Abstract
Objectives:
Adenomatous polyps are precursors of colorectal cancer; their detection and removal is the goal of colon cancer screening programs. However, fecal-based methods identify patients with adenomatous polyps with low levels of sensitivity. The aim or this study was to develop a highly accurate, prototypic, proof-of-concept, spot urine-based diagnostic test using metabolomic technology to distinguish persons with adenomatous polyps from those without polyps.
Methods:
Prospective urine and stool samples were collected from 876 participants undergoing colonoscopy examination in a colon cancer screening program, from April 2008 to October 2009 at the University of Alberta. Colonoscopy reference standard identified 633 participants with no colonic polyps and 243 with colonic adenomatous polyps. One-dimensional nuclear magnetic resonance spectra of urine metabolites were analyzed to define a diagnostic metabolomic profile for colonic adenomas. A urine metabolomic diagnostic test for colonic adenomatous polyps was established using 67% of the samples (un-blinded training set) and validated using the other 33% of the samples (blinded testing set). The urine metabolomic diagnostic test's specificity and sensitivity were compared with those of fecal-based tests.
Results:
Using a two-component, orthogonal, partial least-squares model of the metabolomic profile, the un-blinded training set identified patients with colonic adenomatous polyps with 88.9% sensitivity and 50.2% specificity. Validation using the blinded testing set confirmed sensitivity and specificity values of 82.7% and 51.2%, respectively. Sensitivities of fecal-based tests to identify colonic adenomas ranged from 2.5 to 11.9%.
Conclusions:
We describe a proof-of-concept spot urine-based metabolomic diagnostic test that identifies patients with colonic adenomatous polyps with a greater level of sensitivity (83%) than fecal-based tests.
INTRODUCTION
Early adenomatous polyp detection and removal via colonoscopy has led to non-invasive, population-based colorectal cancer (CRC) screening programs. Nevertheless, current non-invasive methods for detecting polyps rely upon fecal occult blood that suffers from low accuracy and, because of their fecal nature, limited uptake. Furthermore, fecal-based tests target CRC detection and not colonic polyps and thus a non-invasive test to better detect colonic adenomatous polyps would represent an advance to population-based screening programs.
The intestinal microflora has been identified as a participant in the CRC process.1 Fusobacterium nucleatum sequences are enriched in participants with CRC, while those from Bacteroidetes and Firmicutes phyla are depleted.2, 3 Shen et al.4 showed significantly more Proteobacteria, fewer Bacteroidetes, and higher diversity in adenomatous colonic mucosa. Recently, Pagnini et al.5 suggested microflora dysbiosis as a potential causative factor for dysplastic cell proliferation, demonstrating that colonic polyps, compared with normal tissue, expressed high numbers of antimicrobial receptors and antibacterial activity, with a 20-fold reduction in mucosa adherent bacteria.
Metabolomics is the quantification of low molecular weight compounds (metabolites) generated by metabolism.6, 7 The metabolome represents the collection of all metabolites of an organism. Relative to adenomatous polyps and CRC, metabolomics has the capacity to detect, not only dysplastic cellular changes of the human mucosa,8 but also changes in the intestinal microflora.2, 3, 4, 5 To date, one systematic review9 and nine pilot studies have examined metabolomics in identifying CRC9, 10, 11, 12, 13, 14 but only one explored metabolomics for detection of adenomatous polyps.14
The aim of this study was to develop a highly accurate, prototypic, proof-of-concept, urine-based metabolomic diagnostic test to detect the presence of colonic adenomatous polyps. This was accomplished by validating, in a large prospective cohort of participants undergoing screening colonoscopy, the metabolomic profile of a single spot urine sample that, in turn, was diagnostic for colonic adenomatous polyps. The sensitivity and specificity of this urine-based metabolomic diagnostic test was compared with commercially available fecal-based tests.
METHODS
Participants
Study participants were prospectively recruited through a population-based colon cancer screening program of asymptomatic individuals undergoing colonoscopy between April 2008 and October 2009. Participants included both average CRC risk (50–75 years of age and no personal or first-degree family history of CRC or polyps) and increased CRC risk (40–75 years of age with a personal or first-degree family history of CRC or polyps). Participants were excluded if they were under 40 or over 75 years of age, had any findings of inflammatory colonic or ileal disease at the time of colonoscopy, or had only hyperplastic polyps.15 Ethics approval was obtained from the Health Research Ethics Board at the University of Alberta on 21 February 2008. All authors had access to study data and approved the final manuscript.
Urine and fecal collections
On the day of entry, participants provided informed written consent, a midstream urine sample, and a demographic questionnaire.15 No dietary or activity modification was required before the urine collection in containers containing six drops of sodium azide (27.3 mg/ml; Sigma Aldrich, Oakville, ON, Canada). Samples were stored at 4 °C within 4 h and then frozen at −80 °C within 24 h. Within 1 week of providing the urine sample, all participants provided a fecal sample for fecal occult blood testing.
Colonoscopy and polyp detection
Colonoscopy was performed 2–6 weeks after the urine and fecal collections as the reference standard. Each participant followed a low residue diet for 5 days, a clear fluid diet on the day before colonoscopy, and prepped with a 4 l colonic lavage. Colonoscopy was performed by gastroenterologists performing a minimum of 100 colonoscopies yearly. Cecal intubation rate was 98% and polyp detection rate 28%. Polyps were characterized according to location, size, and histology. There were no severe adverse events associated with the colonoscopies.
Fecal occult blood analysis
Fecal samples, collected 2–6 weeks before colonoscopy, underwent fecal occult blood testing using three commercially available tests. The Hemoccult II (Beckman Coulter, Mississauga, ON, Canada) test (non-rehydrated) was positive if at least one test window displayed a blue color within 60 s of developer. The Hemoccult ICT (Beckman Coulter) was positive if a pink line appeared in the test area within 5 min of buffer. The MagStream HemSp/HT (Fujirebio Diagnostics, Malvern, PA, USA) was positive at a level of >67 μg hemoglobin/g stool.
Urine metabolomic analysis
Urine samples, collected approximately 1 week before the fecal samples, underwent metabolomic analysis to define the metabolite profile that could distinguish participants with colonic adenomatous polyps from those without polyps.
Sample processing
Urine samples were thawed at room temperature. A 1:10 ratio of internal standard (2,2-dimethyl-2-silapentane-5-sulfonate) and urine was buffered to pH 6.7–6.8, and then placed in a Wilmad 528-pp 4-inch nuclear magnetic resonance tube (Wilmad, Buena, NJ).
Nuclear magnetic resonance acquisition
Spectra were collected using a 600 MHz nuclear magnetic resonance spectrometer (Oxford Instruments, Oxfordshire, UK) with a VNMRS two-channel console (Varian, Palo Alto, CA) running VNMRJ software version 2.2C on a RHEL 4 (Red Hat, Raleigh, NC, USA) host computer. The spectrometer was equipped with an HX probe with Z-axis gradients. The first increment of a 2D-1H, 1H-NOESY pulse sequence was utilized for the acquisition of 1H-nuclear magnetic resonance data and for suppressing the solvent signal. Experiments used a 100 ms mixing time along with a 990 ms pre-saturation (∼80 Hz gammaB1). Spectra were collected at 25 °C for a total of 32 scans over a period of 3.5 min.
Metabolite quantification
Quantification of 69 validated and previously confirmed metabolites from the spectra was completed using targeted profiling with Chenomx NMRSuite v7.0 software (Chenomx, Edmonton, Alberta, Canada). Quantification was completed by one individual and verified by a second blinded to the initial results.
Metabolomic modeling and statistical analysis
SIMCA-P+ v12.0.1 (Umetrics, Umea, Sweden) was used to perform the projection-based principal component analysis, partial least-squares discriminant analysis, and orthogonal partial-least squares analysis. The area under the curve was calculated using Stata/SE 10.1 (Stata Corporation, College Station, TX). Chi-squared tests were used to compare proportional outcomes and Student's t-tests to compare continuous outcomes. The importance of individual metabolites in separating normal from colonic adenomatous polyps was confirmed by variable importance in the projection plots SIMCA-P+ v12.0.1 (Umetrics). The higher the variable importance in the projection value the more important the metabolite is in separation of persons without adenomatous polyps from those with adenomatous polyps (Figure 4).
Metabolomic study design
To define the urine metabolomic profile that distinguished participants with colonic adenomatous polyps (adenoma) from those with no polyps (normal); training and validation cohorts were defined. Two-thirds of the urine samples from each group (adenoma and normal) were randomly assigned to the un-blinded training set. This un-blinded training set was used to generate the metabolite profile that was diagnostic for colonic adenomatous polyps. The remaining one-third of the urine samples from each group (adenoma and normal) formed the blinded testing set. This blinded testing set was used to validate the metabolite profile diagnostic for colonic adenomatous polyps.
The sensitivity and specificity of the urine metabolomic diagnostic test for colonic adenomatous polyps was calculated using Stata/SE 10.1 (Stata Corporation) with colonoscopy as the reference standard and then compared against the results of the fecal occult blood tests. The negative predictive value and positive predictive value were calculated using the generated sensitivity and specificity values and the prevalence of adenoma in this series. Advanced adenoma, defined as any adenoma >1.0 cm, villous components or high-grade dysplasia on histology, or carcinoma of any size, were further investigated to assess a dose–response relationship between size/histology and metabolites.
RESULTS
Participant characteristics
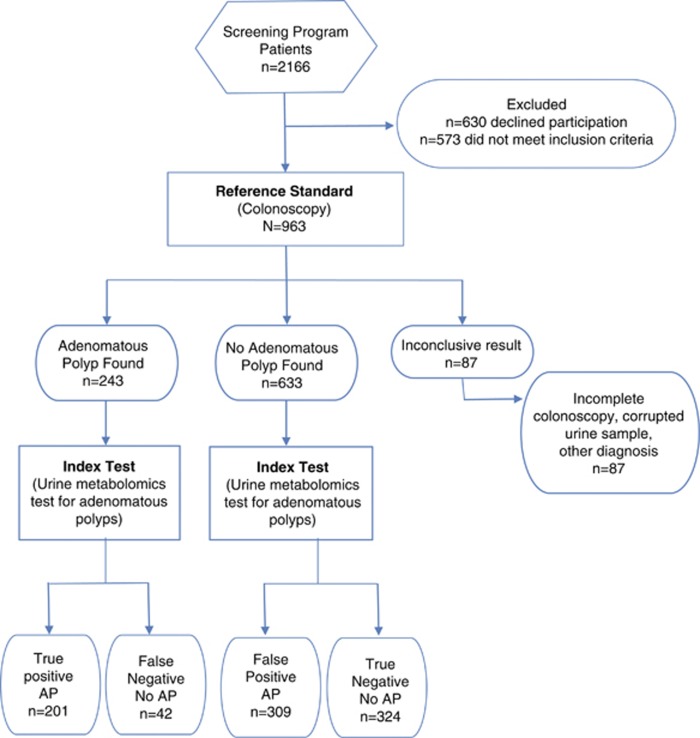
Of the 963 participants sequentially enrolled in the colon cancer screening program and completing colonoscopy, results from 876 were used to determine the urine-based metabolomic diagnostic test for colonic adenomatous polyps. The remaining 87 were excluded because of incomplete colonoscopy, corrupted urine samples, or other diagnoses found at the time of colonoscopy (Figure 1).
Figure 1.
Stard flow diagram. Values for the index test (spot urine metabolomic test for adenomatous polyps) were determined using the 82.7% sensitivity and 51.2% specificity. Nevertheless, these sensitivities and specificity values can be adjusted along the ROC curve to optimize performance. AP, adenomatous polyp; ROC, receiver operating characteristics.
From the 876 participants, an un-blinded training set (n=584) and blinded testing set (n=292) were randomly established. The un-blinded training set, comprising 422 normal participants and 162 participants with adenomatous polyps, established the metabolite profile diagnostic for colonic adenomatous polyps. The blinded testing set, comprising 211 normal participants and 81 participants with adenomatous polyps, validated the metabolite profile diagnostic for colonic adenomatous polyps.
The demographics and clinical characteristics of the un-blinded training set and the blinded testing set are shown in Table 1. In the un-blinded training set, the average age for the adenoma group (59.1±0.6 years) is older than the normal group (55.7±0.4 years, P<0.001) and there are more males in the adenoma group than the normal group (59% vs. 43%, P<0.001). Although there are more participants with a positive family history of CRC in the normal group (66% vs. 56%, P=0.023), there are more smokers in the adenoma group (16% vs. 9%, P=0.021). In the blinded testing set, differences exist for gender and age between the normal and the adenoma group.
Table 1. Participant characteristics of the un-blinded training and blinded testing sets.
| Un-blinded training set | Normal (N=422) N (%) | Adenoma (N=162) N (%) | P value |
|---|---|---|---|
| Male:female | 180 (43):242 (57) | 95 (59):67 (41) | <0.001* |
| Average age (years±s.e.m.) | 55.7±0.4 | 59.1±0.6 | <0.001* |
| Family history of CRC | 261 (66) | 83 (56) | 0.023* |
| Family history of any cancer | 331 (86) | 114 (84) | 0.570 |
| Smoking | 37 (9) | 25 (16) | 0.021* |
| Change in bowel habit | 9 (2) | 0 (0) | 0.060 |
| Blinded testing set | Normal (N=211) N (%) | Adenoma (N=81) N (%) | P value |
|---|---|---|---|
| Male:female | 89 (42):122 (58) | 50 (62):31 (38) | 0.003* |
| Average age (years±s.e.m.) | 56.1±0.6 | 60.4±0.8 | <0.001* |
| Family history of CRC | 128 (64) | 44 (58) | 0.35 |
| Family history of any cancer | 163 (84) | 53 (75) | 0.082 |
| Smoking | 20 (10) | 14 (18) | 0.058 |
| Change in bowel habit | 5 (2) | 1 (1) | 0.537 |
CRC, colorectal cancer.
*P<0.05.
Colonic adenoma polyp characteristics
Of the adenomas removed during colonoscopy, 16% were rectal, 56% were from the distal colon, and 28% were from the proximal colon, and this distribution was similar in the un-blinded and blinded sets. In the un-blinded training set, 89% of the polyps had tubular while 11% had tubulovillous or villous histology. Similarly in the blinded testing set, 88% of the polyps had a tubular and 12% tubulovillous or villous histology.
Establishing the urine metabolomic diagnostic test for colonic adenomatous polyps using the un-blinded training set
The un-blinded training set was utilized to establish a urine metabolomic profile of metabolites that could be converted to a diagnostic test for colonic adenomatous polyps.
Separation of normal from adenoma using the urine metabolomic diagnostic test
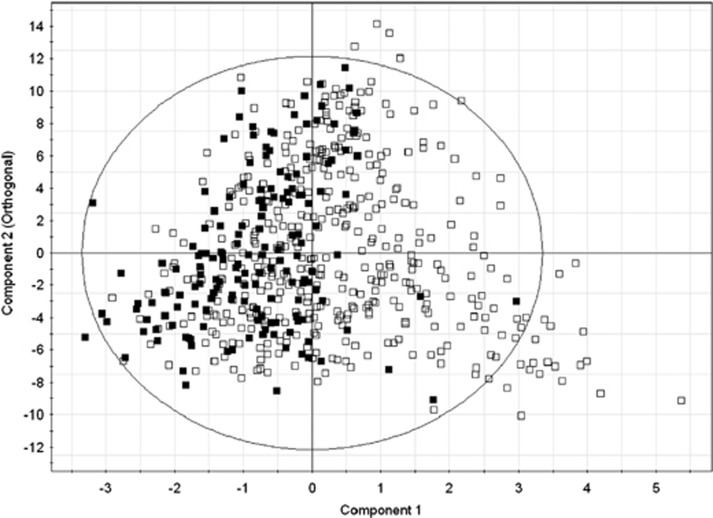
Using two-component separation with 69 urine metabolites, a supervised orthogonal partial least squares model was built with R2Y=0.142 (model's fit of data) and Q2=0.0463 (model's predictability of data in sevenfold cross-validation) to separate normal participants (those without adenomatous polyps) from those with adenomatous polyps. The orthogonal partial-least squares scatter plot (Figure 2) illustrates the separation of normal participants without adenomatous polyps (open squares) and participants with adenomas (solid squares). Internal validation of the partial least-squares discriminant analysis model of the same data, using permutation tests (n=100), showed that the R2Y and Q2 values for the model were higher than those for the randomly generated models; that is, the model is not over-fit.
Figure 2.
Orthogonal partial-least squares (OPLS) plot of results from the urine metabolomic diagnostic test for colonic adenomatous polyps. Using the urine metabolomic diagnostic test, participants without colonic adenomatous polyps (open squares) are compared with participants with adenomatous polyps (solid squares). The X axis represents component one of the partial least square analysis and the Y axis represents the orthogonal component. Clustering of the solid squares to the left of the zero line indicates the urine metabolomic diagnostic test is highly sensitive in determining the presence of colonic adenomatous polyps.
Diagnostic accuracy of the urine metabolomic diagnostic test to detect colonic adenomatous polyps
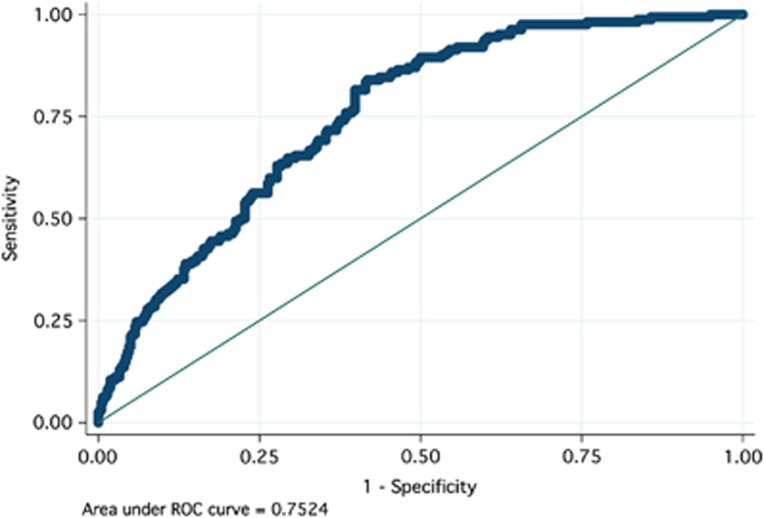
A spectrum of sensitivity and specificity for detection of adenomas from the urine metabolomic profile was calculated using the observed vs. predicted plot at varying threshold cutoff values. The receiver operating characteristics curve was generated (Figure 3) from the sensitivity and specificity values, and the area under the curve was calculated to be 0.752 (95% confidence interval (CI) 0.712–0.793). The clinically relevant pair of sensitivity and specificity values were 88.9% and 50.2%, respectively, corresponding to a cutoff of 0.25947.
Figure 3.
Receiver operating characteristic (ROC) curve of the urine metabolomic diagnostic test for colonic adenomatous polyps. The urine metabolomic diagnostic test was able to detect colonic adenomatous polyps with a sensitivity of 88.9% and a specificity of 50.2%. Area under the curve (AUC)=0.7524.
Unique urine metabolites important in separating participants with adenomatous polyps from normal
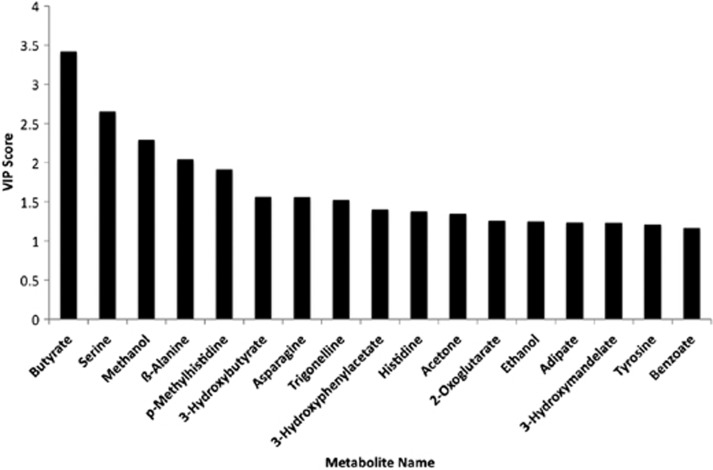
Figure 4 represents the varying importance of 17 metabolites that contribute most to separate participants with colonic adenomatous polyps from those without adenomatous polyps. Interestingly, butyrate, with its potential association to CRC was the metabolite that is most important in separating patients with adenoma from normal.
Figure 4.
Projection plot of the importance of individual metabolites from the urine metabolomic diagnostic test that separate persons without polyps from those with colonic adenomatous polyps. Metabolites analyzed by the urine metabolomic diagnostic test are listed along the X axis. The height of each bar relative to the Y axis, designated as the variable importance in the projection (VIP) Score, represents the importance of that individual metabolite in separating normal form colonic adenomatous polyps. The higher the bar the more important the metabolite is in separation of persons without adenomatous polyps from those with adenomatous polyps.
Validating the urine metabolomic diagnostic test for colonic adenomatous polyps using the blinded testing set and generating test characteristics
Having used the un-blinded training set to determine the metabolite profile and thus the urine metabolomic diagnostic test for colonic adenomatous polyps, we next validated the test using the blinded testing set. Diagnostic accuracies were calculated using the same cutoff value (0.259) used for the training set model. The validated sensitivity and specificity from the blinded testing set were 82.7% and 51.2%, respectively; nearly identical to those identified with the un-blinded training set. Using these values and a prevalence of adenoma as 27.7% (95% CI 24.8–30.8), the negative predictive value of the urine test is calculated to be 88.5% (95% CI 84.1–91.6), and positive predictive value to be 39.4%.(95% CI 35.1–43.8) The true and false positives and negatives at the specificity and sensitivity defined above are shown in Figure 1. The demographics and clinical characteristics of participants with adenomatous polyps and those without adenomatous polyps in the un-blinded training set and the blinded testing set were similar.
Advanced adenoma and increased risk populations
There were 70 patients that had advanced adenoma. The concentration of metabolites that separate patients with polyps from those without polyps in the advanced adenoma group was similar to those in the non-advanced adenoma group (Table 2). Similarly, we did not detect a significant difference in metabolomic profile of those patients with hyperplastic polyps vs. those with adenomatous polyps and average risk compared with high-risk patients (data not shown).
Table 2. Metabolites that separate individuals with polyps from those without polyps in persons with advanced adenoma and those without advanced adenoma.
|
Mean metabolite concentration (μM) |
|||
|---|---|---|---|
| Urine metabolites | Advanced adenoma | Non-advanced adenoma | P value |
| Butyrate | 0.14 | 0.09 | 0.717 |
| Serine | 47.33 | 51.99 | 0.758 |
| Methanol | 40.00 | 33.47 | 0.643 |
| β-Alanine | 3.18 | 1.94 | 0.600 |
| p-Methylhistidine | 213.85 | 240.09 | 0.681 |
| 3-Hydroxybutyrate | 7.04 | 26.74 | 0.325 |
| Asparagine | 48.28 | 40.37 | 0.401 |
| Trigonelline | 215.95 | 284.05 | 0.071 |
| 3-Hydroxyphenylacetate | 12.84 | 14.08 | 0.729 |
| Histidine | 206.41 | 254.40 | 0.223 |
| Acetone | 8.59 | 14.23 | 0.243 |
| 2-Oxoglutarate | 52.64 | 50.64 | 0.862 |
| Ethanol | 242.71 | 42.64 | 0.138 |
| Adipate | 0.57 | 8.63 | 0.120 |
| 3-Hydroxymandelate | 55.30 | 79.88 | 0.176 |
| Tyrosine | 73.28 | 77.03 | 0.748 |
| Benzoate | 16.43 | 44.31 | 0.485 |
Comparison of the urine metabolomic diagnostic test for colonic adenomatous polyps with commercially available fecal-based tests
The diagnostic accuracies of urine-based metabolomic diagnostic test for colonic adenomatous polyps were compared with the three fecal-based (one fecal-guaiac and two fecal-immune) tests. The sensitivity and specificity for each test are summarized in Table 3. The urine-based metabolomic diagnostic test outperformed the fecal guaiac-based Hemoccult II in sensitivity (82.7% vs. 2.5%, respectively) for colonic adenoma detection. The fecal immune-based tests had slightly higher sensitivities than the fecal guaiac-based test (7.6% for Hemoccult ICT and 11.9% for MagStream HemSp/HT) but were still inferior to the urine-based metabolomic diagnostic test for colonic adenomatous polyps. Nevertheless, at maximum sensitivity, the specificity of the urine metabolomic diagnostic test was not as high as those of the fecal-based tests.
Table 3. Diagnostic accuracies of the urine-based metabolomic diagnostic test compared with fecal-based tests in detecting colonic adenomatous polyps.
| Diagnostic test used to detect colonic adenomatous polyps | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Urine-based diagnostic tests | ||
| Urine metabolomics diagnostic test (urine at maximum sensitivity) | 82.7 | 51.2 |
| Urine metabolomics diagnostic test (specificity set at Hemoccult II level) | 4.9 | 99.5 |
| Urine metabolomics diagnostic test (specificity set at Hemoccult ICT level) | 11.1 | 96.8 |
| Urine metabolomics diagnostic test (specificity=MagStream HemSp/HT level) | 23.5 | 94.3 |
| Fecal-based diagnostic tests | ||
| Hemoccult II | 2.5 | 99.5 |
| Hemoccult ICT | 7.6 | 96.8 |
| MagStream HemSp/HT | 11.9 | 94.3 |
Threshold cutoffs for the urine test are presented to match the specificity values of the fecal-based tests.
As the urine metabolomic diagnostic test for colonic adenomatous polyps can be adapted for specificity and sensitivity, we assessed how the urine metabolomic test would function with a higher specificity cutoff. Thus, using the receiver operating characteristics curve, we set the specificity at a level comparable to those of the fecal-based tests. As shown in Table 3, when the specificity values for the urine-based metabolomic diagnostic test for colonic adenomatous polyps are matched to those of the fecal-based tests, the urine-based metabolomics test still outperformed and offered a higher sensitivity than the fecal-based tests.
DISCUSSION
This study examined the performance characteristics of a prototypic, proof-of-concept, spot urine test, using metabolomic technologies, in distinguishing individuals with colonic adenomatous polyps from those individuals without polyps. The un-blinded training set of 422 normal participants and 162 adenoma participants was used to build the orthogonal partial-least squares model, which was internally validated using permutation testing. Receiver operating curve (area under the curve=0.752) generated a clinically relevant sensitivity and specificity of 88.9% and 50.2%, respectively. The model was then validated using a blinded testing set of 292 urine samples; yielding a sensitivity and specificity of 82.7% and 51.2%, respectively, which mirrored the results from the training set. Furthermore, the sensitivity of the urine-based metabolomic test for detecting colonic adenomatous polyps was superior when compared with the commercially available fecal-based tests. Moreover, the negative predictive value of this urine test in detecting colonic adenomas is 88.5%, suggesting a potential future role for the test to be used as a screening tool to exclude adenomas. The positive predictive value in this prototype diagnostic test described in this study is only 39.4% and thus further metabolomic refinements to enhance this value will be required before the test can be fully integrated into population-based clinical care screening algorithms.
This study also compared one fecal guaiac and two fecal immune tests against both the urine metabolomic test and the reference standard colonoscopy. The performance accuracy of fecal tests is known to vary between significantly between manufactures and generally demonstrates less accuracy for adenomas than for CRC.16 Nevertheless, given the fecal tests selected for comparison, the urine metabolomic test had a higher sensitivity for adenomatous polyps than did the fecal tests. This relationship was also maintained when specificity for the urine and fecal tests were matched (Figure 4).
For population-based colon cancer screening programs that rely upon the non-invasive detection of pre-cancerous adenomatous polyps, a high test sensitivity is critical in order to accurately predict the presence or absence of a adenomatous polyp and limit false-negative testing. Balancing the importance of a high sensitivity, however, is the need for specificity to limit the number of false-positive detections. In this study, we presented the results reflecting the highest sensitivities for each of the tests (Table 3). However, as the urine-based metabolomic diagnostic test for colonic adenomatous polyps is a quantitative one, the model cutoff value can be transformed to generate a higher specificity at the expense of the sensitivity. This is in contrast to the fecal-based tests that are points along the receiver operating characteristics curve and cannot be adjusted. The urine metabolomics test can thus be tailored to the clinical requirements of the population-based screening program. Indeed, when the urine metabolomics diagnostic test was set to match the specificities of the fecal-based tests (Table 3), its sensitivity for detecting colonic adenomas was still higher than that of the fecal-based tests.
Adherence and population uptake of the fecal-based screening tests for colonic polyps is less than optimal and indeed is a weakness of all population-based colon cancer screening programs that rely on their positivity to trigger a colonoscopy.17 A single spot urine-based test that accurately detects colonic adenomatous polyps would markedly improve adherence in screening programs.
Interestingly, the metabolomic profile for adenoma and advanced adenoma were similar (Table 2). Although this may simply be a result of the relatively small numbers of advanced adenomas in the cohort, it is also interesting to speculate that the metabolite drivers, and perhaps the pathogenesis, for both classifications of adenomas is from a similar root.
The top 10 metabolites that separated the normal from those participants with colonic adenomatous polyps were butyrate, serine, methanol, β-alanine, π-methylhistidine, 3-hydroxybutyrate, asparagine, trigonelline, 3-hydroxyphenylacetate, and histidine. The majority of these metabolites tracked their origin to the intestinal microflora, implicating an association of the microbiome in the adenoma-cancer sequence. It can be hypothesized that the metabolite profile and thus the urine-based diagnostic test for colonic adenomatous polyps are not detecting the adenoma themselves, but instead the associated changes in the colonic microbiome. Indeed, recent publications in inflammatory bowel disease, cancer, and obesity have highlighted the significant role of the intestinal microbiota in these disorders.1, 18, 19
Butyrate was the metabolite that contributed the most to distinguishing the normal subjects from those with adenoma. Butyrate, a short-chain fatty acid generated by microbial fermentation of dietary fiber,20 has anti-inflammatory potential,21 affects the intestinal barrier,22 and has a role in satiety23 and oxidative stress.24 Butyrate has been shown in in vitro studies to increase apoptosis in both colon adenoma and cancer, thus contributing to protection against CRC.25 However, butyrate did not have the same chemopreventative effects in in vivo studies.26, 27
Serine, another metabolite more abundant in the normal group than in the adenoma group, is an amino acid derived from glycine that has a central role in cellular proliferation. Indeed, serine is an active component of the serine proteases, a group of enzymes that cleave peptides. Certain serine proteases act as tumor suppressors and promote angiogenesis, induce tumor cell migration, and enhance the invasive potential of pancreatic, breast, and lung cancer cells.28, 29, 30, 31, 32, 33 Serine is also involved in microbial metabolism, including that of Fusobacterium nucleatum,34 which has been implicated in CRC.2, 3 Similarly, methanol, which also contributed to the detection of adenoma, is mainly a product of microbial metabolism. The differences in its presence may reflect the differences in the microbiota of the two groups.34 The metabolism of β-alanine, π-methylhistidine, and histidine are closely associated to each other and with carnosine. Carnosine is an amino acid found in red meat that has a known protective effect against oxidative stress in intestinal epithelial cells.35 Overall, 4 of the top 10 metabolites likely represent products of microbial metabolism, a finding that emphasizes the developing association of the intestinal microbiome in the adenoma-CRC sequence.
This is the largest prospective study, with colonoscopy as the reference standard, to highlight the identification and use of metabolomics to distinguish between individuals with colonic adenomatous polyps and those without. Furthermore, this metabolomic diagnostic test offers a much higher sensitivity than do the standard fecal-based tests and a specificity that can be adjusted to minimize false positives. Preliminary follow-up studies by our group have also demonstrated that once colonic adenomatous polyps are removed, the metabolomic fingerprint reverts back to a “normal” state, implying that the metabolomic fingerprint is likely a reflection of the changes in human and microbial metabolism in the presence of adenoma(s) rather than an innate fingerprint.36
Future refinement and development will need to enhance the sensitivity and positive predictive value of this prototypic urine-based metabolomics diagnostic test and to confirm that its accuracy with advanced adenomas as well as non-advanced adenomas. Nevertheless, a highly specific and sensitive urine-based screening test for colonic adenomatous polyps will likely represent a paradigm shift in global CRC screening.
Study Highlights
Guarantor of the article: Richard N. Fedorak, MD.
Specific author contributions: H.W. co-designed and performed the majority of the experiments, as well as analyzed the data and wrote the first draft of the manuscript. V.T. provided technical assistance and participated in data analyses and manuscript drafting and review. C.W. and D.S. shared in the protocol development and edited the manuscript. R.N.F. co-developed the protocol and co-designed the experiments, oversaw all activities, and co-wrote and edited the manuscript.
Financial support: The authors received financial support from the Royal College of Physicians and Surgeons Clinical Investigator's Program, AHFMR Clinical Fellowship, Alberta Health Services, and the Centre of Excellence for Gastrointestinal Inflammation and Immunity Research (CEGIIR).
Potential competing interests: H.W. and R.N.F. are co-founders and majority shareholders and V.T. is a shareholder and employee in Metabolomic Technologies, Edmonton, Alberta, Canada. Metabolomic Technologies is commercializing the urine-based polyp detection diagnostic test. C.W. and D.S. are executive leads in the Alberta Colon Cancer Screening Program.
References
- Arthur JC, Jobin C. The struggle within: microbial influences on colorectal cancer. Inflamm Bowel Dis. 2011;17:396–409. doi: 10.1002/ibd.21354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22:299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–298. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen XJ, Rawls JF, Randall T, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1:138–147. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagnini C, Corleto VD, Mangoni ML, et al. Alteration of local microflora and alpha-definsins hyper-production in colonic adenoma mucosa. J Clin Gastroenterol. 2011;45:602–610. doi: 10.1097/MCG.0b013e31820abf29. [DOI] [PubMed] [Google Scholar]
- Claudino WM, Quattrone A, Biganzoli L, et al. Metabolomics: available results, current research projects in breast cancer, and future applications. J Clin Oncol. 2007;25:2840–2846. doi: 10.1200/JCO.2006.09.7550. [DOI] [PubMed] [Google Scholar]
- Patel NR, McPhail MJ, Shariff MI, et al. Biofluid metabonomics using (1)H NMR spectroscopy: the road to biomarker discovery in gastroenterology and hepatology. Expert Rev Gastroenterol Hepatol. 2012;6:239–251. doi: 10.1586/egh.12.1. [DOI] [PubMed] [Google Scholar]
- Yoshie T, Nishiumi S, Izumi Y, et al. Regulation of the metabolite profile by an APC gene mutation in colorectal cancer. Cancer Sci. 2012;103:1010–1021. doi: 10.1111/j.1349-7006.2012.02262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tso VK, Slupsky CM, et al. Metabolomics and detection of colorectal cancer in humans: a systematic review. Future Oncol. 2011;6:1395–1406. doi: 10.2217/fon.10.107. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Xie G, Chen T, et al. Distinct urinary metabolic profile of human colorectal cancer. J Proteome Res. 2012;11:1354–1363. doi: 10.1021/pr201001a. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhang P, Wang F, et al. An integrated proteomics and metabolomics approach for defining oncofetal biomarkers in the colorectal cancer. Ann Surg. 2012;255:720–730. doi: 10.1097/SLA.0b013e31824a9a8b. [DOI] [PubMed] [Google Scholar]
- Mal M, Koh PK, Cheah PY, et al. Metabotyping of human colorectal cancer using two-dimensional gas chromatography mass spectrometry. Anal Bioanal Chem. 2012;403:483–493. doi: 10.1007/s00216-012-5870-5. [DOI] [PubMed] [Google Scholar]
- Nishiumi S, Kobayashi T, Ikeda A, et al. A novel serum metabolomics-based diagnostic approach for colorectal cancer. PLoS One. 2012;7:e40459. doi: 10.1371/journal.pone.0040459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Feng B, Li X, et al. Urinary metabolic profiling of colorectal carcinoma based on online affinity solid phase extraction-high performance liquid chromatography and ultra performance liquid chromatography-mass spectrometry. Mol Biosyst. 2010;6:1947–1955. doi: 10.1039/c004994h. [DOI] [PubMed] [Google Scholar]
- Wong CK, Fedorak RN, Prosser CI, et al. The sensitivity and specificity of guaiac and immunochemical fecal occult blood tests for the detection of advanced colonic adenomas and cancer. Int J Colorectal Dis. 2012;27:1657–1664. doi: 10.1007/s00384-012-1518-3. [DOI] [PubMed] [Google Scholar]
- Burch JA, Soares-Weiser K, St, John DJ, et al. Diagnostic accuracy of faecal occult blood tests used in screening for colorectal cancer: a systematic review. J Med Screen. 2007;14:132–137. doi: 10.1258/096914107782066220. [DOI] [PubMed] [Google Scholar]
- Birkenfeld S, Belfer RG, Chared M, et al. Factors affecting compliance in faecal occult blood testing: a cluster randomized study of the faecal immunochemical test versus the guaiac faecal occult test. J Med Screen. 2011;18:135–141. doi: 10.1258/jms.2011.010147. [DOI] [PubMed] [Google Scholar]
- Cani PD, Delzenne NM, Amar J, et al. Role of gut microflora in the development of obesity and insulin resistance following high-fat diet feeding. Pathol Biol (Paris) 2008;56:305–309. doi: 10.1016/j.patbio.2007.09.008. [DOI] [PubMed] [Google Scholar]
- Takaishi H, Matsuki T, Nakazawa A, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol. 2008;298:463–472. doi: 10.1016/j.ijmm.2007.07.016. [DOI] [PubMed] [Google Scholar]
- McMillan L, Butcher SK, Pongracz J, et al. Opposing effects of butyrate and bile acids on apoptosis of human colon adenoma cells: differential activation of PKC and MAP kinases. Br J Cancer. 2003;88:748–753. doi: 10.1038/sj.bjc.6600793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segain JP, de la Bletiere DR, Bourreille A, et al. Butyrate inhibits inflammatory responses through NF kappa B inhibition: implications for Crohn's disease. Gut. 2000;47:397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnie IA, Dwarakanath AD, Taylor BA, et al. Colonic mucin synthesis is increased by soidum-butyrate. Gut. 1995;36:93–99. doi: 10.1136/gut.36.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbut C. Motor effects of short-chain fatty acids and lactate in the gastrointestinal tract. Proc Nutr Soc. 2003;62:95–99. doi: 10.1079/PNS2002213. [DOI] [PubMed] [Google Scholar]
- Rosignoli P, Fabiani R, De Bartolomeo A, et al. Protective activity of butyrate on hydrogen peroxide-induced DNA damage in isolated human colonocytes and HT29 tumour cells. Carcinogenesis. 2001;22:1675–1680. doi: 10.1093/carcin/22.10.1675. [DOI] [PubMed] [Google Scholar]
- Hague A, Manning AM, Hanlon KA, et al. Sodium-butyrate induces apoptosis in human colonic tumor-cell lines in a p53-independent pathway-implications for the possible role of dietary fiber in the prevention of large-bowel cancer. Int J Cancer. 1993;55:498–505. doi: 10.1002/ijc.2910550329. [DOI] [PubMed] [Google Scholar]
- Freeman HJ. Effects of differing concentrations of sodium butyrate on 1,2-dimethylhydrazine-induced rat intestinal neoplasia. Gastroenterology. 1986;91:596–602. doi: 10.1016/0016-5085(86)90628-1. [DOI] [PubMed] [Google Scholar]
- Zoran DL, Turner ND, Taddeo SS, et al. Wheat bran reduces tumor incidence in a rat model of colon cancer independent of effects on distal luminal butyrate concentrations. J Nutr. 1997;127:2217–2225. doi: 10.1093/jn/127.11.2217. [DOI] [PubMed] [Google Scholar]
- Bajou K, Noel A, Gerard RD, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4:923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- Bajou K, Peng H, Laug WE, et al. Plasminogen activator inhibitor-1 protects endothelial cells from FasL-mediated apoptosis. Cancer Cell. 2008;14:324–334. doi: 10.1016/j.ccr.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu GH, Shuman MA, Cohen RL. Coexpression of urokinase, urokinase receptor and PAI-1 is necessary for optimum invasiveness of cultured lung-cancer cells. Int J Cancer. 1995;60:501–506. doi: 10.1002/ijc.2910600413. [DOI] [PubMed] [Google Scholar]
- Buchholz M, Biebl A, Neessbe A, et al. SERPINE2 (protease nexin I) promotes extracellular matrix production and local invasion of pancreatic tumors in vivo. Cancer Res. 2003;63:4945–4951. [PubMed] [Google Scholar]
- Candia BJ, Hines WC, Heaphy CM, et al. Protease nexin-1 expression is altered in human breast cancer. Cancer Cell Int. 2006;6:16. doi: 10.1186/1475-2867-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SF, Dong QG, Yao M, et al. Establishment of an experimental human lung adenocarcinoma cell line SPC-A-1BM with high bone metastases potency by Tc-99m-MDP bone scintigraphy. Nucl Med Biol. 2009;36:313–321. doi: 10.1016/j.nucmedbio.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Kyoto Encyclopedia of Genes and Genomes (KEGG) 2011. Accessed November 2010 to January 2011; Available from http://www.genome.jp/kegg/ .
- Son DO, Satsu H, Kiso Y, et al. Characterization of carosine uptake and its physiological function in human intestinal epithelial Caco-2 cells. Biofactors. 2004;21:395–398. doi: 10.1002/biof.552210177. [DOI] [PubMed] [Google Scholar]
- Wang H, Gies N, Tso VK, et al. The highly accurate urine metabolomics diagnostic test for colonic adenoma reverts to normal following adenoma removal—abstract. Gastroenterology. 2012;142:S–771. [Google Scholar]