BD FACSCalibur, Becton Dickinson, Franklin Lakes, NJ, USA) and Cell Quest Pro Software (Becton Dickinson).
Gene knockdown by small interfering RNA (siRNA)
SphK1 siRNA that targets 5′-CTGCCTATGTAAGGCCTTCTA-3′ was synthesized from Qiagen (Singapore, Singapore). The siRNA transfection was conducted in a 6-well format (for immunoblotting) or 96-well format (for MTT cell viability assay), using Lipofectamine RNAiMAX (Invitrogen) following the manufacturer's instructions. In all, 20 nM of siRNA is used for each transfection. At 24 h after transfection, the transfection media (Opti-MEM) was replaced with complete media to allow cells to recover. At 72 h post-transfection, cells in the 6-well plate were harvested for western blot analysis and cells in the 96-well plate were treated with 5-FU for another 48 h before carrying out MTT assay to measure cell viability. In all experiments, the AllStars Negative Control siRNA (Qiagen) was used as a negative control sequence. This negative control siRNA has no homology to any known mammalian gene.
Statistical analysis for in vitro studies
The results were expressed as means±s.e. The difference between the means of two independent samples was evaluated using the unpaired Student's t-test. All the above descriptive analysis was based on two-sided test, and the level of significance was set at P<0.05 for each time point.
RESULTS
SphK1 expression correlates with late tumor stages and it is an independent predictor of mortality in colon cancer patients while taking into account disease recurrence
To ascertain the contribution of SphK1 to the malignant behavior of colon adenocarcinomas, we examined its expression in 303 cases by immunohistochemistry. The median survival time for the patient cohort is 3.11 years and the median disease-free survival time is 1.43 years (Table 2). The patients' demographics, chemo responses, TNM staging, histology grades, disease progression, vascular/lymphatic invasion, mortality, and recurrence status were similar in the absence and presence of SphK1 expression (Table 2). However, the presence of SphK1 expression correlated with late AJCC tumor stages (P=0.042). In addition, the number of deaths is reported to be 206 of total 303 cases (67.99%). Out of these 206 cases, 193 of them (93.7%) had positive SphK1 expression (1–2–3+ scores).
Table 2. Characteristics of demographic, clinical status, pathological features, response of treatment options, and clinical outcomes by the expression of SphK1.
| Absence of SphK1 (N=23) | Presence of SphK1 (N=280) | Total | P value | |
|---|---|---|---|---|
| Age groupa | ||||
| Age ≤65 years | 14 (60.87) | 144 (51.43) | 158 (52.15) | 0.384 |
| Age >65 years | 9 (39.13) | 136 (48.57) | 145 (47.85) | |
| Gender | ||||
| Male (n, %) | 9 (39.13) | 127 (45.36) | 136 (44.88) | 0.564 |
| Female (n, %) | 14 (60.87) | 153 (54.64) | 167 (55.12) | |
| Ethnic group | ||||
| Chinese (n, %) | 20 (86.96) | 250 (89.29) | 270 (89.11) | 0.817 |
| Malays (n, %) | 2 (8.7) | 18 (6.43) | 20 (6.6) | |
| Indians (n, %) | 1 (4.35) | 8 (2.86) | 9 (2.97) | |
| Others (n, %) | 0 (0) | 4 (1.43) | 4 (1.32) | |
| AJCC stage | ||||
| Stage 1 (n, %) | 0 | 21 (7.5) | 21 (6.93) | 0.042 |
| Stage 2 (n, %) | 10 (43.48) | 95 (33.93) | 105 (34.65) | |
| Stage 3 (n, %) | 10 (43.48) | 78 (27.86) | 88 (29.04) | |
| Stage 4 (n, %) | 3 (13.04) | 86 (30.71) | 89 (29.37) | |
| Chemo response | ||||
| Complete (n, %) | 6 (50) | 31 (27.68) | 37 (29.84) | 0.284 |
| Partial (n, %) | 1 (8.33) | 8 (7.14) | 9 (7.26) | |
| Stable disease (n, %) | 3 (25) | 29 (25.89) | 32 (25.81) | |
| Disease progression (n, %) | 2 (16.67) | 44 (39.29) | 46 (37.1) | |
| T stage | ||||
| Early (1 and 2) | 1 (4.35) | 27 (9.64) | 28 (9.24) | 0.707 |
| Late (3 and 4) | 22 (95.65) | 253 (90.36) | 275 (90.76) | |
| N status | ||||
| 0 | 11 (47.83) | 137 (48.93) | 148 (48.84) | 0.415 |
| 1 | 9 (39.13) | 79 (28.21) | 88 (29.04) | |
| 2 | 3 (13.04) | 64 (22.86) | 67 (22.11) | |
| Nodal involvement | ||||
| 0 | 11 (47.83) | 137 (48.93) | 148 (48.84) | 0.919 |
| 1 | 12 (52.17) | 143 (51.07) | 155 (51.16) | |
| M status | ||||
| 0 | 20 (86.96) | 194 (69.29) | 214 (70.63) | 0.095 |
| 1 | 3 (13.04) | 86 (30.71) | 89 (29.37) | |
| Histology grade | ||||
| Well differentiated | 1 (4.35) | 10 (3.57) | 11 (3.63) | 0.591 |
| Moderately differentiated | 21 (91.3) | 238 (85) | 259 (85.48) | |
| Poorly differentiated | 1 (4.35) | 32 (11.43) | 33 (10.89) | |
| Disease progression | ||||
| Non-complete response | 2 (16.67) | 44 (39.29) | 46 (37.1) | 0.207 |
| Complete response | 10 (83.33) | 68 (60.71) | 78 (62.9) | |
| Vascular invasion | ||||
| No | 19 (82.61) | 250 (89.29) | 269 (88.78) | 0.307 |
| Yes | 4 (17.39) | 30 (10.71) | 34 (11.22) | |
| Lymphatic invasion | ||||
| No | 22 (95.65) | 251 (89.64) | 273 (90.1) | 0.713 |
| Yes | 1 (4.35) | 29 (10.36) | 30 (9.9) | |
| Gene by outcome status | ||||
| Alive | 10 (43.48) | 87 (31.07) | 97 (32.01) | 0.22 |
| Death | 13 (56.52) | 193 (68.93) | 206 (67.99) | |
| Disease recurrence | ||||
| No recurrence | 14 (63.64) | 197 (71.9) | 211 (71.28) | 0.418 |
| Recurrence | 0 (0) | 12 (4.38) | 12 (4.05) | |
| Distant recurrence | 8 (36.36) | 65 (23.72) | 73 (24.66) | |
DSF, disease-free survival; SphK1, sphingosine kinase 1.
Median survival time=3.11 (years) (2.23–3.69).
Median DSF time=1.43 (years) (0.01–19.29).
Age (mean=65.23±13.10).
Conventional analysis such as Cox regression (Tables 3 and 5) and Kaplan–Meier survival analysis (Figure 1d and e) were carried out alongside the competing risk regression approach (Tables 4 and 6, Figure 1b and c) to investigate the impact of SphK1 expression on mortality and recurrence in the patient cohort. The adjusted hazard ratios obtained in the Cox regression were comparable to the SHRs obtained in the competing risk regression and both analysis showed similar trends (Tables 3, 4, 5, 6). In both approaches, there was a positive correlation between SphK1 expression and mortality, but not recurrence. However, the adjusted hazard ratio estimates for AJCC stages 3 and 4 (reference group AJCC stages 1 and 2) for mortality was 2.0 (95% CI: 1.29–3.11, P=0.002) using Cox regression, in which competing event recurrence was not taken into account. On the other hand, the SHR estimates for AJCC stages 3 and 4 (reference group AJCC stages 1 and 2) for mortality in the competing risk regression was 1.29 (95% CI: 0.71–2.32, P=0.402) after taking into account the competing event of disease recurrence. The Kaplan–Meier survival analysis also showed an increased cumulative incidence of mortality in patients with positive SphK1 expression compared with patients with negative SphK1 expression (P=0.1314 by log-rank test; Figure 1d). However, this did not reach statistical significance.
Table 3. Adjusted HR estimates for SphK1 expression and colon cancer-specific mortality using multivariate Cox regression analysis.
|
Multivariate analysis |
|||
|---|---|---|---|
| Variables | Adjusted SHR | 95% CI | P value |
| SphK1 expression | |||
| Absence (Ref) | 1.0 | — | — |
| Presence | 2.50 | 1.10–5.67 | 0.029 |
| Age | |||
| ≤65 years (Ref) | 1.0 | — | — |
| >65 years | 2.51 | 1.72–3.67 | <0001 |
| Ethnicity | |||
| Chinese (Ref) | 1.0 | — | — |
| Malays | 4.13 | 2.60–6.56 | <0.001 |
| Indians | 1.77 | 0.66–4.76 | 0.261 |
| Others | 0.41 | 0.11–1.48 | 0.172 |
| Pre-op CEA levels | 1.00 | 1.00–1.00 | <0.001 |
| AJCC stage | |||
| Stages 1 and 2 (Ref) | 1.0 | — | — |
| Stages 3 and 4 | 2.00 | 1.29–3.11 | 0.002 |
| Vascular invasion | |||
| Absence (Ref) | 1.0 | — | — |
| Presence | 2.98 | 1.92–4.61 | <0.001 |
CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; SHR, sub-hazard ratio; SphK1, sphingosine kinase 1.
Table 5. Adjusted HR estimates for SphK1 expression and recurrence of colon cancer-specific using multivariate Cox regression analysis.
|
Multivariate analysis |
|||
|---|---|---|---|
| Variables | Adjusted SHR | 95% CI | P value |
| SphK1 expression | |||
| Absence (Ref) | 1.0 | — | — |
| Presence | 1.35 | 0.48–3.77 | 0.564 |
| Age | |||
| ≤65 years (Ref) | 1.0 | — | — |
| >65 years | 1.33 | 0.73–2.42 | 0.344 |
| Ethnicity | |||
| Chinese (Ref) | 1.0 | — | — |
| Malays | 3.29 | 1.35–8.02 | 0.009 |
| Indians | 2.17 | 0.45–10.50 | 0.336 |
| Others | 0.86 | 0.14–5.23 | 0.873 |
| Pre-op CEA levels | 1.002 | 1.00–1.001 | 0.647 |
| AJCC stage | |||
| Stages 1 and 2 (Ref) | 1.0 | — | — |
| Stages 3 and 4 | 2.28 | 1.22–4.29 | 0.01 |
| Vascular invasion | |||
| Absence (Ref) | 1.0 | — | — |
| Presence | 1.52 | 0.55–4.23 | 0.422 |
CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; SHR, sub-hazard ratio; SphK1, sphingosine kinase 1.
Table 4. Adjusted SHR estimates for SphK1 expression and colon cancer-specific mortality using univariate and multivariate competing risk regression analysis.
|
Univariate analysis |
Multivariate analysisa |
|||||
|---|---|---|---|---|---|---|
| Variables | Unadjusted SHR | 95% CI | P value | Adjusted SHR | 95% CI | P value |
| SphK1 expression | ||||||
| Absence (Ref) | 1.0 | — | — | 1.0 | — | — |
| Presence | 3.41 | 1.05–11.03 | 0.041 | 7.99 | 1.53–41.80 | 0.014 |
| Age | ||||||
| ≤65 years (Ref) | 1.0 | — | — | — | — | — |
| >65 years | 2.85 | 2.18–3.72 | <0.001 | 3.09 | 1.66–5.74 | <0.001 |
| Ethnicity | ||||||
| Chinese (Ref) | 1.0 | — | 1.0 | — | — | |
| Malays | 1.15 | 0.71–1.86 | 0.563 | 5.19 | 2.01–13.35 | 0.001 |
| Indians | 1.82 | 0.95–3.47 | 0.070 | 0.85 | 0.08–8.89 | 0.892 |
| Others | 0.44 | 0.15–1.32 | 0.143 | 1.53 | 0.46–5.11 | 0.488 |
| Pre-op CEA levels | 1.001 | 1.001–1.002 | <0.001 | 1.02 | 1.001–1.004 | <0.001 |
| AJCC stage | ||||||
| Stages 1 and 2 (Ref) | 1.0 | — | — | 1.0 | — | — |
| Stages 3 and 4 | 1.08 | 0.84–1.37 | 0.559 | 1.29 | 0.71–2.32 | 0.402 |
| Vascular invasion | ||||||
| Absence (Ref) | 1.0 | — | — | 1.0 | — | — |
| Presence | 1.43 | 0.98–2.09 | 0.062 | 2.23 | 1.08–4.60 | 0.029 |
CEA, carcinoembryonic antigen; CI, confidence interval; SHR, sub-hazard ratio; SphK1, sphingosine kinase 1.
The SHR estimates for SphK1 expression was adjusted for age, ethnicity, pre-op CEA levels, AJCC staging, and presence of vascular invasion in the multivariate analysis.
Table 6. Adjusted SHR estimates for SphK1 expression and recurrence of colon cancer using multivariate competing risk regression analysis.
|
Multivariate analysisa |
|||
|---|---|---|---|
| Variables | Adjusted SHR | 95% CI | P value |
| SphK1 expression | |||
| Absence (Ref) | 1.0 | — | — |
| Presence | 0.84 | 0.31–2.24 | 0.726 |
| Age | |||
| ≤65 years (Ref) | 1.0 | — | — |
| >65 years | 0.92 | 0.51–1.67 | 0.779 |
| Ethnicity | |||
| Chinese (Ref) | 1.0 | — | — |
| Malays | 0.78 | 0.17–3.53 | 0.750 |
| Indians | 1.33 | 0.18–9.89 | 0.781 |
| Others | 1.12 | 0.24–5.12 | 0.886 |
| Pre-op CEA levels | 0.99 | 0.97–1.001 | 0.217 |
| AJCC stage | |||
| Stages 1 and 2 (Ref) | 1.0 | — | — |
| Stages 3 and 4 | 2.12 | 1.13–3.96 | 0.019 |
| Vascular invasion | |||
| Absence (Ref) | 1.0 | — | — |
| Presence | 0.84 | 0.31–2.32 | 0.735 |
CEA, carcinoembryonic antigen; CI, confidence interval; HR, hazard ratio; SHR, sub-hazard ratio; SphK1, sphingosine kinase 1.
The SHR estimates for SphK1 expression was adjusted for age, ethnicity, pre-op CEA levels, AJCC staging, and presence of vascular invasion in the multivariate analysis.
On the basis of the competing risk regression model, SphK1 is a significant independent predictor of mortality after adjusting for other significant risk factors in the model while taking into account disease recurrence as the other competing risk event (Table 4, Figure 1b). As for the prediction of prognosis for disease recurrence using competing risk regression approach, AJCC late stages (stages 3 and 4) was the only significant prognosis factor for disease recurrence in the presence of the competing risk event—death due to colon cancer (Table 6). Adjusted SHR for AJCC (late stages) was 2.1 (95% CI: 1.13–3.93; P=0.019). In this competing risk regression model, SphK1 failed to predict the disease recurrence in patients with colon cancer (Table 6, Figure 1c). We also adjusted for ethnicity, pre-operative carcinoembryonic antigen levels, AJCC stages and the presence of vascular invasion in this model. The AJCC stage was forcefully added in the model because of the strong biological relationship with cancer mortality.
SphK1 inhibition by 5c reduces the viability of colon cancer cells
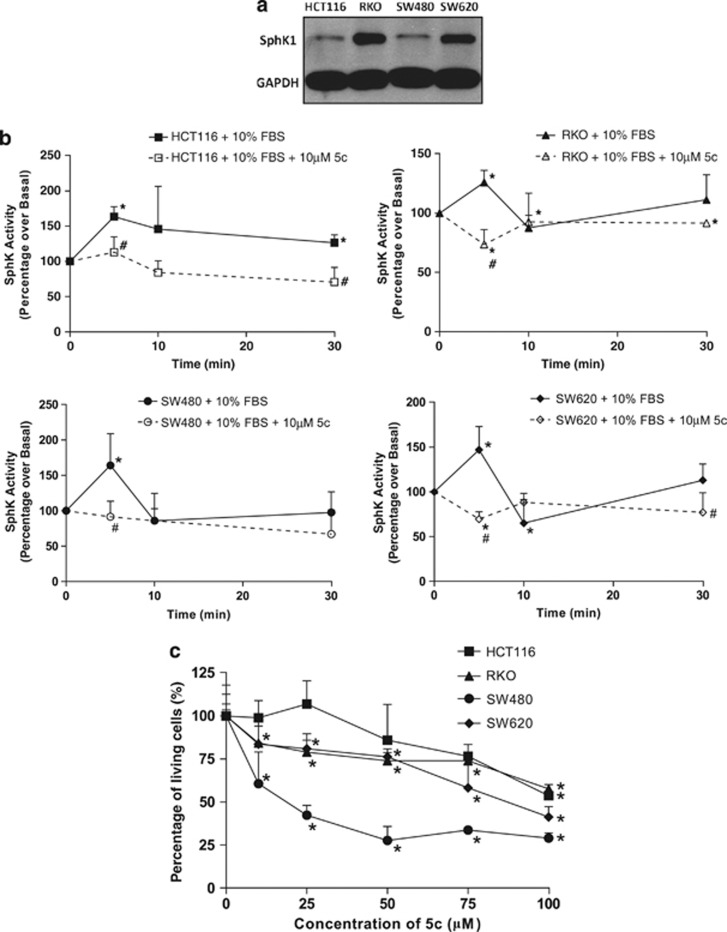
The expression and activity of SphK1 were investigated in a panel of colon cancer cell lines, which included HCT116, RKO, SW480, and SW620 (Figure 2a). SphK1 expression was detected in all cell lines, with highest expression in RKO, consistent with previous reports.22 In all, 10% FBS stimulated the SphK activity in these cell lines, with peak activities ranging from 50 to 100% above the basal non-serum stimulated levels at 5–10 min after treatment (Figure 2b). However, the serum-induced peaks in SphK activity were significantly attenuated with the addition of the SphK1-specific inhibitor 5c, indicating that SphK1 is a major determinant of serum-induced SphK activity in these cells (Figure 2b). The SphK1 inhibitor, compound 5c (5c), was previously developed by our group and validated for specificity to SphK1.20 The contribution of SphK1 to colon cancer cell survival was investigated by ascertaining cell viability after treatment of cell lines with 10–100 μM 5c for 48 h, using the MTT assay. We found that 10–100 μM 5c induced a dose-dependent reduction in cell viability, and 100 μM 5c compromised cell viability by up to 70% (Figure 2c), demonstrating a crucial requirement for SphK1 activity in maintaining colon cancer cell survival.
Figure 2.
Sphingosine kinase 1 (SphK1) inhibition by compound 5c (5c) reduces the viability of colon cancer cells. (a) SphK1 protein levels in HCT116, RKO, SW480, and SW620 cells were measured by western blot with antibodies against SphK1. Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. (b) Whole-cell lysates were prepared respectively from HCT116, RKO, SW480, and SW620 cells pre-treated with or without 10 μM 5c, and stimulated with 10% fetal bovine serum (FBS) for up to 30 min. Equal amounts of protein lysates were measured for SphK activity using the fluorometric SphK Assay. *P<0.05, comparing 0 min with FBS stimulation. #P<0.05, comparing FBS stimulation (closed symbols) with inhibitor treated (open symbols). (c) HCT116, RKO, SW480, and SW620 cells were treated with 10–100 μM of 5c for 48 h in 10% FBS, and then subjected to 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Each point was measured in triplicate and data are expressed as means±s.d. *P<0.05, comparing 0 μm with increasing doses of 5c. Results are representative of three independent experiments.
SphK1 inhibition by 5c induces colon cancer cell death and inhibits signaling through the Akt pathway
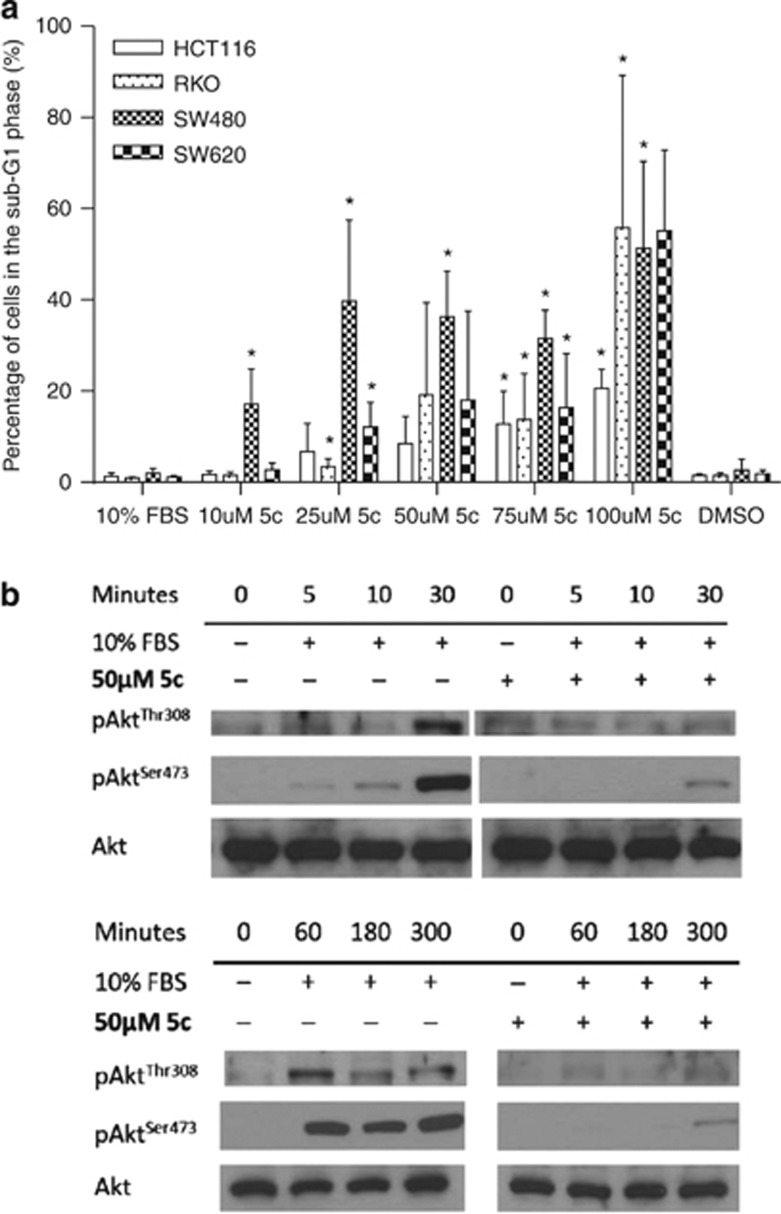
Propidium iodide staining of fixed cells was performed on HCT116, RKO, SW480, and SW620 cell lines treated with 10–100 μM 5c for 24 h. We observed significant increases in the sub-G1 population, indicating nuclear fragmentation and cell death, with up to 55% of cells in sub-G1 using 100 μM 5c (Figure 3a). The sensitivity of colon cancer cells to SphK1 inhibitors such as 5c is consistent with previous work demonstrating that SphK1 knockdown increases caspase activity in RKO cells.22
Figure 3.
Sphingosine kinase 1 (SphK1) inhibition by compound 5c (5c) induces colon cancer cell death and inhibits signaling through the Akt pathway. (a) HCT116, RKO, SW480, and SW620 cells were treated with 10–100 μM of 5c for 24 h in 10% fetal bovine serum (FBS), then fixed and stained with propidium iodide (PI). Dimethylsulfoxide was added as vehicle control. *P<0.05, comparing 10% FBS with increasing doses of 5c. (b) HCT116 cells were pre-treated with 50 μM 5c, and stimulated with 10% FBS for up to 300 min. Equal amounts of protein lysates were immunoblotted with anti-phospho-AktThr308/Ser473 or anti-total-Akt antibodies. 5c inhibited the phosphorylation of Akt at Thr308 and Ser473. Results are representative of three independent experiments.
The phosphoinositide 3-kinase (PI3K) pathway and its major effector, protein kinase B (PKB/Akt), promote tumor cell growth and survival. Upon stimulation by growth factors, Akt is recruited to the plasma membrane and phosphorylated by 3-phosphoinositide-dependent kinase (PDK-1) at threonine residue 308 (Thr308), followed by PDK-2-mediated phosphorylation at serine residue 473 (Ser473) for full activation.23 HCT116 cells were used in this experiment because they have the highest increase in SphK activity upon serum stimulation (Figure 2b) and they also have an activating mutation in the catalytic subunit of PI3K, which results in the hyper-activity of PI3K.24 We found that 10% FBS stimulated the phosphorylation of Akt at Thr308 and Ser473 in HCT116 cells (Figure 3b). Serum-induced Akt activation was observed within 5 min and sustained up to 5 h. Both the early and sustained phosphorylation of Akt at Thr308 and Ser473 induced by serum could be attenuated by 50 μM 5c (Figure 3b), indicating that 5c effectively inhibits the SphK1-dependent response to survival-promoting factors including growth factors present in serum, and is potentially useful in counteracting SphK1-directed oncogenic signaling. In all, 50 μM 5c was used to inhibit SphK1 activity in HCT116 cells because as observed from the MTT results (Figure 2c), 50 μM of 5c was the minimum concentration required to induce a significant loss of cell viability. As Akt blocks the activity of pro-apoptotic proteins and also cross-activates other survival pathways,23 its inhibition by 5c represents an effective mechanism for inducing cell death in colon cancer cells.
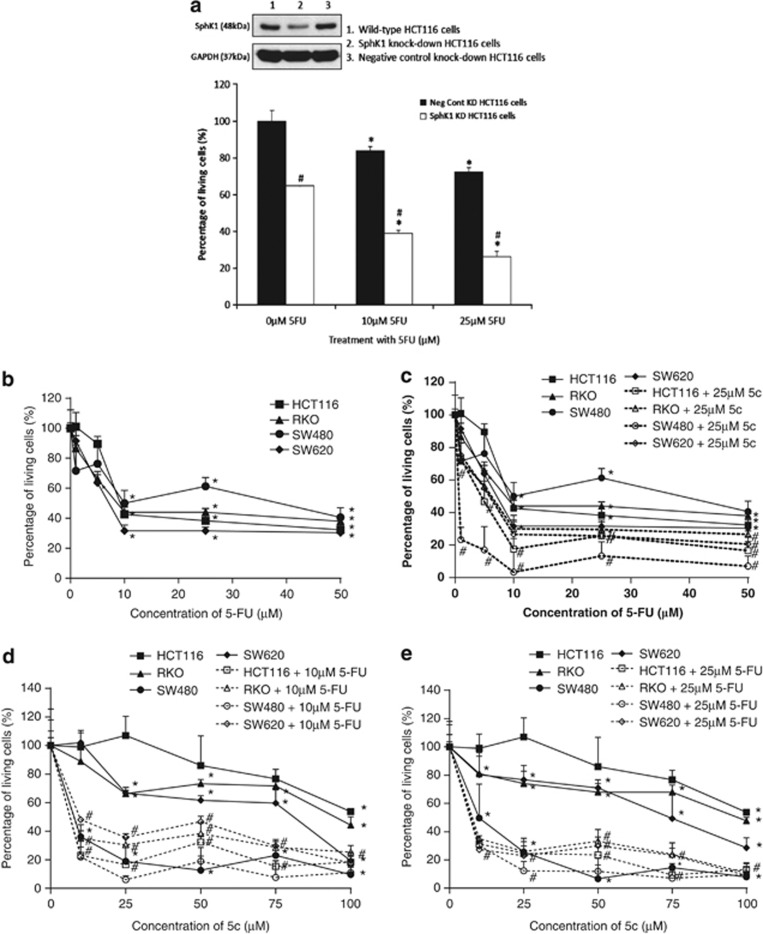
The cytotoxicity of 5-FU in colon cancer cells is augmented by SphK1 knockdown by siRNA and inhibition by 5c
As SphK1 activity appears to be a critical survival mechanism in colon tumor cells, we determined if the loss of SphK1 expression by siRNA knockdown, as well as inhibition of SphK1 activity by 5c may render colon tumor cells more susceptible to the cytotoxic effects of the chemotherapeutic drug, 5-FU. Cell viability studies were performed using the MTT assay. In the absence of 5-FU, the knockdown of SphK1 in HCT116 cells by siRNA reduced cell viability by 35% compared with treatment with control siRNA, indicating that SphK1 normally maintains colon tumor cell survival (Figure 4a). With 5-FU treatment (10–25 μM), the control siRNA-treated HCT116 cells exhibited dose-dependent cytotoxicities, with up to 28% reduction in cell viability using 25 μM 5-FU (Figure 4a). The dose-dependent cytotoxicity of 5-FU was further enhanced by SphK1 knockdown, with a reduction in cell viability by up to 74% when SphK1 knockdown was combined with 25 μM 5-FU (Figure 4a). We also tested the effectiveness of combining 5-FU with inhibition of SphK1 activity by 5c in the HCT116, RKO, SW480, and SW620 cell lines. We found that 1–50 μM 5-FU used as a single agent compromises the viability of cells, with up to 60–70% reduction in viability at 50 μM 5-FU (Figure 4b). However, the combination of 5-FU with 25 μM 5c significantly enhances cytotoxicity, with a further reduction in viability by up to 90%, indicating that Sphk1 inhibition sensitizes colon cancer cells to 5-FU (Figure 4c). In all, 25 μM 5c was used for the combination drug studies as it is the minimum concentration that increases the proportion of cells in the sub-G1 phase, representing cell death, in the panel of cell lines tested (Figure 3a). Conversely, the cytotoxicity of 5c is also enhanced by combination with 10 μM and 25 μM 5-FU, with up to 90% reduction in viability of the cell lines when 100 μM 5c is combined with 25 μM 5-FU (Figure 4d and e). Taken together, these data suggest that addition of SphK1 inhibitors to current chemotherapeutic therapies for colon cancer is likely to augment clinical responses by sensitizing tumor cells that are otherwise poorly responsive to cytotoxic drugs.
Figure 4.
The cytotoxicity of 5-fluorouracil (5-FU) in colon cancer cells is augmented by sphingosine kinase 1 (SphK1) inhibition. Cell viability for treatment with drugs was ascertained by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. All readings were compared with control cells that are not subjected to drug treatment (cell viability taken as 100% at “0” drug concentration). Each point was measured in triplicate and data are expressed as means±s.d. (a) HCT116 cells were transfected with 20 nM of SphK1 small interfering RNA (siRNA) for 72 h, followed by 48 h incubation with 10–25 μm of 5-FU. *P<0.05, comparing untreated with 5-FU-treated cells; #P<0.05, comparing control siRNA control-transfected cells with SphK1 siRNA-transfected cells. (b) HCT116, RKO, SW480, and SW620 cells were treated with 1–50 μM 5-FU, for 48 h. *P<0.05, comparing 0 μm with increasing doses of 5-FU. (c) HCT116, RKO, SW480, and SW620 cells were treated with 25 μM compound 5c (5c), together with increasing doses of 5-FU for 48 h. Combination with 5c enhanced the cytotoxicity of 5-FU. *P<0.05, comparing 0 μm–100 μm 5c; #P<0.05, comparing 5c with 5c+25 μm 5-FU. (d) HCT116, RKO, SW480, and SW620 cells were treated with 10 μM 5-FU, together with increasing doses of 5c for 48 h. *P<0.05, comparing 0 μm–50 μm 5-FU; #P<0.05, comparing 5-FU with 5-FU+25 μm 5c. (e) HCT116, RKO, SW480, and SW620 cells were treated with 25 μM 5-FU, together with increasing doses of 5c for 48 h. *P<0.05, comparing 0 μm–50 μm 5-FU; #P<0.05, comparing 5-FU with 5-FU+25 μm 5c. Combination with 5-FU with SphK1 inhibition significantly enhances colon cancer cell death. Results are representative of three independent experiments and data are expressed as means±s.d.
DISCUSSION
Our results clearly show the pro-survival role of SphK1 in colon cancer. SphK1 expression correlates with late AJCC tumor stages. Using a competing risk analysis model to take into account disease recurrence, we showed that SphK1 independently predicts poor survival in colon cancer patients. In vitro, the inhibition of SphK1 activity impaired colon cancer cell survival. These findings implicate SphK1 as a potentially important contributing factor to colon cancer progression. The antagonism of SphK1 activity inhibited the Akt-mediated survival signaling. In addition, SphK1 knockdown and inhibition sensitized colon cancer cells to treatment with 5-FU, suggesting that SphK1 inhibitors may be explored for their potency as adjunct agents in current chemotherapeutic regimes.
Studies to date have found that the generation of S1P in the colon is largely dependent on SphK1, the predominant SphK isoform in the colon, which is upregulated upon neoplastic transformation of colon cells.8, 9 Previous observations by Kawamori et al.9 found increased SphK1 expression in 61 human colonic tumors comprising adenomas, primary carcinomas, and metastastic lesions, compared with normal tissues. In this study, 100% of the adenocarcinomas had detectable expression of SphK1. This is in line with our findings whereby 92.4% of the adenocarcinoma tissues had detectable expression of SphK1. This upregulation of SphK1 promotes colon cancer cell survival8, 9 and adenoma progression by enhancing cell proliferation.17 The contribution of SphK1 activity to tumor growth is supported by work in the Apc Min/+ mouse model of intestinal tumorigenesis, which exhibits a critical requirement for SphK1 in tumor cell proliferation and intestinal polyp growth.17 Our data support these findings by further showing that SphK1 predicts colon cancer patient prognosis. The correlation between high SphK1 expression and advanced tumor stages as well as patient mortality emphasize the critical roles of SphK1 in colon tumor growth and metastasis.
Mortality and disease recurrences are important clinical outcomes of interest and eminent competing risks in cancer research. Emerging evidence now strongly recommends the competing risk approach, which used to estimate the probability of occurrence of the event of interest in the presence of other competing events. In comparison, the conventional Cox regression and Kaplan–Meier survival analysis ignored the presence of competing risk event(s). As such, they may substantially overestimate the absolute risk of the event of interest because subjects with a competing (and thus censored) event are treated as if they could experience the event of interest in the future.25 The competing risk approach removes such bias by taking into account for the presence of such competing events when estimating the primary outcome of interest.25, 26 Our results, based on the competing risk approach, in which disease recurrence was treated as a competing event, clearly demonstrated that SphK1 expression has a significant role in predicting mortality in addition to other well-known important classical risk factors such as age, late AJCC tumor stages, pre-op carcinoembryonic antigen levels, and presence of vascular invasion in our colon cancer patients. However, SphK1 was not a significant predictor for the recurrence of colon cancer when death was treated as a competing risk event, and after adjusting for the above well-known important classical risk factors. In this model, late AJCC stages was an important prognostic indicator for the recurrence of colon cancer. In Table 2, we showed that the presence of SphK1 correlates with advanced disease (AJCC stage, P=0.042). This suggests that the SphK1 pathway is associated with the progression of colon cancer, which is consistent with poorer survival observed in the presence of SphK1 expression. Out of 89 patients with stage 4 tumors, 86 of them (96.6%) had positive SphK1 expression while only 33 (37.1%) of them had disease recurrence. Therefore, although SphK1 expression does not correlate with disease recurrence, it is still a potential prognostic biomarker to predict mortality of colon cancer patients.
Previous work demonstrated that the sensitivity to oxaliplatin in colon cancer cells could be enhanced by combining oxaliplatin with a non-isoform-specific SphK inhibitor, SKI.22 The inhibition of SphK1 also helped to overcome the resistance of colon cancer cells to cetuximab.27 In prostate cancer, specific inhibition of the SphK1/S1P pathway also sensitizes prostate cancer cells to docetaxel and camptothecin.28 Furthermore, SphK1 overexpression in breast cancer cells induces endocrine resistance, whereas inhibition of SphK1 restores endocrine responsiveness.29 It thus appears that the upregulation or sustained activity of SphK1 in cancer cells may be a critical “escape” mechanism leading to tumor cell resistance and poor response to chemotherapy. In fact, it has been suggested that the reliance of tumor cells on SphK1 activity to sustain survival signaling pathways contributes to non-oncogene addiction.30 Moreover, SphK1 and S1P are mediators for the transactivation of several growth factor receptor signaling, including epidermal growth factor receptor in breast cancer,29 transforming growth factor-β in esophageal cancer31 and HER2 in gastric cancer.32 It is thus conceivable that through transactivation, SphK1 activity in tumors not only amplifies proliferative pathways, but also provides alternative survival pathways when growth signals are antagonized by drugs. In addition to tumor cells, the pro-survival roles of SphK1 have also been shown to encompass other cell types including endothelial cells, through regulation of Akt and Bcl pathways.33, 34
Akt has been shown to be a downstream factor of SphK1, promoting cell proliferation and survival in several cancers such as leukemia,35 glioblastoma,36, 37 and prostate cancer.38 The SphK1 product, S1P, has been shown to exhibit anti-apoptotic roles and enhance Akt activation in intestinal epithelial cells,15 pointing to a reliance on SphK1 activity for survival of the gut epithelium and epithelial tumors. Nemoto et al.22 showed that treatment of colon cancer cells with the anticancer drug oxaliplatin activates SphK1 and Akt. The combination of oxaliplatin together with knockdown of SphK1 downregulated the levels of phosphorylated Akt, thus overcoming resistance of cells to oxaliplatin treatment. This is also demonstrated by Rosa et al.27 whereby the knockdown of SphK1, as well as treatment with fingolimod, a S1P receptor antagonist, reduce Akt phosphorylation thus increasing the sensitivity of colon cancer cells to cetuximab treatment. Consistent with these studies, we found that under serum stimulation, the early activation and subsequent sustained phosphorylation of Akt can both be inhibited by the blockade of SphK1. This suggests that in colon cancer cells, growth stimuli such as serum upregulate the activity of SphK1 over prolonged periods to activate proliferative and survival signaling through Akt. It is therefore likely that SphK1-targeted drugs would be effective in counteracting colon tumor growth and resistance to chemotherapy.
It has been hypothesized that the ability of cancer cells to survive is governed by a threshold level of SphK1 activity,39 suggesting that a minimal level of SphK1 activity may allow cancer cells to survive and proliferate. The persistence of survival pathways, such as those mediated by SphK1, may in turn contribute to drug resistance as well as cancer recurrence. Targeting such survival pathways present new strategies to counteract the progression and recurrence of cancer, such as the combination of current chemotherapeutic drugs with SphK1 inhibitors. Our data suggest that combination of SphK1 inhibitors with 5-FU is a promising approach to chemosensitization and merits further exploration into its feasibility for treatment of advanced colon cancer.
In conclusion, this study has further strengthened the emerging importance of SphK1 in colon cancer and provided evidence for the potential effectiveness of SphK1 inhibitors in colon cancer. In view of the importance of SphK1 as a prognostic and oncogenic determinant in colon cancer, drugs that target SphK1, if developed with acceptable toxicity, may be promising agents in improving patient survival.
Study Highlights

Acknowledgments
The work on human tissues has been approved by the NUS Institutional Review Board (references 10–137).
Guarantor of the article: Sheryl S.L. Tan, PhD.
Specific author contributions: S.S.L.T. and C.T.Y. were responsible for the study design and interpretation of data, and C.T.Y. is the principal investigator. In addition, S.S.L.T. was responsible for all the in vitro experiments and data analysis, while A.D. contributed to the study design. M.S-T. provided the colon tissue microarrays and C.W.O. provided patients information from the National University Health System in Singapore. B.Y. was responsible for the analysis of the immunohistochemical staining for SphK1, including scoring. L.W.K. (Quantitative Epidemiologist) conducted the data analysis including competing risk regression. L.W. and Y.L. synthesized compound 5c for this project.
Financial support: This work was funded by the Biomedical Research Council and National Medical Research Council in Singapore.
Potential competing interests: None.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Johnston P, Kaye S. Capecitabine: a novel agent for the treatment of solid tumors. Anticancer Drugs. 2001;12:639–646. doi: 10.1097/00001813-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Knijn N, Tol J, Punt CJ. Current issues in the targeted therapy of advanced colorectal cancer. Discov Med. 2010;9:328–336. [PubMed] [Google Scholar]
- Kolesnick R, Hannun Y.Ceramide and apoptosis Trends Biochem Sci 199924224–225.author reply 227. [DOI] [PubMed] [Google Scholar]
- Cuvillier O, Pirianov G, Kleuser B, et al. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- Cuvillier O. Sphingosine kinase-1—a potential therapeutic target in cancer. Anticancer Drugs. 2007;18:105–110. doi: 10.1097/CAD.0b013e328011334d. [DOI] [PubMed] [Google Scholar]
- Shida D, Takabe K, Kapitonov D, et al. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori T, Osta W, Johnson K, et al. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- Kawamori T, Kaneshiro T, Okumura M, et al. Role for sphingosine kinase 1 in colon carcinogenesis. FASEB J. 2009;23:405–414. doi: 10.1096/fj.08-117572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruckhäberle E, Rody A, Engels K, et al. Microarray analysis of altered sphingolipid metabolism reveals prognostic significance of sphingosine kinase 1 in breast cancer. Breast Cancer Res Treat. 2008;112:41–52. doi: 10.1007/s10549-007-9836-9. [DOI] [PubMed] [Google Scholar]
- Li W, Yu C, Xia J, et al. Sphingosine kinase 1 is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res. 2009;15:1393–1399. doi: 10.1158/1078-0432.CCR-08-1158. [DOI] [PubMed] [Google Scholar]
- Ahn E, Schroeder J. Sphingoid bases and ceramide induce apoptosis in HT-29 and HCT-116 human colon cancer cells. Exp Biol Med (Maywood) 2002;227:345–353. doi: 10.1177/153537020222700507. [DOI] [PubMed] [Google Scholar]
- Selzner M, Bielawska A, Morse M, et al. Induction of apoptotic cell death and prevention of tumor growth by ceramide analogues in metastatic human colon cancer. Cancer Res. 2001;61:1233–1240. [PubMed] [Google Scholar]
- Oskouian B, Saba J. Sphingosine-1-phosphate metabolism and intestinal tumorigenesis: lipid signaling strikes again. Cell Cycle. 2007;6:522–527. doi: 10.4161/cc.6.5.3903. [DOI] [PubMed] [Google Scholar]
- Greenspon J, Li R, Xiao L, et al. Sphingosine-1-phosphate protects intestinal epithelial cells from apoptosis through the Akt signaling pathway. Dig Dis Sci. 2009;54:499–510. doi: 10.1007/s10620-008-0393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskouian B, Sooriyakumaran P, Borowsky AD, et al. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is down-regulated in colon cancer. Proc Natl Acad Sci USA. 2006;103:17384–17389. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno M, Momoi M, Oo M, et al. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salto-Tellez M, Nga ME, Han HC, et al. Tissue microarrays characterise the clinical significance of a VEGF-A protein expression signature in gastrointestinal stromal tumours. Br J Cancer. 2007;96:776–782. doi: 10.1038/sj.bjc.6603551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- Wong L, Tan SS, Lam Y, et al. Synthesis and evaluation of sphingosine analogues as inhibitors of sphingosine kinases. J Med Chem. 2009;52:3618–3626. doi: 10.1021/jm900121d. [DOI] [PubMed] [Google Scholar]
- Billich A, Ettmayer P. Fluorescence-based assay of sphingosine kinases. Anal Biochem. 2004;326:114–119. doi: 10.1016/j.ab.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Nemoto S, Nakamura M, Osawa Y, et al. Sphingosine kinase isoforms regulate oxaliplatin sensitivity of human colon cancer cells through ceramide accumulation and Akt activation. J Biol Chem. 2009;284:10422–10432. doi: 10.1074/jbc.M900735200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnero A. The PKB/AKT pathway in cancer. Curr Pharm Des. 2010;16:34–44. doi: 10.2174/138161210789941865. [DOI] [PubMed] [Google Scholar]
- Samuels Y, Wang Z, Bardelli A, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- Lin DY. Non-parametric inference for cumulative incidence functions in competing risks studies. Stat Med. 1997;16:901–910. doi: 10.1002/(sici)1097-0258(19970430)16:8<901::aid-sim543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa R, Marciano R, Malapelle U, et al. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res. 2013;19:138–147. doi: 10.1158/1078-0432.CCR-12-1050. [DOI] [PubMed] [Google Scholar]
- Pchejetski D, Doumerc N, Golzio M, et al. Chemosensitizing effects of sphingosine kinase-1 inhibition in prostate cancer cell and animal models. Mol Cancer Ther. 2008;7:1836–1845. doi: 10.1158/1535-7163.MCT-07-2322. [DOI] [PubMed] [Google Scholar]
- Sukocheva O, Wadham C, Holmes A, et al. Estrogen transactivates EGFR via the sphingosine 1-phosphate receptor Edg-3: the role of sphingosine kinase-1. J Cell Biol. 2006;173:301–310. doi: 10.1083/jcb.200506033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadas M, Xia P, McCaughan G, et al. The role of sphingosine kinase 1 in cancer: oncogene or non-oncogene addiction. Biochim Biophys Acta. 2008;1781:442–447. doi: 10.1016/j.bbalip.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Miller AV, Alvarez SE, Spiegel S, et al. Sphingosine kinases and sphingosine-1-phosphate are critical for transforming growth factor beta-induced extracellular signal-regulated kinase 1 and 2 activation and promotion of migration and invasion of esophageal cancer cells. Mol Cell Biol. 2008;28:4142–4151. doi: 10.1128/MCB.01465-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shida D, Kitayama J, Yamaguchi H, et al. Lysophospholipids transactivate HER2/neu (erbB-2) in human gastric cancer cells. Biochem Biophys Res Commun. 2005;327:907–914. doi: 10.1016/j.bbrc.2004.12.088. [DOI] [PubMed] [Google Scholar]
- Limaye V, Li X, Hahn C, et al. Sphingosine kinase-1 enhances endothelial cell survival through a PECAM-1-dependent activation of PI-3K/Akt and regulation of Bcl-2 family members. Blood. 2005;105:3169–3177. doi: 10.1182/blood-2004-02-0452. [DOI] [PubMed] [Google Scholar]
- Xia P, Wang L, Gamble JR, et al. Activation of sphingosine kinase by tumor necrosis factor-alpha inhibits apoptosis in human endothelial cells. J Biol Chem. 1999;274:34499–34505. doi: 10.1074/jbc.274.48.34499. [DOI] [PubMed] [Google Scholar]
- Le Scolan E, Pchejetski D, Banno Y, et al. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106:1808–1816. doi: 10.1182/blood-2004-12-4832. [DOI] [PubMed] [Google Scholar]
- Radeff-Huang J, Seasholtz T, Chang J, et al. Tumor necrosis factor-alpha-stimulated cell proliferation is mediated through sphingosine kinase-dependent Akt activation and cyclin D expression. J Biol Chem. 2007;282:863–870. doi: 10.1074/jbc.M601698200. [DOI] [PubMed] [Google Scholar]
- Kapitonov D, Allegood J, Mitchell C, et al. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayon A, Brizuela L, Martin C, et al. Sphingosine kinase-1 is central to androgen-regulated prostate cancer growth and survival. PLoS One. 2009;4:e8048. doi: 10.1371/journal.pone.0008048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne N, Pyne S. Sphingosine 1-phosphate and cancer. Nat Rev Cancer. 2010;10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]