Abstract
Previous studies have shown that pharmacologic inhibition of poly (ADP-ribose) polymerase (PARP), a nuclear protein that is crucial in signaling single-strand DNA breaks, is synthetically lethal to cancer cells from patients with genetic deficiency in the DNA repair proteins BRCA1 and BRCA2. Herein, we demonstrate that depletion of the mitochondrial genome (mtDNA) in breast, prostate and thyroid transformed cells resulted in elevated steady-state cytosolic calcium concentration and activation of calcineurin/PI3-kinase/AKT signaling leading to upregulation of miR-1245 and the ubiquitin ligase Skp2, two potent negative regulators of the tumor suppressor protein BRCA2, thus resulting in BRCA2 protein depletion, severe reduction in homologous recombination (HR) and increased sensitivity to the PARP inhibitor rucaparib. Treatment of mtDNA-depleted cells with the PI3-kinase inhibitor LY294002, the calmodulin antagonist W-7, the calcineurin inhibitor FK506, the calcium chelator BAPTA-AM, or suppression of AKT activity by AKT small-interfering RNA (siRNA) enhanced BRCA2 protein levels as well as HR. Decreasing the intracellular calcium levels using BAPTA, or direct reconstitution of BRCA2 protein levels either by recombinant expression or by small molecule inhibition of both Skp2 and miR-1245 restored sensitivity to rucaparib to wild-type levels. Furthermore, by studying prostate tissue specimens from prostate carcinoma patients we found a direct correlation between the presence of mtDNA large deletions and loss of BRCA2 protein in vivo, suggesting that mtDNA status may serve as a marker to predict therapeutic efficacy to PARP inhibitors. In summary, our results uncover a novel mechanism by which mtDNA depletion restrains HR, and highlight the role of mtDNA in regulating sensitivity to PARP inhibitors in transformed cells.
Keywords: PARP inhibitors, mitochondrial DNA, BRCA2, calcium, cancer
Introduction
In recent years, poly (ADP-ribose) polymerase (PARP) inhibitors have emerged as a novel class of anticancer drugs that function through a mechanism known as synthetic lethality, whereby two defective genes or pathways with negligible effect on cell viability turn lethal when combined in the same cell.1 PARP-1 and -2 have an important role in signaling single-strand breaks2 and their inhibition results in accumulation of single-strand breaks, double-strand breaks (DSBs), stalled S-phase replication forks and apoptosis unless rescued by upstream homologous recombination (HR).3 Effective HR depends upon BRCA1 and BRCA2, whose major function is to complex with Rad51 to orchestrate DNA repair. They are encoded by the tumor suppressor genes BRCA1 and BRCA2 that, when mutated, result in familial predisposition to breast and ovarian cancer in women and prostate cancer in men.4 These neoplasias characteristically lack BRCA1 or BRCA2 activity and thus, upstream inhibition of PARP would result in cancer cell apoptosis. Indeed, cells that are deficient in BRCA1 or BRCA2 are about 1000-fold more sensitive to PARP inhibitors.5 This model of synthetic lethality by PARP inhibitors is being proven to be effective in clinical trials for treatment of cancers that result from inherited mutations in BRCA1 or BRCA2.6, 7 However, the potential value of these novel drugs in sporadic cancers has not yet been investigated.
Mitochondrial dysfunction has been implicated in tumorigenesis because of the vital role of mitochondria in energy production, regulation of apoptosis, nucleus-to-mitochondria and mitochondria-to-nucleus signal integration and a plethora of metabolic pathways.8, 9, 10, 11, 12, 13 Mitochondrial dysfunction leads to resistance to apoptosis,14, 15, 16, 17 promotion of metastasis15, 16, 18, 19 and chromosomal instability,20 and a number of genetic and metabolic mitochondrial abnormalities have been reported in cancer.9, 11 Mitochondria contain their own genome (mtDNA), a circular 16 569-bp molecule containing a regulatory region (the D-loop), which controls mtDNA replication and transcription, 13 protein-encoding genes, 22 tRNA and 2 rRNA. Mutations in the mtDNA have been reported in all cancers examined to date9, 17 although their functional effect is still unclear. In particular, mutations in the D-loop region can result in altered binding of nuclear proteins involved in mtDNA replication, thus promoting depletion of the mtDNA content.21 Actually, mtDNA depletion appears to be a common feature of a variety of cancer types.15, 16, 22, 23, 24 The purpose of the present study was to determine whether, and by what mechanism, mtDNA depletion or large deletions might cooperate with PARP inhibition to induce cell death in cancer cells.
Results
MtDNA depletion decreases HR and sensitizes cells to the PARP inhibitor AG014699
In this study, we used a panel of cell lines depleted of mtDNA by culturing cells in the presence of 100 ng/ml ethidium bromide for 40 days, essentially as described before by us and other groups.15, 16, 24, 25, 26, 27, 28 Ethidium bromide selectively inhibits mtDNA replication and, at the low levels used in this study, has no detectable effect on nuclear DNA replication or cell cycle. We have previously shown that mtDNA depletion promotes a migratory and anoikis-resistant phenotype in transformed cells.16 Here, we first tested whether mtDNA depletion may affect HR in wild-type and mtDNA-depleted [Rho(0)] prostate, breast and thyroid cell lines by analyzing the formation of γH2AX foci (markers of DSBs occurrence), and Rad51 foci (markers of DSBs repair), which arise spontaneously during normal DNA metabolism. As shown in Figure 1a, Rho(0) cells exhibited a significant decrease in spontaneous Rad51 foci formation in comparison with parent cells regardless of tissue derivation, suggesting that mtDNA-depleted cells may have defective HR. We also detected a slight increase in the number of spontaneous γH2AX foci in Rho(0) cells, consistently with previous studies.28, 29
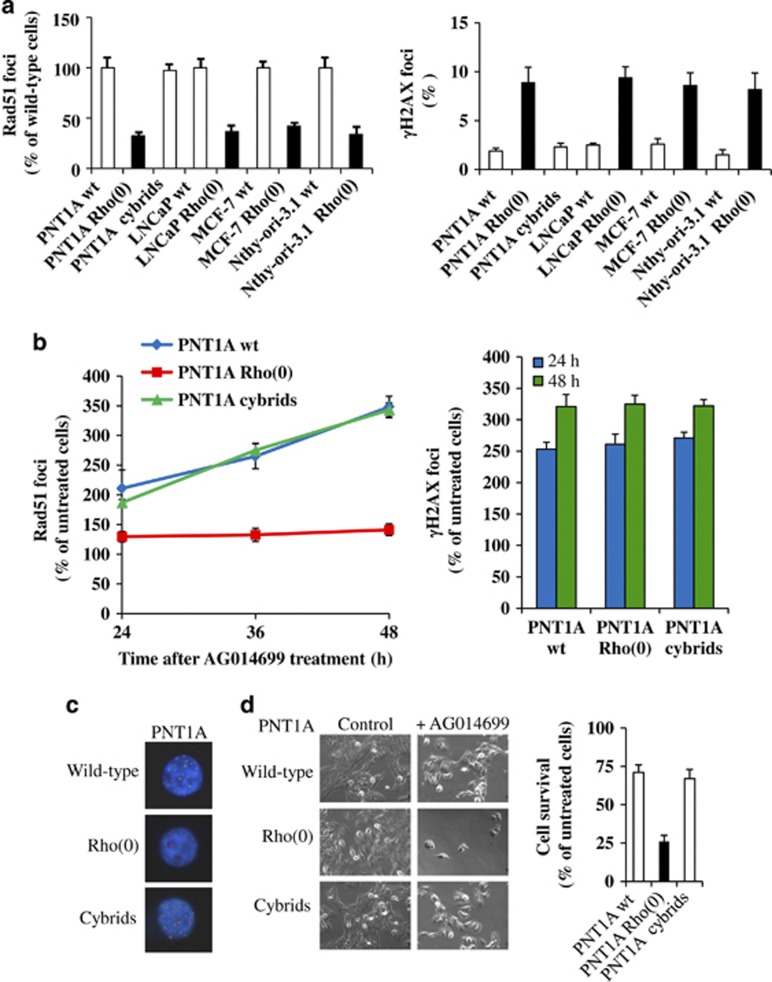
Figure 1.
MtDNA depletion decreases HR and sensitizes cells to AG014699. (a) Wild-type (wt), cybrids and Rho(0) cells were analyzed for spontaneous DSBs formation and repair by γH2AX and Rad51 immunofluorescence, respectively. The number of Rad51 foci was expressed as the percentage of wild-type cells. γH2AX foci were reported in percentage by counting the number of γH2AX-positive cells on a total of 100 cells in a randomly selected field. (b, c) Cells were treated with 10 μM AG014699 or solvent up to 48 h, before incubation with fluorescently labeled antibodies against γH2AX for DSBs induction (red foci) and Rad51 for DSBs undergoing repair (green foci). Graphs and bar chart summarize the results as the percentage of untreated control cells (b). Representative photographs are shown for PNT1A wild type, Rho(0) and cybrids at 24 h (c). (d) Wild-type and mtDNA-mutant cells treated with 10 μM AG014699 for 24 h were cultured in drug-free medium for 21 days, fixed and counted. Cell survival was calculated as the percentage of untreated controls. A representative experiment after 21 days is shown for PNT1A cells.
Because HR-deficient cells fail to form Rad51 foci in response to DNA damage, we then tested the effect of the potent PARP inhibitor AG01469930 on Rad51 and γH2AX foci formation in Rho(0) cells. As shown in Figures 1b and c, PARP inhibition with 10 μmol/l AG014699 for 24–48 h induced DSBs (γH2AX foci) at comparable levels in all cell lines, followed by a time-dependent increase in Rad51 repair foci in wild-type cells (3.5-fold increase at 48 h) but not in Rho(0) PNT1A cells, indicating that defective mtDNA may hinder nuclear DNA DSB repair by HR. A similar pattern was observed in the other Rho(0) cell lines (not shown). This peculiar behavior was not dependent on a different inhibition of PARP activity in wild-type and Rho(0) cells after AG014699 treatment because residual PARP activity was comparable among different cell types (Supplementary Figure 1). Of note, though we observed increased spontaneous γH2AX foci in Rho(0) cells (Figure 1a), induction of DSBs upon treatment with a DNA damaging agent, like AG014699, is such a brisk event that eventual differences in a γH2AX background are virtually undetectable. We then tested whether reduced HR repair after AG014699 treatment may affect survival of Rho(0) cells. As shown in Figure 1d, survival of mtDNA-depleted cells 21 days after AG014699 treatment was decreased by >50% as assessed by clonogenic assay (P<0.005). To prove a causal link between depletion of mtDNA and reduced HR after PARP inhibition, and rule out the possibility of a spurious behavior due to nuclear DNA damage, we repeated these experiments using PNT1A cytoplasmic hybrid cells (cybrids), in which the mtDNA of PNT1A Rho(0) cells was replenished by inducing cell membrane fusion with human platelets obtained from a healthy blood donor (PNT1A cybrids16). As shown in Figure 1, the abnormal phenotype was completely rescued in cybrids. Overall, these results indicate that mtDNA depletion may sensitize cells to PARP inhibitors by hindering HR.
MtDNA depletion reduces BRCA2 protein levels
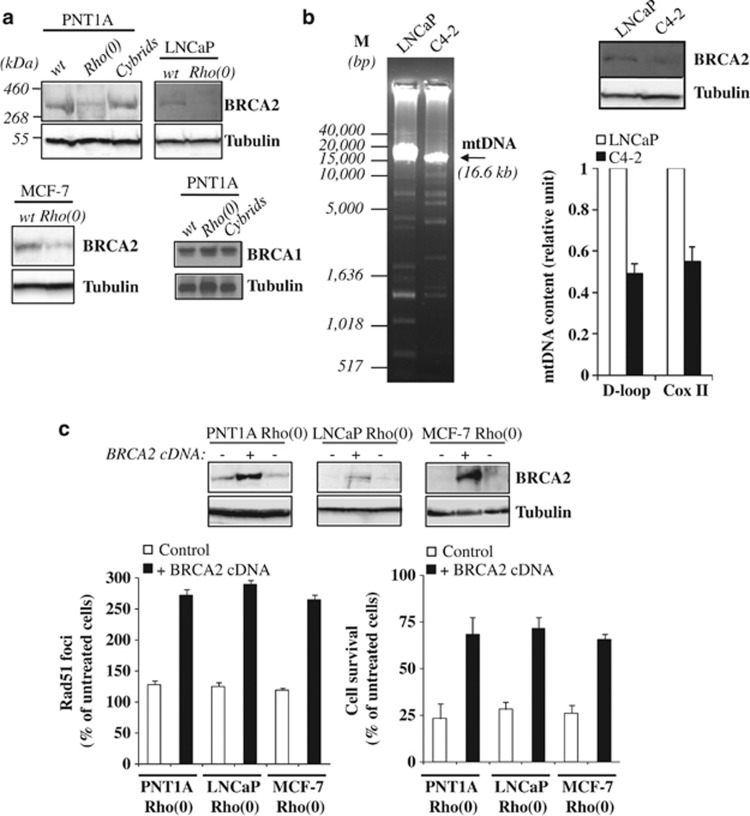
We then investigated whether the decreased formation of Rad51 foci in mtDNA-depleted cells after PARP inhibition resulted from changes in BRCA2 or BRCA1 levels, key components of the HR DNA repair pathway.31 As shown in Figure 2a, mtDNA-depleted cells exhibited >50% reduction in BRCA2 protein expression but no significant change in BRCA1 protein levels. We also tested C4-2 cells, an invasive androgen-independent cell line derived from low-grade LNCaP prostate carcinoma (PCa) cells with decreased mtDNA and harboring multiple mtDNA-deletion mutants (Figure 2b).15, 16, 24 As shown in Figure 2b, BRCA2 protein levels in C4-2 versus LNCaP wild-type cells replicate those of LNCaP Rho(0) cells.
Figure 2.
MtDNA depletion decreases BRCA2 protein expression. (a) Wild-type and mtDNA-depleted cells were assessed for BRCA2 and BRCA1 protein expression by western blotting. (b) Total DNA was extracted from LNCaP and C4-2 prostate carcinoma cells, and mtDNA was amplified by long PCR (left panel) or quantified by real-time PCR (right bar graph) using primers for D-loop or Cox II. At the top right panel, BRCA2 levels were analyzed by western blotting. (c) MtDNA-depleted cells were transiently transfected with BRCA2 cDNA or empty vector (control), treated with AG014699 for 48 h, and Rad51 foci (left panel) and cell survival (right panel) were measured by immunofluorescence and clonogenic assays, respectively. Data are expressed as the percentage of untreated controls. Representative blots of BRCA2 protein levels in transiently transfected cells are shown.
Subsequently, we expressed recombinant BRCA2 and tested the ability of cells to engage in HR DNA repair after PARP inhibition. As shown in Figure 2c, BRCA2 expression was able to rescue the abnormal phenotype by increasing Rad51 HR-repair foci and cell survival to wild-type levels. These data indicate that mtDNA-depleted cells become HR incompetent and die after PARP inhibition as a result of decreased BRCA2 levels.
BRCA2 protein translation and stability are reduced in mtDNA-depleted cells
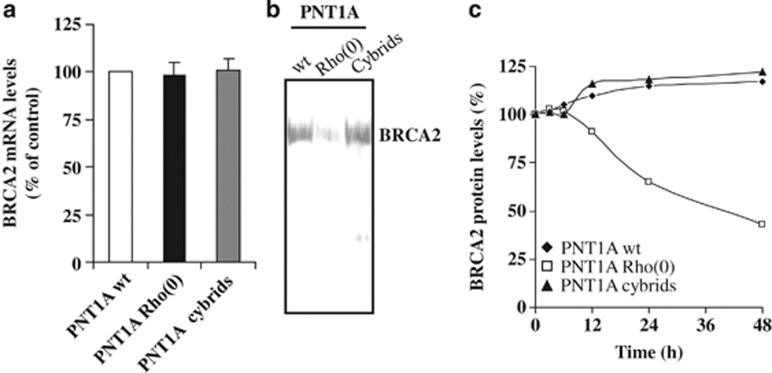
It has been previously reported that mtDNA depletion affects the expression of nuclear genes by modifying their mRNA levels.25, 26, 32 To investigate whether this mechanism was involved in the downregulation of BRCA2 in Rho(0) cells, we examined BRCA2 expression at the transcriptional, translational and post-translational level. As shown in Figure 3a, BRCA2 mRNA content in Rho(0) cells was similar to wild-type cells. However, the BRCA2 translational rate was reduced by 78% in PNT1A Rho(0) (Figure 3b) and a similar decrease was observed in the other Rho(0) cell lines (not shown). Subsequently, we assessed BRCA2 protein stability after cell exposure to the protein synthesis inhibitor cycloheximide (in the absence of rucaparib). While BRCA2 protein was stable up to 48 h in wild-type cells, its half-life decreased in PNT1A Rho(0) cells (Figure 3c), suggesting that BRCA2 levels are modulated by mtDNA depletion also through a post-translational mechanism.
Figure 3.
BRCA2 protein translation and stability are reduced in mtDNA-depleted cells. (a) BRCA2 mRNA levels were quantified by real-time RT–PCR and expressed as the percentage of wild-type cells. (b) Cells were metabolically labeled with 35S-methionine/cysteine, and proteins were immunoprecipitated with an antibody to BRCA2. 35S-labeled immunoprecipitated proteins were visualized by fluorography. (c) BRCA2 steady-state protein levels were determined by western blotting up to 48 h after cycloheximide addition (20 μg/ml) and reported as the percentage of BRCA2 protein levels at time 0.
MtDNA depletion promotes an increase in Skp2 protein and miR-1245 levels
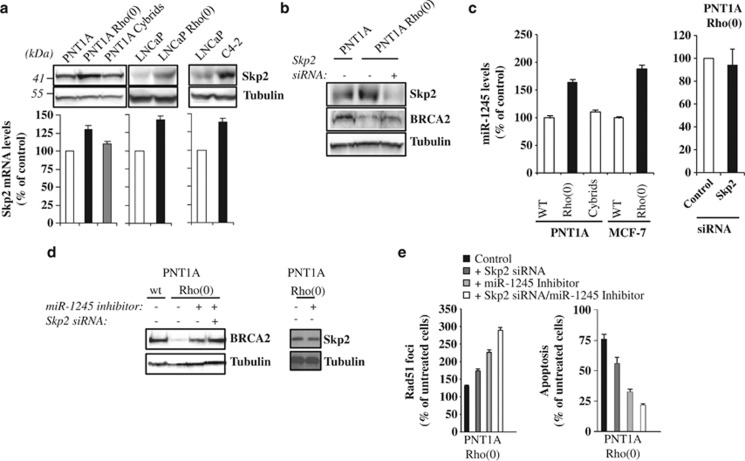
We next tested whether mtDNA mutations may promote BRCA2 protein instability by altering Skp2 levels, an E3 ubiquitin ligase able to bind and degrade BRCA2.33, 34 As shown in Figure 4a, mtDNA-depleted cells exhibited a significant increase in Skp2 protein and mRNA levels compared with wild-type controls. Reduction in Skp2 levels by small-interfering RNA (siRNA) in Rho(0) cells resulted in a partial recovery of BRCA2 protein levels (Figure 4b). Since we also detected a reduction in BRCA2 protein translation in mtDNA-mutated cells, we next investigated whether mtDNA mutations may affect the levels of miR-1245, a micro-RNA that suppresses BRCA2 translation by binding to the 3′UTR.35 MtDNA-mutated cells showed a 70–80% increase in miR-1245 levels (P<0.005; Figure 4c) compared with wild-type controls and reconstitution of the mtDNA pool in PNT1A Rho(0) restored miR-1245 levels to those of wild-type cells (PNT1A cybrids; Figure 4c). Suppression of Skp2 protein by specific siRNA did not alter miR-1245 levels (Figure 4c). Inhibition of miR-1245 by transfection of a specific inhibitory miRNA-1245 lentiviral system resulted in about 70% increase in BRCA2 protein levels in Rho(0) cells without affecting Skp2 levels (Figure 4d), and the combination of miR-1245 inhibitor with Skp2 siRNA completely restored BRCA2 protein levels (Figure 4d). Consistently, Skp2 siRNA and miR-1245 inhibitor increased the number of Rad51 foci and decreased cell apoptosis in Rho(0) cells treated with AG014699, with anti-miR-1245 proven to be more effective than Skp2 siRNA in restoring DNA repair capability (Figure 4e).
Figure 4.
MtDNA depletion increases Skp2 protein and miR-1245 levels. (a) Skp2 protein and mRNA levels were analyzed in wild-type and Rho(0) cells by western blotting and real-time RT–PCR, respectively. (b) Wild-type and Rho(0) cells were transiently transfected with Skp2 siRNA or non-specific siRNA (−) and, after 48 h, analyzed for Skp2 and BRCA2 protein levels by western blotting. (c) miR-1245 levels were monitored in wild-type (WT) and Rho(0) cells by real-time RT–PCR and expressed as the percentage of wild-type cells. On the right, PNT1A Rho(0) cells were transiently transfected with Skp2 siRNA for 48 h before analysis of miR-1245 levels. (d, e) Wild-type and Rho(0) cells were transiently transfected with a miR-1245 inhibitor and/or Skp2 siRNA or non-specific siRNA (−). Forty-eight hours after transfection, BRCA2 or Skp2 levels were analyzed by western blotting (d) or cells were treated with AG014699 for 48 h, and then Rad51 foci (e, left panel) and cell apoptosis (e, right panel) were measured. Data are expressed as the percentage of untreated controls.
Suppression of BRCA2 levels and increased sensitivity to PARP inhibitors under mitochondrial dysfunction is dependent on calcineurin/PI3-kinase/Akt pathway
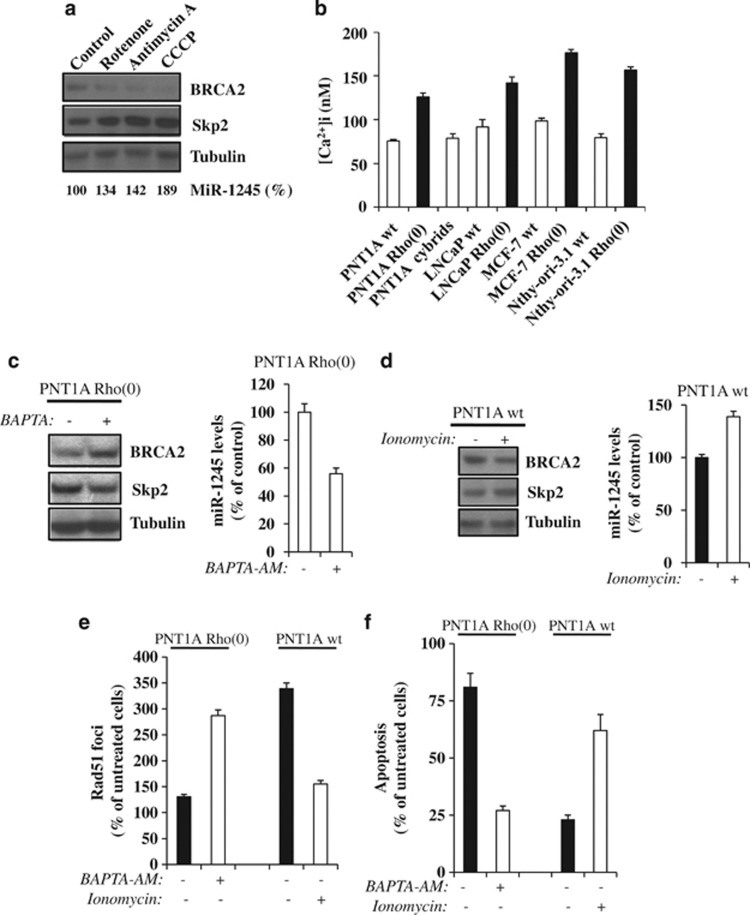
We next explored how mtDNA depletion may modulate the expression of the BRCA2 regulators Skp2 and miRNA-1245. To assess whether suppression of BRCA2 expression is induced by inhibition of mitochondrial respiration or collapse of mitochondrial membrane potential, PNT1A cells were cultured in the presence or absence of rotenone (Complex I inhibitor), Antimycin A (Complex III inhibitor) or CCCP (uncoupler). As shown in Figure 5a, rotenone, antimycin A and CCCP suppressed BRCA2 levels, but enhanced Skp2 and miR-1245 expression. Because inhibition of mitochondrial respiration or mitochondrial membrane depolarization is reported to increase the cytosolic calcium concentration ([Ca2+]i), we measured the amount of cytosolic calcium levels and found that mtDNA-mutated cells showed higher [Ca2+]i than control cells (Figure 5b), in agreement with previous studies.25, 26 All Rho(0) cell lines exhibited reduction in ATP levels (Supplementary Figure 2), consistently with a reduction in mitochondrial respiratory activity in mtDNA-deficient cells.
Figure 5.
Suppression of BRCA2 levels and increased sensitivity to PARP inhibitors under mitochondrial dysfunction involves increased cytosolic calcium concentration. (a) BRCA2, Skp2 and miR-1245 levels were evaluated in total protein extracts or miRNA preparations of PNT1A cells cultured 6 h in the presence or absence of rotenone (350 nM), antimycin A (150 ng/ml), CCCP (2 μM) or solvent alone (Control). (b) Steady-state [Ca2+]i was measured using Fura-2AM. (c) PNT1A Rho(0) cells were treated with the calcium chelator BAPTA-AM for 1 h, incubated in fresh medium for 24 h, harvested and analyzed for BRCA2 and Skp2 protein levels by immunoblotting, and for miR-1245 levels by real-time quantitative PCR. (d) PNT1A wild-type cells were treated with the calcium ionophore ionomycin for 3 h, incubated in fresh medium for 24 h, harvested and analyzed for BRCA2 and Skp2 protein levels by immunoblotting, and for miR-1245 levels by real-time quantitative PCR. (e, f) Cells were treated with BAPTA-AM or ionomycin as described in (b) and (c), then added with fresh medium containing 10 μM AG014699. After 48 h, Rad51 foci formation (e) and apoptosis (f) were measured.
To investigate whether changes in [Ca2+]i had a role in regulating BRCA2 levels and cell sensitivity to PARP inhibitors, mtDNA-mutated cells were exposed to BAPTA-AM, an intracellular calcium chelator. As shown in Figures 5c–f, treatment of PNT1A Rho(0) cells with BAPTA-AM resulted in increased BRCA2 protein levels, reduction in the BRCA2 negative regulators Skp2 and miR-1245, as well as decreased apoptosis and increased number of Rad51 foci after treatment with rucaparib. Consistently, transient treatment of wild-type PNT1A cells with the Ca2+ ionophore ionomycin, which resulted in about twofold elevation in steady-state [Ca2+]i, increased both Skp2 and miR-1245 levels, decreased BRCA2 protein and enhanced sensitivity to rucaparib-induced apoptosis.
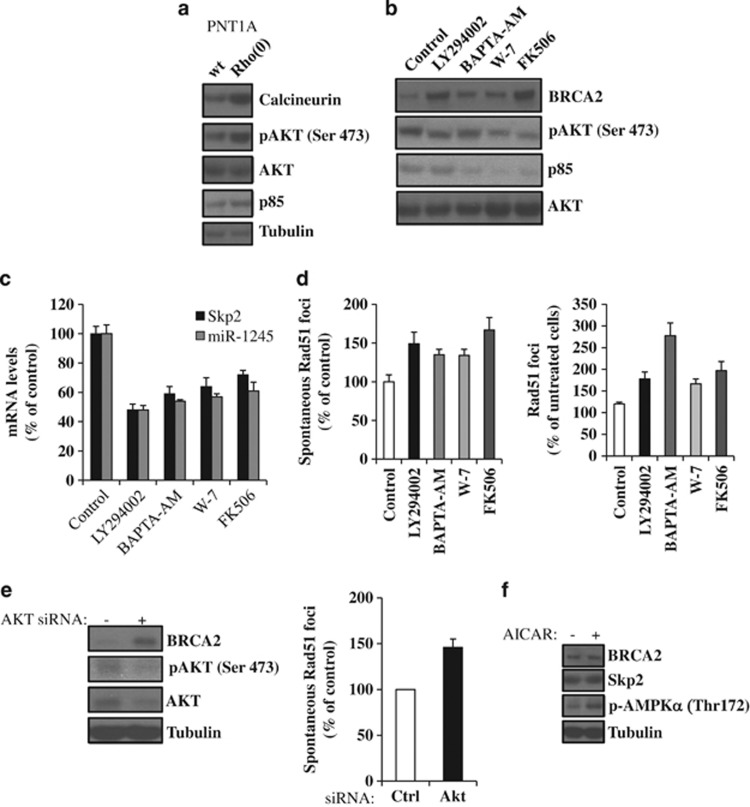
To investigate the involvement of calcium-dependent factors in the suppression of BRCA2 protein, we first tested the expression levels of calcineurin, a serine/threonine phosphatase controlled by calcium and calmodulin, previously shown to be activated in mtDNA-depleted cells.26, 36 Calmodulin was upregulated in PNT1A Rho(0) cells (Figure 6a), as well as pAKT and the PI3-kinase regulatory subunit p85, as previously reported.16 To assess the potential involvement of calcineurin and PI3-kinase/AKT in the regulation of BRCA2 levels in Rho(0) cells, we selectively inhibited PI3-kinase with LY294002, calmodulin with W-7 and calcineurin with FK506 in Rho(0) cells. All three inhibitors increased BRCA2 protein levels (Figure 6b) and suppressed Skp2 and miR-1245 levels (Figure 6c). Interestingly, inhibition of calmodulin or calcineurin also resulted in reduced AKT phosphorylation (Ser473) and p85 levels, suggesting that calmodulin/calcineurin are upstream activators of PI3-kinase/AKT in Rho(0) cells. Finally, all three inhibitors increased both spontaneous and rucaparib-induced Rad51 foci formation (Figure 6d).
Figure 6.
Suppression of BRCA2 levels and inhibition of HR under mitochondrial dysfunction is dependent on a calcineurin/PI3-kinase/Akt pathway. (a) Wild-type and Rho(0) PNT1A cells were assessed for calcineurin, pAKT, total AKT and p85 levels by immunoblotting. (b–d) PNT1A Rho(0) cells were incubated for 1 h with 10 μM LY294002, 2 μM BAPTA-AM, 25 μM W-7, 10 nM FK506 or solvent alone (control), incubated for 24 h in fresh medium and then assessed for BRCA2, pAKT, total AKT and p85 levels by immunoblotting (b), for Skp2 and miR-1245 RNA levels by quantitative RT–PCR (c), and for spontaneous and rucaparib-induced Rad51 foci by immunofluorescence (d). (e) PNT1A Rho(0) cells were transiently transfected with AKT siRNA or non-specific siRNA (−) and after 48 h assessed for BRCA2, pAKT and AKT protein levels by immunoblotting (left) and for spontaneous Rad51 foci formation by immunofluorescence (right). (f) PNT1A wild-type cells were incubated for 6 h with the AMPK activator AICAR (0.5mM), incubated in fresh medium for 24 h, then analyzed for BRCA2 and Skp2 protein levels by immunoblotting.
Since AKT is a downstream effector of calcium and PI3-kinase, and we have previously shown that its activation may suppress BRCA2 protein levels,33 we next tested the role of AKT in the modulation of BRCA2 levels and HR in Rho(0) cells. Suppression of AKT by siRNA enhanced BRCA2 protein (Figure 6e), downregulated Skp2 and miRNA-1245 (not shown) and increased HR in Rho(0) cells (Figure 6e). As a control, we also tested whether activation of the metabolic sensor AMPK may be involved in the regulation of BRCA2 protein. To this extent, we activated AMPK in wild-type PNT1A cells with AICAR and then tested BRCA2 and Skp2 protein levels, as well as AMPK activation (pAMPK (Thr172) levels). Neither BRCA2 nor Skp2 protein was affected by AMPK (Figure 6f). Overall, these results suggest that a calcineurin/PI3-kinase/AKT pathway modulates suppression of BRCA2 protein and HR in mtDNA-depleted cells.
Large deletions in mtDNA are associated with reduced BRCA2 in human PCa
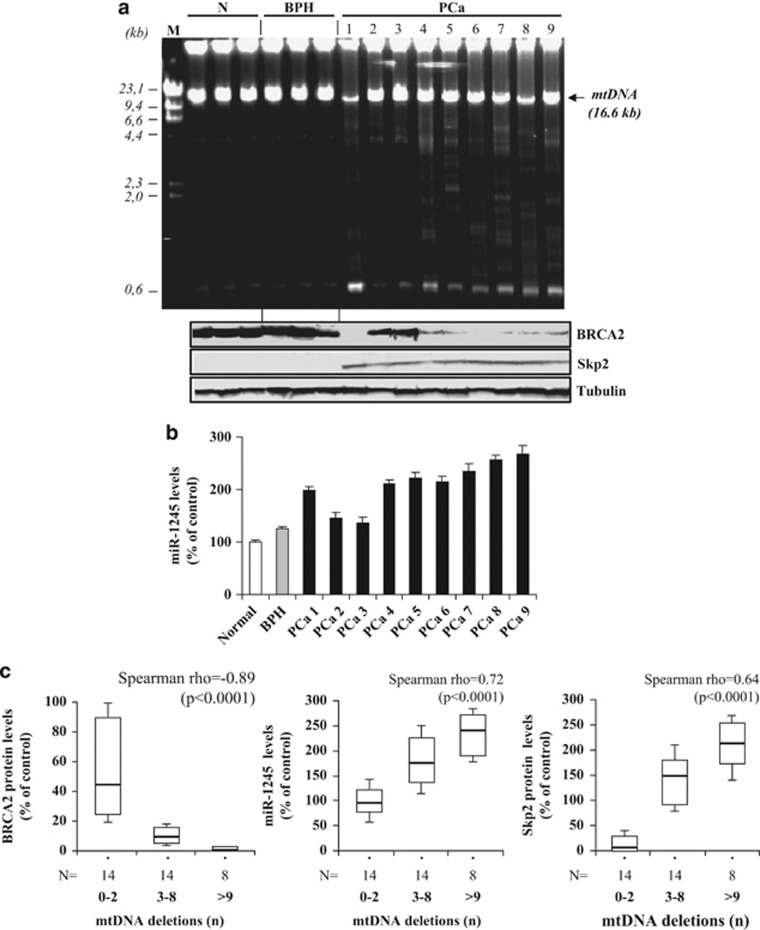
To assess the biologic relevance of our findings in vivo, we used long PCR to screen for large deletions of mtDNA in 12 benign and 24 PCa specimens. Large mtDNA deletions, absent or present in low number (⩽2) in benign prostates, were significantly increased in PCa but not in the corresponding mtDNA from peripheral blood lymphocytes, confirming their somatic origin (Figure 7a; Supplementary Figure 3). In the same specimens, BRCA2 and Skp2 protein levels were quantified by western blotting, and miR-1245 levels were measured by real-time RT–PCR. As shown in Figure 7a (bottom panel), BRCA2 protein was virtually undetectable in the majority of prostate cancers, confirming previous observations,34 whereas Skp2 was upregulated. Similarly, miR-1245 levels were significantly increased in PCa samples (Figure 7b). The number of mtDNA deletions was inversely correlated with BRCA2 protein levels (rho=−0.89) but directly correlated with both miR-1245 and Skp2 levels (rho=0.72 and rho=0.64, respectively) (Figure 7c). On the other hand, mtDNA point mutations in 15 PCa samples did not show any correlation with BRCA2 protein levels (Table 1). From the 15 samples, only 6 were found to harbor point mutations (40%), as follows: 4 had a single missense mutation affecting a mtDNA-encoded protein and 2 (PCa 7 and PCa 14) showed 2 concurrent point mutations affecting either a mtDNA-encoded protein or a tRNA. None of the PCa specimens analyzed in this study exhibited mutations in the D-loop region and 6/15 exhibited a significant reduction in mtDNA content. Moreover, 75% of the mtDNA point mutations in PCa were not somatic as they were also present in the patient's peripheral blood lymphocytes. Interestingly, mtDNA point mutations were also found in benign prostates, indicating that they may not be specific for prostate neoplastic transformation.
Figure 7.
MtDNA large deletions are associated with reduced BRCA2 protein in human prostate carcinoma (PCa). (a) Total DNA extracted from normal prostate (N; n=3), BPH (n=3) and PCa (n=9) specimens was subjected to long PCR analysis of the complete mtDNA. M=molecular weight marker. Bottom panel: Total protein extracts obtained from the same specimens used for PCR analysis were analyzed for BRCA2 and Skp2 protein expression by western blotting. Tubulin was used as a loading control. (b) Total miRNA extracted from samples used in (a) was subjected to real-time PCR to measure miR-1245 levels. Data are expressed as the percentage of normal prostate levels set at 100. (c) BRCA2 protein levels (left) were negatively correlated with the number of mtDNA deletions in prostate cancer specimens (P<0.0001, Spearman's correlation coefficient testing). MiR-1245 (middle) and Skp2 protein (right) levels were positively correlated with the number of mtDNA deletions in prostate cancer specimens (P<0.0001). Each box and the associated bars represent the values of the middle 50% and the range of data, respectively. N: number of specimens.
Table 1. mtDNA point mutations, mtDNA large deletions and BRCA2 protein levels in prostate carcinoma specimens.
|
Point mutations |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Base change | Amino-acid change | Gene | Het | PSIC | Somatic | Variability | ID | Large deletions (n) | mtDNA content (%) | BRCA2 protein (%) |
| BPH 1 | A15929G | — | MT-TT | − | n.a. | 0.002 | PA_XX_XX_183 | 0 | 83 | 45 | |
| BPH 2 | T12713C | I126T | MT-ND5 | − | 0.003 | n.a. | 0.000 | PA_XX_XX_188 | 2 | 98 | 61 |
| BPH 3 | G9300A | A32T | MT-COIII | − | 0.000 | n.a. | 0.019 | PA_XX_XX_184 | 0 | 100 | 62 |
| BPH 4 | T14501C | I58T | MT-ND6 | − | 0.514 | n.a. | 0.000 | PA_XX_XX_185 | 0 | 98 | 89 |
| BPH 5 | A12634G | I100V | MT-ND5 | − | 0.925 | n.a. | 0.010 | PA_XX_XX_186 | 1 | 100 | 84 |
| A13630G | T432A | MT-ND5 | − | 0.527 | n.a. | 0.006 | |||||
| T8668C | W48R | MT-ATP6 | − | 0.999 | n.a. | 0.004 | |||||
| BPH 6 | T5160C | S231P | MT-ND2 | − | 0.986 | n.a. | 0.000 | PA_XX_XX_187 | 2 | 91 | 100 |
| PCa 1 | None | PA_XX_XX_168 | 7 | 34 | 5 | ||||||
| PCa 2 | T11204C | F149L | MTND4 | − | 0.000 | Y | 0.020 | PA_XX_XX_169 | 6 | 98 | 5 |
| PCa 3 | None | PA_XX_XX_170 | 5 | 56 | 13 | ||||||
| PCa 4 | None | PA_XX_XX_171 | 5 | 89 | 11 | ||||||
| PCa 5 | C9739T | A178V | MTCOIII | − | 0.018 | N | 0.000 | PA_XX_XX_172 | 5 | 76 | 16 |
| PCa 6 | T11253C | I165T | MTND4 | − | 0.001 | N | 0.021 | PA_XX_XX_173 | 6 | 98 | 16 |
| PCa 7 | G15428A | D228N | MTCYTB | + | 0.999 | Y | 0.000 | PA_XX_XX_174 | 13 | 100 | 8 |
| T12631G | S99A | MTND5 | − | 0.003 | N | 0.001 | |||||
| PCa 8 | None | PA_XX_XX_175 | 9 | 39 | 0.5 | ||||||
| PCa 9 | None | PA_XX_XX_176 | 10 | 79 | 0.5 | ||||||
| PCa 10 | None | PA_XX_XX_177 | 11 | 82 | 0.5 | ||||||
| PCa 11 | None | PA_XX_XX_178 | 13 | 60 | 0.5 | ||||||
| PCa 12 | G9738A | A178T | MTCOIII | − | 0.219 | N | 0.007 | PA_XX_XX_179 | 14 | 65 | 0.5 |
| PCa 13 | None | PA_XX_XX_180 | 7 | 81 | 7 | ||||||
| PCa 14 | A14220C | L152P | MTND6 | − | 0.999 | N | 0.000 | PA_XX_XX_181 | 9 | 80 | 10 |
| G5849A | — | MT-TY | + | N | 0.000 | ||||||
| PCa 15 | None | PA_XX_XX_182 | 16 | 31 | 0.25 | ||||||
Abbreviations: BPH, benign prostate hyperplasia; Het, heteroplasmy; n.a., information not available; PCa, prostate carcinoma.
Discussion
Growing evidence suggests that mtDNA mutations may be associated with tumor development and progression in a variety of cancers, including breast, thyroid and prostate carcinoma.16, 18, 37, 38, 39, 40 However, the mechanisms involved are not completely understood. Herein, using cell models of mtDNA depletion, as well as tissue samples from patients affected with PCa, we have shed light on a novel biologic relationship between mitochondrial dysfunction and loss of BRCA2 protein in sporadic tumors, which conceivably leads to nuclear genomic instability, cumulative mutations and tumor progression, but also enhances cancer cell sensitivity to apoptosis induced by PARP inhibitors.
BRCA2 is a potent tumor suppressor protein mostly recognized as part of the hereditary breast and ovarian cancer syndrome. Indeed, inheritance of a single mutated allele results in genomic instability that predisposes to familial breast and ovarian cancer, as well as other malignancies, including prostate cancer in men.41, 42 Recent evidence demonstrated that its role in tumorigenesis goes well beyond the familial cancer syndrome. Indeed, loss of BRCA2 protein has been reported in sporadic cancers.34 While the role of mtDNA depletion in BRCA2 regulation had not been previously explored, reports in the literature have linked mtDNA mutations with chromosomal instability due to increased DSBs. For example, DSBs and chromosomal instability have been shown to occur spontaneously upon mtDNA depletion in cancer cells.28, 29 Our study provides strong evidence supporting a direct linkage between mtDNA large deletions/depletion and downregulation of BRCA2, with the resulting increase in DSBs and chromosomal instability that is observed in mtDNA-mutated tumors. Furthermore, we have confirmed these observations in PCa samples in vivo, where we have also shown that unlike mtDNA point mutations, only mtDNA large deletions would be able to affect BRCA2, as they occur in carcinoma but not in benign prostate hyperplasia (BPH) or normal prostate. MtDNA point mutations appear largely non-tissue specific as 75% of the point mutations detected in carcinoma samples were also present in the corresponding blood lymphocytes (germline origin of the mutations).
Since the resulting effect of mtDNA depletion on chromosomal instability appears to be at least in part mediated by BRCA2 downregulation, we hypothesized that mtDNA-depleted cells ought to have an increased sensitivity to PARP inhibitors, a new class of anticancer substances that rely on the synthetic lethality of PARP inhibition in the presence of a defective BRCA1-2 pathway, resulting in DSBs, DNA fragmentation and cell death. Indeed, we demonstrate that mtDNA depletion, a condition that recapitulates mtDNA large deletions,21 does enhance cell sensitivity to PARP inhibitors by hindering HR, as assessed by generation of Rad51 foci. Several prior studies including from our own group have shown that mtDNA depletion/mutations in low-grade cancer cells promote chromosomal instability, a metastatic cellular phenotype, and resistance to apoptosis.15, 16, 19, 29, 43 To our knowledge, this is the first time that mtDNA depletion is shown to be synthetically lethal with inhibition of a nuclear-encoded protein, that is, PARP. A direct clinical application of these findings is that some tumors featuring mtDNA large deletions, such as prostate carcinomas, would potentially benefit from therapeutic regimens encompassing PARP inhibitors.
Our investigations on the mechanisms whereby mtDNA mutations result in downregulation of BRCA2 protein unveiled two different pathways, which in combination were able to abate BRCA2 levels: a translational mechanism mediated by miR-1245 and a post-translational one mediated by the E3 ubiquitin ligase Skp2, both mechanisms dependent on a mitochondrial stress-induced increase in [Ca2+]i. The finding of increased [Ca2+]i in mtDNA-depleted cells is consistent with previous studies demonstrating a 2- to 3-fold increase in calcium levels after mtDNA depletion, associated with upregulation of anti-apoptotic and pro-invasive genes.25, 27 However, this is the first time that mitochondrial-stress related calcium signaling is shown to affect the expression of BRCA2 as well as of a miRNA. Furthermore, by studying prostate tissue specimens from PCa patients we found a direct correlation between the presence of mtDNA large deletions and increased Skp2 and miR-1245 levels in vivo. Of note, two PCa specimens reported in this study (PCa 2 and 3; Figure 7) exhibited Skp2 upregulation but only a mild suppression of BRCA2 levels compared with normal prostates. This may be ascribed to lack of increased miR-1245 levels in these tumors, due possibly to the low number of mtDNA large deletions and/or to other regulators of miR-1245 levels at present unknown, as well as to other factors, including a possible aberrant localization of Skp2 in the cytoplasm,44, 45 which could prevent or weaken the interaction with its substrates, including BRCA2.
The PI3-kinase/AKT pathway is known to modulate a multitude of cellular processes, including cell proliferation and survival, cancer metastasis and transcriptional regulation,46 and a rise in calcium concentration has been reported to activate AKT.47 We have previously shown that activation of PI3-kinase/AKT promotes resistance to anoikis in mtDNA-depleted cells.16 We demonstrate here that a calcium/calcineurin-dependent activation of the PI3-kinase/AKT pathway suppresses also BRCA2 protein and enhances both spontaneous and rucaparib-induced HR in Rho(0) cells. Our findings that calcium/calcineurin are modulators of AKT activity in mtDNA-depleted cells are consistent with a previous report.48 Moreover, a recent study has shown that AKT impairs DNA repair by HR and that AKT suppression restores DNA damage processing,49 thus supporting our conclusions that AKT activation plays an important role in mediating BRCA2 downregulation and the resulting accumulation of DSBs and chromosomal instability in mtDNA-depleted cells. However, we cannot exclude that other factors including the hypoxic-to-normoxic shift recently described in prostate and breast Rho(0) cells50 or activation of calcineurin-dependent IkBβ signaling described in mtDNA-depleted C2C12 myoblasts51 may participate in the regulation of BRCA2 levels and HR in mtDNA-depleted cells. Reactive oxygen species are unlikely modulators of BRCA2 levels as mtDNA-deficient cells have reduced superoxide levels.52 We also cannot exclude that other proteins, besides BRCA2, modulated by the E3 ubiquitin ligase Skp2 and/or by miR-1245 may contribute to reduced HR in mtDNA-deficient cells.
While biological and clinical evidence have demonstrated that cancers arising from inherited BRCA2-deficient tissues are sensitive to PARP inhibitors, we suggest here that sporadic tumors harboring decreased BRCA2 protein resulting from mtDNA mutations may too be responsive to PARP inhibitors. Our work also highlights the potential role for miR-1245 and Skp2 as possible therapeutic targets in alternative strategies aimed at preventing tumor progression.
Materials and methods
Cell lines
Normal immortalized prostate epithelial cells PNT1A, the PCa cell lines LNCaP and C4-2 and the breast carcinoma cell line MCF-7 were kept in culture as previously described.15, 16, 53 Nthy-ori-3.1 normal immortalized thyroid cells were obtained from ECACC (Salisbury, UK). MtDNA depletion by exposing cells to low concentration of ethidium bromide is a powerful strategy that has been widely used in the characterization of cellular processes that may be influenced by alterations in the mtDNA.25, 26, 28 In this study, we used mtDNA-less [Rho(0)] PNT1A cells, PNT1A cybrids (that is, PNT1A Rho(0) cells in which the mtDNA pool was restored by fusion with platelets) and LNCaP Rho(0) cells that have been previously described.16 MCF-7 Rho(0) and Nthy-ori-3.1 Rho(0) cells were generated by cellular exposure to ethidium bromide at low concentration (100 ng/ml) for 40 days and kept in culture in DMEM supplemented with 10% fetal bovine serum, 1 mM pyruvate and 50 μg/ml uridine.
RNA extraction and real-time quantitative PCR
Total miRNA was extracted using the mirVana miRNA Isolation Kit (Life Technologies, Monza, Italy) according to the manufacturer's instructions. cDNA was synthesized using the Taqman miRNA reverse transcription kit (Life Technologies), and the expression levels of miR-1245 were quantified using the miRNA-specific TaqMan MiRNA Assay Kit (Life Technologies). The expression of miR-1245 was defined based on the threshold cycle (Ct), and relative expression levels were calculated as 2−[(Ct of miR-1245)−(Ct of U6)] after normalization with reference to the expression of U6 small nuclear RNA.
To assess BRCA2 mRNA levels, total RNA was extracted from cell lines using the Trizol reagent (Life Technologies) and BRCA2 mRNA levels were assessed by real-time PCR using the QuantiFast SYBR Green RT-PCR kit (Qiagen, Milan, Italy). The primers used for BRCA2 are 5′-GCGCGGTTTTTGTCAGCTTA-3′ (forward) and 5′-TGGTCCTAAATCTGCTTTGTTGC-3′ (reverse). GAPDH was used as a reference control (primers: 5′-ACCACAGTCCATGCCATCAC-3′ forward, 5′-TCCACCACCCTGTTGCTGTA-3′ reverse).
Prostate tissue specimens
Primary cancer (Gleason grade 6–9) and BPH were obtained from the Tissue Bank of UT Southwestern Medical Center (Dallas, TX, USA). The Institutional Review Board approved the tissue procurement protocol in this study, and informed consent was obtained from all patients. Six normal prostate specimens from organ donors who died from 9 May 2003 to 26 November 2004 were obtained from the National Disease Research Interchange (Philadelphia, PA, USA). Blood samples collected from the Department of Urology at the time of prostate cancer patients' hospitalization/surgery have been used for mtDNA analysis to exclude individual/genotype variability in the mtDNA profile. Blood samples were not available for normal and BPH specimens.
γH2AX/Rad51 immunofluorescence
The histone protein H2AX becomes rapidly phosphorylated leading to accumulation of γH2AX at nascent DSBs, thus creating a focus where proteins involved in DNA repair and chromatin remodeling assemble. This amplification makes it possible to detect DSBs with an antibody to γH2AX, with the number of DSBs estimated from the number of foci.54 Rad51 is a crucial downstream protein involved in HR repair, which is relocalized within the nucleus in response to DNA damage to form distinct foci that can be visualized by immunofluorescence. These foci are thought to represent assemblies of proteins at these sites of HR repair. Therefore, quantification of Rad51 serves as a marker of HR function to distinguish between HR-proficient and HR-deficient cell lines.55 Analysis of γH2AX and Rad51 foci was performed by immunofluorescence essentially as described by Mukhopadhyay et al.56 For more details, see Supplementary Information.
Clonogenic cell survival assays and apoptosis
Cells were seeded into six-well plates for 24 h and added with medium containing 10 μM AG014699 for 24 h. Following harvesting, cells were seeded in drug-free medium into 90-mm Petri dishes (8000 per dish). Cells were fixed (methanol/acetic acid, 3:1) after 14–21 days. When distinct colonies were not formed, cells in monolayer were counted across three fields by two independent reviewers. Apoptosis was analyzed 48 h after addition of 10 μM AG014699 using the Cell Death Detection ELISAPlus according to the manufacturer's instructions (Roche Applied Science, Milan, Italy).
ATP levels
ATP levels were measured in cell extracts as previously described.57
Western blotting
Total protein extracts were analyzed by immunoblotting as described previously.16 Where indicated, cells were incubated with the protein synthesis inhibitor cycloheximide (20 μg/ml; Sigma, Milan, Italy) or vehicle alone (ethanol), before lysis. BRCA1 antibody (D20) was from Santa Cruz Biotechnology (Dallas, TX, USA). p-AMPKα antibody was from Cell Signaling (Danvers, MA, USA).
Determination of [Ca2+]i
[Ca2+]i was measured in live cells using the fluorescent intracellular calcium indicator Fura-2AM (Life Technologies) as previously described.58 For manipulation of intracellular calcium levels, cells were preincubated for 1 h in medium supplemented with 2 μM BAPTA-AM (Sigma) or for 3 h in medium supplemented with 0.5 μM ionomycin (Sigma), then washed and added with fresh medium containing 10 μM AG014699 for 48 h.
Detection of mtDNA large deletions by long PCR
High molecular weight DNA was isolated from cells or tissue specimens (50–100 mg) using the DNeasy Blood and Tissue kit (Qiagen, Valencia, CA, USA) as described by the manufacturer. The entire mtDNA genome (16.6 kb) was amplified using a single back-to-back primer set as previously described.16
Analysis of mtDNA content
The mtDNA content was analyzed by real-time PCR using specific primers for the D-loop and cytochrome oxidase II (Cox II) mtDNA regions as previously described.15
Mitochondrial DNA sequencing and analysis
The entire mtDNA sequence of tumor and hyperplastic samples was obtained with the MitoAll re-sequencing kit (Applera, Foster City, CA, USA) and analyzed with the SeqScape2.5 software (Applied Biosystems, Foster City, CA, USA) as previously described.39 Prediction of pathogenic potential of missense mutations was performed with PolyPhen259 as previously described.39, 60 Variability was estimated as previously described,39 from public database HmtDB.61
Translation assay
BRCA2 translation rate was assessed using [35S]protein labeling mix and the amount of BRCA2 nascent protein analyzed by immunoprecipitation as previously described.62
Transient transfection
Transient transfections were performed as previously described.16 Rad51 and Skp2 siRNAs were from Santa Cruz Biotechnologies. Inhibitory hsa-miR-1245 miRNA/microRNA Lentivector was transfected according to the manufacturer's protocol (Applied Biological Materials Inc, Richmond, Canada).
Statistical analysis
Differences were compared among normal prostate, BPH and cancer specimens using the Mann–Whitney U-test and Fisher's exact text, with similar results. The relationship of BRCA2, Skp2 and miR-1245 with the number of mtDNA large deletions was evaluated using the Spearman correlation coefficient. The ANOVA test was used for experiments with cell lines.
Acknowledgments
This work was supported by grants from the Italian Ministry of Economy and Finance to the CNR-Project ‘FaReBio di Qualita', MIUR Merit RBNE08YFN3_005 and PRIN 2009R8LJPS_002 (to LM), from the United States Army Grant W81XWH-10-1-0176 (to J-TH), from FIRB ‘Futuro in Ricerca' J31J10000040001 to GG and partially from Fondazione Umberto Veronesi. FG is supported by an AIRC biennial fellowship ‘Maria Antonietta Carluccio'.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis).
Supplementary Material
References
- Kaelin WG., Jr The concept of synthetic lethality in the context of anticancer therapy. Nat Rev Cancer. 2005;5:689–698. doi: 10.1038/nrc1691. [DOI] [PubMed] [Google Scholar]
- Dantzer F, de La Rubia G, Menissier-De Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is impaired in mammalian cells lacking Poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39:7559–7569. doi: 10.1021/bi0003442. [DOI] [PubMed] [Google Scholar]
- Schultz N, Lopez E, Saleh-Gohari N, Helleday T. Poly(ADP-ribose) polymerase (PARP-1) has a controlling role in homologous recombination. Nucleic Acids Res. 2003;31:4959–4964. doi: 10.1093/nar/gkg703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Filippo J, Sung P, Klein H. Mechanism of eukaryotic homologous recombination. Annu Rev Biochem. 2008;77:229–257. doi: 10.1146/annurev.biochem.77.061306.125255. [DOI] [PubMed] [Google Scholar]
- Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–251. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- Tutt A, Robson M, Garber JE, Domchek SM, Audeh MW, Weitzel JN, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- Singh KK, Russell J, Sigala B, Zhang Y, Williams J, Keshav KF. Mitochondrial DNA determines the cellular response to cancer therapeutic agents. Oncogene. 1999;18:6641–6646. doi: 10.1038/sj.onc.1203056. [DOI] [PubMed] [Google Scholar]
- Modica-Napolitano JS, Kulawiec M, Singh KK. Mitochondria and human cancer. Curr Mol Med. 2007;7:121–131. doi: 10.2174/156652407779940495. [DOI] [PubMed] [Google Scholar]
- Penta JS, Johnson FM, Wachsman JT, Copeland WC. Mitochondrial DNA in human malignancy. Mutat Res. 2001;488:119–133. doi: 10.1016/s1383-5742(01)00053-9. [DOI] [PubMed] [Google Scholar]
- Wallace DC. Mitochondria and cancer. Nat Rev Cancer. 2012;12:685–698. doi: 10.1038/nrc3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Dasgupta S, Sidransky D. Mitochondrial subversion in cancer. Cancer Prev Res (Phila) 2011;4:638–654. doi: 10.1158/1940-6207.CAPR-10-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon EA, Dimauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet. 2012;13:878–890. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicano H, Xu RH, Du M, Feng L, Sasaki R, Carew JS, et al. Mitochondrial respiration defects in cancer cells cause activation of Akt survival pathway through a redox-mediated mechanism. J Cell Biol. 2006;175:913–923. doi: 10.1083/jcb.200512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro L, Arbini AA, Marra E, Greco M. Mitochondrial DNA depletion reduces PARP-1 levels and promotes progression of the neoplastic phenotype in prostate carcinoma. Cell Oncol. 2008;30:307–322. doi: 10.3233/CLO-2008-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro L, Arbini AA, Yao JL, di Sant'Agnese PA, Marra E, Greco M. Mitochondrial DNA depletion in prostate epithelial cells promotes anoikis resistance and invasion through activation of PI3K/Akt2. Cell Death Differ. 2009;16:571–583. doi: 10.1038/cdd.2008.178. [DOI] [PubMed] [Google Scholar]
- Ohta S. Contribution of somatic mutations in the mitochondrial genome to the development of cancer and tolerance against anticancer drugs. Oncogene. 2006;25:4768–4776. doi: 10.1038/sj.onc.1209602. [DOI] [PubMed] [Google Scholar]
- Imanishi H, Hattori K, Wada R, Ishikawa K, Fukuda S, Takenaga K, et al. Mitochondrial DNA mutations regulate metastasis of human breast cancer cells. PLoS One. 2011;6:e23401. doi: 10.1371/journal.pone.0023401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K, Takenaga K, Akimoto M, Koshikawa N, Yamaguchi A, Imanishi H, et al. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320:661–664. doi: 10.1126/science.1156906. [DOI] [PubMed] [Google Scholar]
- Desler C, Munch-Petersen B, Stevnsner T, Matsui S, Kulawiec M, Singh KK, et al. Mitochondria as determinant of nucleotide pools and chromosomal stability. Mutat Res. 2007;625:112–124. doi: 10.1016/j.mrfmmm.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Higuchi M. Regulation of mitochondrial DNA content and cancer. Mitochondrion. 2007;7:53–57. doi: 10.1016/j.mito.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Yin PH, Lin JC, Wu CC, Chen CY, Wu CW, et al. Mitochondrial genome instability and mtDNA depletion in human cancers. Ann NY Acad Sci. 2005;1042:109–122. doi: 10.1196/annals.1338.011. [DOI] [PubMed] [Google Scholar]
- Wu CW, Yin PH, Hung WY, Li AF, Li SH, Chi CW, et al. Mitochondrial DNA mutations and mitochondrial DNA depletion in gastric cancer. Genes Chromosomes Cancer. 2005;44:19–28. doi: 10.1002/gcc.20213. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Kudo T, Suzuki S, Evans TT, Sasaki R, Wada Y, et al. Mitochondrial DNA determines androgen dependence in prostate cancer cell lines. Oncogene. 2006;25:1437–1445. doi: 10.1038/sj.onc.1209190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas G, Adebanjo OA, Freedman BD, Anandatheerthavarada HK, Vijayasarathy C, Zaidi M, et al. Retrograde Ca2+ signaling in C2C12 skeletal myocytes in response to mitochondrial genetic and metabolic stress: a novel mode of inter-organelle crosstalk. EMBO J. 1999;18:522–533. doi: 10.1093/emboj/18.3.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuthan G, Biswas G, Ananadatheerthavarada HK, Vijayasarathy C, Shephard HM, Avadhani NG. Mitochondrial stress-induced calcium signaling, phenotypic changes and invasive behavior in human lung carcinoma A549 cells. Oncogene. 2002;21:7839–7849. doi: 10.1038/sj.onc.1205983. [DOI] [PubMed] [Google Scholar]
- Amuthan G, Biswas G, Zhang SY, Klein-Szanto A, Vijayasarathy C, Avadhani NG. Mitochondria-to-nucleus stress signaling induces phenotypic changes, tumor progression and cell invasion. EMBO J. 2001;20:1910–1920. doi: 10.1093/emboj/20.8.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh KK, Kulawiec M, Still I, Desouki MM, Geradts J, Matsui S. Inter-genomic cross talk between mitochondria and the nucleus plays an important role in tumorigenesis. Gene. 2005;354:140–146. doi: 10.1016/j.gene.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kulawiec M, Safina A, Desouki MM, Still I, Matsui S, Bakin A, et al. Tumorigenic transformation of human breast epithelial cells induced by mitochondrial DNA depletion. Cancer Biol Ther. 2008;7:1732–1743. doi: 10.4161/cbt.7.11.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew Y, Mulligan EA, Vong WT, Thomas HD, Kahn S, Kyle S, et al. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. J Natl Cancer Inst. 2011;103:334–346. doi: 10.1093/jnci/djq509. [DOI] [PubMed] [Google Scholar]
- Davies AA, Masson JY, McIlwraith MJ, Stasiak AZ, Stasiak A, Venkitaraman AR, et al. Role of BRCA2 in control of the RAD51 recombination and DNA repair protein. Mol Cell. 2001;7:273–282. doi: 10.1016/s1097-2765(01)00175-7. [DOI] [PubMed] [Google Scholar]
- Butow RA, Avadhani NG. Mitochondrial signaling: the retrograde response. Mol Cell. 2004;14:1–15. doi: 10.1016/s1097-2765(04)00179-0. [DOI] [PubMed] [Google Scholar]
- Moro L, Arbini AA, Marra E, Greco M. Up-regulation of Skp2 after prostate cancer cell adhesion to basement membranes results in BRCA2 degradation and cell proliferation. J Biol Chem. 2006;281:22100–22107. doi: 10.1074/jbc.M604636200. [DOI] [PubMed] [Google Scholar]
- Arbini AA, Greco M, Yao JL, Bourne P, Marra E, Hsieh JT, et al. Skp2 overexpression is associated with loss of BRCA2 protein in human prostate cancer. Am J Pathol. 2011;178:2367–2376. doi: 10.1016/j.ajpath.2011.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Dai T, Xie Y, Wang C, Lin C, Wu Z, et al. Up-regulation of miR-1245 by c-myc targets BRCA2 and impairs DNA repair. J Mol Cell Biol. 2012;4:108–117. doi: 10.1093/jmcb/mjr046. [DOI] [PubMed] [Google Scholar]
- Biswas G, Tang W, Sondheimer N, Guha M, Bansal S, Avadhani NG. A distinctive physiological role for IkappaBbeta in the propagation of mitochondrial respiratory stress signaling. J Biol Chem. 2008;283:12586–12594. doi: 10.1074/jbc.M710481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros JA, Baumann AK, Ruiz-Pesini E, Amin MB, Sun CQ, Hall J, et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc Natl Acad Sci USA. 2005;102:719–724. doi: 10.1073/pnas.0408894102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparre G, Porcelli AM, Bonora E, Pennisi LF, Toller M, Iommarini L, et al. Disruptive mitochondrial DNA mutations in complex I subunits are markers of oncocytic phenotype in thyroid tumors. Proc Natl Acad Sci USA. 2007;104:9001–9006. doi: 10.1073/pnas.0703056104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra F, Kurelac I, Cormio A, Zuntini R, Amato LB, Ceccarelli C, et al. Placing mitochondrial DNA mutations within the progression model of type I endometrial carcinoma. Hum Mol Genet. 2011;20:2394–2405. doi: 10.1093/hmg/ddr146. [DOI] [PubMed] [Google Scholar]
- Giannattasio S, Guaragnella N, Arbini AA, Moro L. Stress-related mitochondrial components and mitochondrial genome as targets of anticancer therapy. Chem Biol Drug Des. 2013;81:102–112. doi: 10.1111/cbdd.12057. [DOI] [PubMed] [Google Scholar]
- Wooster R, Weber BL. Breast and ovarian cancer. N Engl J Med. 2003;348:2339–2347. doi: 10.1056/NEJMra012284. [DOI] [PubMed] [Google Scholar]
- Sundararajan S, Ahmed A, Goodman OB., Jr The relevance of BRCA genetics to prostate cancer pathogenesis and treatment. Clin Adv Hematol Oncol. 2011;9:748–755. [PubMed] [Google Scholar]
- Dasgupta S, Hoque MO, Upadhyay S, Sidransky D. Mitochondrial cytochrome B gene mutation promotes tumor growth in bladder cancer. Cancer Res. 2008;68:700–706. doi: 10.1158/0008-5472.CAN-07-5532. [DOI] [PubMed] [Google Scholar]
- Lin HK, Wang G, Chen Z, Teruya-Feldstein J, Liu Y, Chan CH, et al. Phosphorylation-dependent regulation of cytosolic localization and oncogenic function of Skp2 by Akt/PKB. Nat Cell Biol. 2009;11:420–432. doi: 10.1038/ncb1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao D, Inuzuka H, Tseng A, Chin RY, Toker A, Wei W. Phosphorylation by Akt1 promotes cytoplasmic localization of Skp2 and impairs APCCdh1-mediated Skp2 destruction. Nat Cell Biol. 2009;11:397–408. doi: 10.1038/ncb1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog. 2012;17:69–95. doi: 10.1615/critrevoncog.v17.i1.60. [DOI] [PubMed] [Google Scholar]
- Deb TB, Coticchia CM, Dickson RB. Calmodulin-mediated activation of Akt regulates survival of c-Myc-overexpressing mouse mammary carcinoma cells. J Biol Chem. 2004;279:38903–38911. doi: 10.1074/jbc.M405314200. [DOI] [PubMed] [Google Scholar]
- Guha M, Fang JK, Monks R, Birnbaum MJ, Avadhani NG. Activation of Akt is essential for the propagation of mitochondrial respiratory stress signaling and activation of the transcriptional coactivator heterogeneous ribonucleoprotein A2. Mol Biol Cell. 2010;21:3578–3589. doi: 10.1091/mbc.E10-03-0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu N, Hegarat N, Black EJ, Scott MT, Hochegger H, Gillespie DA. Akt/PKB suppresses DNA damage processing and checkpoint activation in late G2. J Cell Biol. 2010;190:297–305. doi: 10.1083/jcb.201003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook CC, Kim A, Terao S, Gotoh A, Higuchi M. Consumption of oxygen: a mitochondrial-generated progression signal of advanced cancer. Cell Death Dis. 2012;3:e258. doi: 10.1038/cddis.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Chowdhury AR, Guha M, Huang L, Van Winkle T, Rustgi AK, et al. Silencing of IkBbeta mRNA causes disruption of mitochondrial retrograde signaling and suppression of tumor growth in vivo. Carcinogenesis. 2012;33:1762–1768. doi: 10.1093/carcin/bgs190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Manna SK, Sasaki R, Aggarwal BB. Regulation of the activation of nuclear factor kappaB by mitochondrial respiratory function: evidence for the reactive oxygen species-dependent and -independent pathways. Antioxid Redox Signal. 2002;4:945–955. doi: 10.1089/152308602762197489. [DOI] [PubMed] [Google Scholar]
- Moro L, Arbini AA, Marra E, Greco M. Down-regulation of BRCA2 expression by collagen type I promotes prostate cancer cell proliferation. J Biol Chem. 2005;280:22482–22491. doi: 10.1074/jbc.M414091200. [DOI] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol. 2000;10:886–895. doi: 10.1016/s0960-9822(00)00610-2. [DOI] [PubMed] [Google Scholar]
- Lee SA, Roques C, Magwood AC, Masson JY, Baker MD. Recovery of deficient homologous recombination in Brca2-depleted mouse cells by wild-type Rad51 expression. DNA Repair (Amst) 2009;8:170–181. doi: 10.1016/j.dnarep.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, Elattar A, Cerbinskaite A, Wilkinson SJ, Drew Y, Kyle S, et al. Development of a functional assay for homologous recombination status in primary cultures of epithelial ovarian tumor and correlation with sensitivity to poly(ADP-ribose) polymerase inhibitors. Clin Cancer Res. 2010;16:2344–2351. doi: 10.1158/1078-0432.CCR-09-2758. [DOI] [PubMed] [Google Scholar]
- Moro L, Arbini AA, Hsieh JT, Ford J, Simpson ER, Hajibeigi A, et al. Aromatase deficiency inhibits the permeability transition in mouse liver mitochondria. Endocrinology. 2010;151:1643–1652. doi: 10.1210/en.2009-1450. [DOI] [PubMed] [Google Scholar]
- Vacca RA, Moro L, Petragallo VA, Greco M, Fontana F, Passarella S. The irradiation of hepatocytes with He-Ne laser causes an increase of cytosolic free calcium concentration and an increase of cell membrane potential, correlated with it, both increases taking place in an oscillatory manner. Biochem Mol Biol Int. 1997;43:1005–1014. doi: 10.1080/15216549700204821. [DOI] [PubMed] [Google Scholar]
- Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra F, Perrone AM, Kurelac I, Santini D, Ceccarelli C, Cricca M, et al. Mitochondrial DNA mutation in serous ovarian cancer: implications for mitochondria-coded genes in chemoresistance. J Clin Oncol. 2012;30:e373–e378. doi: 10.1200/JCO.2012.43.5933. [DOI] [PubMed] [Google Scholar]
- Rubino F, Piredda R, Calabrese FM, Simone D, Lang M, Calabrese C, et al. HmtDB, a genomic resource for mitochondrion-based human variability studies. Nucleic Acids Res. 2012;40:D1150–D1159. doi: 10.1093/nar/gkr1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro L, Perlino E, Marra E, Languino LR, Greco M. Regulation of beta1C and beta1A integrin expression in prostate carcinoma cells. J Biol Chem. 2004;279:1692–1702. doi: 10.1074/jbc.M307857200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.