Abstract
Agents targeting the PI3K/mTOR signaling axis have shown promise in early-phase clinical trials and are currently being studied in later stages of clinical development in multiple indications. Experience with other targeted agents suggests that clinical responses may be short-lived because of acquired resistance to therapy. Here, we report preclinical modeling of acquired resistance in a HER2-positive, PIK3CA mutant breast cancer cell line, KPL-4. We identified a heretofore-unreported mechanism of resistance, specifically high-level amplification of the mutant allele of PIK3CA, which resulted in a marked upregulation of PI3K signaling, enabling resistant cells to regain proliferative capacity at clinically relevant concentrations of the PI3K inhibitor, GDC-0941. We show that knockdown of the amplified PIK3CA mutant allele in these cells by small interfering RNA restored pathway signaling and sensitivity to PI3K inhibition at levels comparable to parental cells. These novel preclinical findings suggest that, in addition to assessment of other previously reported mechanisms of resistance, evaluation of PI3K copy number variation should be integrated into the exploratory analysis of biopsies obtained at disease progression.
Keywords: breast cancer, biomarkers, PI3K, resistance, amplification
Introduction
Somatic activation of the PI3K pathway is common in cancer, suggesting widespread potential for agents targeting this pathway in the management of various cancers. Inhibitors designed to target p110α, mTOR or AKT, as well as dual PI3K/mTOR inhibitors, are in various stages of clinical development.1 mTOR is a clinically validated target in renal cell carcinoma2 and hormone receptor-positive breast cancer,3 and early clinical trials of PI3K or dual PI3K/mTOR inhibitors have shown promise in a number of malignancies.4 However, experience with other successful targeted agents suggests that clinical resistance is likely to variably reduce the durability of clinical benefit.5 As with other targeted agents, understanding the diversity of mechanisms that give rise to PI3K inhibitor resistance through preclinical modeling is likely to help guide clinical hypothesis testing. Ultimately, understanding clinical mechanisms of resistance can provide the rationale for therapeutic combinations, sequencing or alternative therapies in these settings that can overcome resistance mechanisms. For example, preclinical modeling of acquired resistance to BRAF and MEK inhibitors has provided the rationale for clinical testing of combination therapy involving BRAF and MEK inhibitors.6, 7
Previous preclinical studies of acquired resistance to PI3K inhibitors have identified several mechanisms, many of which center on activation of the Myc oncogene. For example, studies in a mouse mammary tumor model engineered to express an activated PIK3CA allele (H1047R) demonstrated that activation of the Myc oncogene rendered these tumors resistant to selective PI3K inhibitors, independent of the PI3K pathway.8 A chemical genetic screen identified Myc and Notch pathway activation as mechanisms of resistance to PI3K inhibitors in breast cancer cell lines.9 A third study of acquired resistance in genetically defined mammary epithelial cells also identified Myc amplification as a resistance mechanism to the dual PI3K/mTOR inhibitor, BEZ-235;10 the same study also demonstrated that amplification of the downstream effector, eIF4E elicited similar effects, conferring resistance to pharmacological inhibition of PI3K and mTOR.10 In addition, overexpression of the kinases RSK3 and RSK4 has also been shown to confer resistance to PI3K inhibitors via attenuation of apoptotic effects and upregulation of protein translation.11
Remarkably, studies of resistance to PI3K inhibitors have not identified mechanisms acting at, or near the level of, the target itself. This contrasts with drug targets such as BRAF, MEK, BCR-ABL or EGFR, where mutations or genomic amplification of the target itself lead to preclinical and clinical drug resistance to the targeted agent, either by blocking compound binding or by increasing intrinsic kinase activity, or in some cases, both.5 Scanning mutagenesis screens have identified a second site mutation within PIK3CA that does confer modest resistance to PI3K inhibition but surprisingly, found that engineered ‘gatekeeper' mutations in PIK3CA failed to confer resistance.12
Here, we sought to understand mechanisms of acquired resistance to PI3K inhibition in PIK3CA mutant KPL-4 cells by selecting pools and single cell clones that were able to grow in the presence of high concentrations (>1 μM) of the selective PI3K inhibitor, GDC-0941. Genome-wide copy number analyses revealed high-level amplification of the PIK3CA locus. Analysis of mutant and wild-type alleles by quantative PCR and deep sequencing revealed that amplification specifically affected only the mutant H1047R allele. Functional studies showed that knockdown of amplified PIK3CA in these cells restored pathway signaling and sensitivity to PI3K inhibition to levels comparable to parental cells. Our results suggest a novel mechanism of resistance involving amplification of an activating mutant PIK3CA allele in breast cancer cells and may thus be a clinically relevant way in which breast cancer cells, and possibly other cell types, evade PI3K inhibition.
Results
KPL-4 PR cells show specific resistance to inhibition along the HER2/PI3K axis
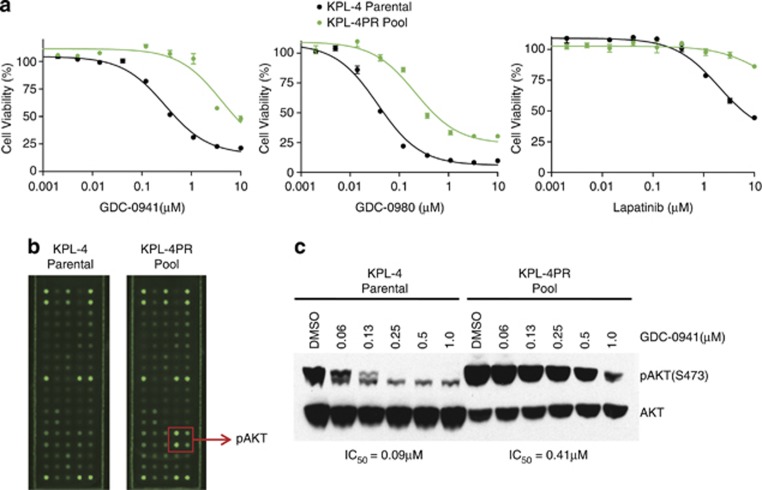
We set out to model resistance to a PI3K inhibitor, GDC-0941, currently in clinical development using a breast carcinoma cell line, KPL-4, which harbors HER2 amplification and an activating PIK3CA mutation. This cell line has previously been shown to be particularly sensitive to the selective PI3K inhibitor GDC-0941 in vitro and in vivo.13 Resistant derivatives of KPL-4 were selected by culturing in gradually increasing concentrations of GDC-0941 until a pool of cells was isolated that was able to grow at a concentration of 1 μM drug. The resulting pool, termed KPL-4PR for ‘PI3K inhibitor resistance', showed in vitro resistance to GDC-0941 in cell viability assays, evidenced by a 15-fold shift in half-maximal inhibitory concentration (IC50) relative to parental cells in an adenosine triphosphate-based cell viability assay (Figure 1a). KPL-4PR cells showed pathway cross-resistance to the dual PI3K/mTOR inhibitor GDC-0980 and also were resistant to upstream inhibition by the dual HER2/HER3 inhibitor lapatinib, as well as a more selective PI3K inhibitor (GDC-0032) that shows strong isoform selectivity for PI3Kα over PI3Kβ14 (Figure 1a, Supplementary Figure S1A). Resistance was specific to PI3K-/HER2-targeted inhibitors, as we found that KPL-4PR cells showed sensitivity comparable to parental cells to MEK and EGFR inhibitors, a proteasome inhibitor and chemotherapeutics such as doxorubicin (Supplementary Figure S1A and data not shown). Because resistant pools can show marked heterogeneity, we isolated two clonal derivatives of KPL-4PR, termed KPL-4PR.5 and KPL-4PR.18, and showed that they exhibited a similar spectrum of resistance to PI3K pathway inhibitors (Supplementary Figure S1B). The KPL-4PR clones were also largely refractory to the inhibitory effects of GDC-0941 on doubling time when compared with parental cells (Supplementary Figure S2), confirming resistance through an independent assay methodology. The clones stably maintained resistance in the absence of PI3K inhibitor for up to 20 passages, suggesting a stable genetic mechanism (data not shown).
Figure 1.
(a) Adenosine triphosphate-based cell viability experiments comparing sensitivity of KPL-4 parental (black) and KPL-4PR pool cells (green) to the selective PI3K inhibitor GDC-0941, the dual PI3K/mTOR inhibitor GDC-0980 and the dual HER2/EGFR inhibitor lapatinib. (b) Phospho-protein arrays probed with lysates from KPL-4 parental and KPL-4PR cells showing elevated phosphorylation of two phospho-sites on AKT in KPL-4PR cells. (c) Western blot of lysates from cells treated with increasing concentrations of GDC-0941, showing elevated basal pAKT in KPL-4PR cells and a shift in the IC50 for AKT inhibition in KPL-4PR cells.
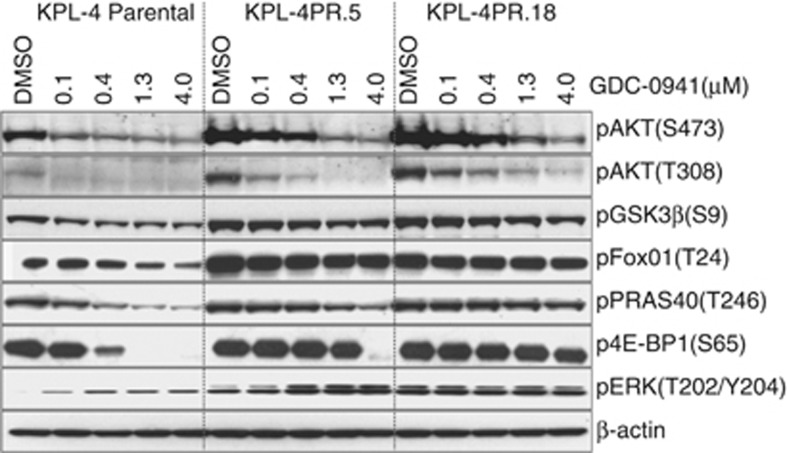
To investigate whether KPL-4PR cells showed activation of specific signaling pathways, protein lysates were analyzed using a fluorescence-based phospho-protein array that enables simultaneous detection of 28 receptor tyrosine kinases and 11 intracellular signaling nodes when phosphorylated at tyrosine or other residues. The complete list of analytes is shown in (Supplementary Figure S3). The only epitopes that showed significant differences between parental and KPL-4PR cells were pAKT(S473) and pAKT(T308), which were 6.6-fold and 2.5-fold higher in KPL-4PR cells, respectively (Figure 1b). In addition to higher baseline levels, dose response studies and densitometric quantitation showed that substantially more GDC-0941 was required for half-maximal pAKT inhibition in KPL-4PR cells compared with parental cells, with IC50 values of 0.41 μM versus 0.09 μM, respectively (Figure 1c). We extended this analysis to the resistant clones and showed by immunoblotting that they were broadly resistant to the inhibitory effects of PI3K inhibition on multiple pathway nodes, including pAKT(S473), pAKT(T308), pPRAS40, p4EB-P1, FOXO1 and pGSK-3β (Figure 2).
Figure 2.
Western blot analysis of lysates from KPL-4 parental cells and resistant clones KPL-4PR.5 and KPL-4PR.18 treated with increasing doses of GDC-0941 and immunoblotted for signaling pathway components.
Genome-wide copy number profiling reveals copy number gains at chromosome 3q and focal amplification of mutant PIK3CA in KPL-4PR cells
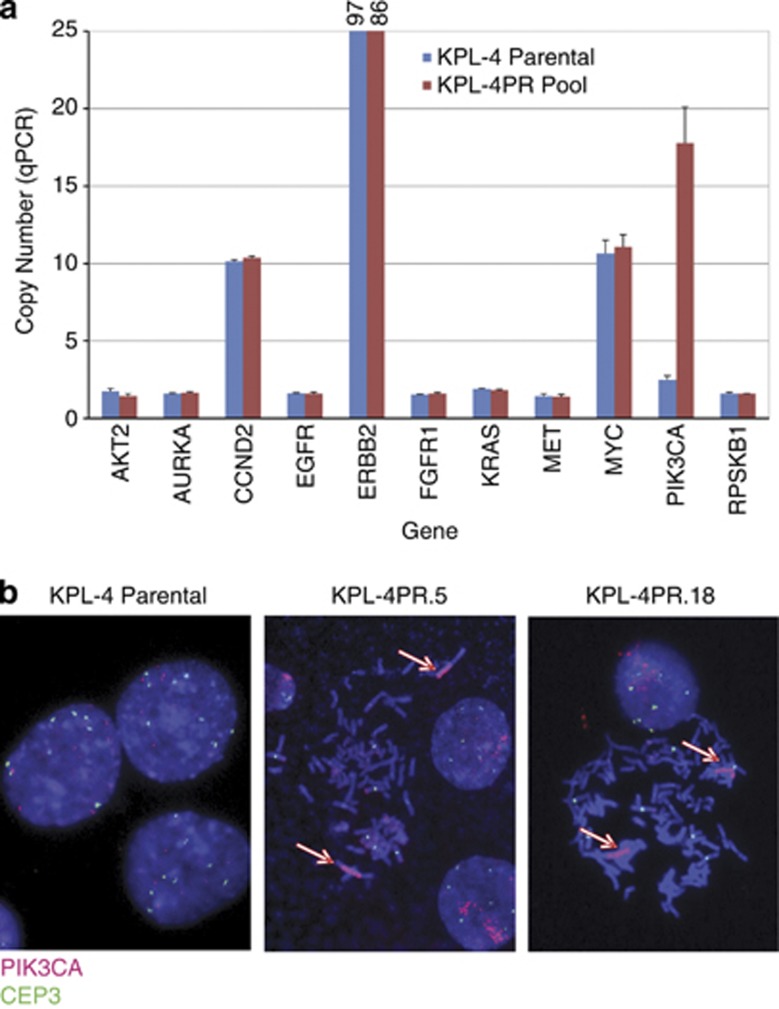
The KPL-4PR resistant pool was profiled using molecular inversion probe (MIP) arrays. We identified a prominent amplification event in the region of chromosome 3q26 that harbors the PIK3CA locus (Supplementary Figure S4). We did not detect amplification of other PI3K isoforms or pathway-related candidate genes in KPL-4PR cells in this analysis (data not shown). To confirm specific amplification of the PIK3CA gene, a panel of quantative PCR assays that detect copy number alterations in PIK3CA and other cancer-related genes was assessed using genomic DNA from parental and KPL-4PR cells. This assay revealed that KPL-4PR cells contained >15 copies of PIK3CA, far in excess of the parental cells (Figure 3a). Other loci examined showed equivalent copy numbers between parental and KPL-4PR cells. Notably, equivalent levels of Myc amplification are seen between parental and resistant cells (Figure 3a), suggesting Myc is not a driver of resistance in KPL-4PR cells. Similar results were obtained for KPL-PR.5 and KPL-4PR.18 (Table 1). Comparison of threshold cycle (Ct) values from the wild-type and mutant assays suggested that most of the additional copies harbored the activating allele. Ct values in the clones were markedly lower for the mutant allele compared with wild type, and the wild-type allele was undetectable or barely detectable (Ct of 37.5 or 40) (Table 1). To confirm this selective enrichment of the mutant allele at the sequence level, as well as to rule out second site mutations that could confer resistance, we performed next-generation sequencing of the entire PIK3CA-coding sequence. This analysis revealed no other alterations aside from the original mutation that results in the H1047R amino-acid alteration and showed that KPL-PR.5 and KPL-4PR.18 clones harbored the mutant allele at a frequency of 92% and 95%, respectively, compared with 44% in the parental line (Table 1). To visualize the copy number gains at a cellular level, a previously described fluorescence in situ hybridization assay15 was used to assess PIK3CA copy number and chromosome 3 centromeric copies in parental cells and the KPL-4PR.5 and KPL-4PR.18 clones. This analysis showed that parental cells have evidence of polysomy or duplication of chromosome 3, with six copies of PIK3CA but an overall ratio of PIK3CA/CEP3 of 1.0 (Figure 3b, Table 1). In contrast, close to 100% of cells derived from KPL-4PR.5 and KPL-4PR.18 showed evidence of high-level gene amplification, with PIK3CA copy numbers from 38 and 77 copies per cell, respectively (Table 1). The overall ratio of PIK3CA/CEP3 was greater than five for both resistant clones (Table 1). The additional copies of PIK3CA were colocalized and appear to comprise an intrachromosomal homogeneously staining region (Figure 3b). To determine whether the PIK3CA homogeneously staining region was present in parental cells, we screened 3000 nuclei and were not able to identify a cell with evidence of amplification, suggesting that it is either not present or occurs at a frequency of less than 0.03%.
Figure 3.
(a) Quantative PCR assessment of chromosomal copy number for 11 cancer-related genes conducted on genomic DNA from KPL-4 parental and KPL-4PR cells. Copy number was calculated by normalizing to the RNAaseP gene, as described in the text. (b) FISH assay showing PIK3CA copy number in KPL-4 parental and two resistant clones. Chromosomal copies of PIK3CA are shown in red, and copies of the centromeric control gene CEP3 are shown in green. KPL-4PR.5 and KPL-4PR.18 clones show high-level amplification of the PIK3CA locus present in homogeneously staining regions, indicated by arrows.
Table 1. Mutational and copy number analysis of KPL-4 parental and PI3K inhibitor resistant clones.
| PIK3CA (H1047R) Mutation % next-generation sequencing | H1047R Mutant allele Ct value | H1047 WT allele Ct value | PIK3CA copy by qPCR | MYC copy by qPCR | PIK3CA copy number by FISH | Ratio PIK3CA/CEP3 | FISH result | Tumor cells with clusters (FISH) | |
|---|---|---|---|---|---|---|---|---|---|
| KPL-4 Parental | 44% (879/2029)a | 30.3 | 32.8 | 2.6 | 12.7 | 6 | 1 | High polysomy | 0% (n=3000) |
| KPL-4PR.5 | 95% (1946/2040)a | 22.7 | 40 | 34.6 | 7.7 | 38 | 5.43 | Gene amplification | 93% (n=800) |
| KPL-4PR.18 | 92% (1738/1892)a | 23.9 | 37.5 | 25.3 | 10.7 | 77 | 9.63 | Gene amplification | 96% (n=800) |
Abbreviations: Ct, threshold cycle; FISH, fluorescence in situ hybridization; qPCR, quantitative PCR; WT, wild type.
(Mutant reads/total).
Knockdown of PIK3CA in resistant cells decreases pathway activation and restores sensitivity to PI3K inhibition
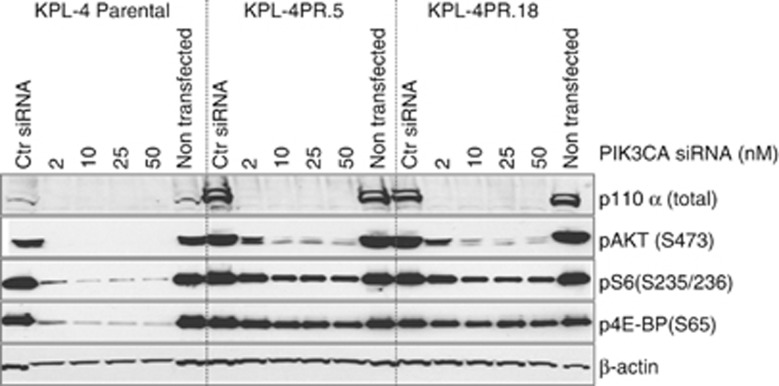
We used small interfering RNA (siRNA) targeting PIK3CA to knock down p110α expression in parental and KPL-4PR clones. Western blotting of control-treated cells showed that KPL-4PR.5 and KPL-5PR.18 cells substantially overexpress p110α relative to parental cells (Figure 4), consistent with an interpretation wherein amplification is driving protein overexpression of the mutant allele. Knockdown by siRNA resulted in 80–90% reduction of p110α protein and PIK3CA mRNA (Figure 4, Supplementary Figure S5). Similar effects were observed with independent siRNA duplexes, suggesting an on-target effect (Supplementary Figure S5). Knockdown of PIK3CA was associated with diminished levels of pAKT, pS6 and p4EB-P1, suggesting the elevated signaling observed in the resistant clones is due to p110α overexpression (Figure 4).
Figure 4.
Western blot analysis of lysates from KPL-4 parental cells and resistant clones KPL-4PR.5 and KPL4-PR.18 harvested after siRNA knockdown of the p110α subunit. siRNA concentrations tested were 2, 10, 25 and 50 nM. Ctr, control (non-targeting scrambled) siRNA.
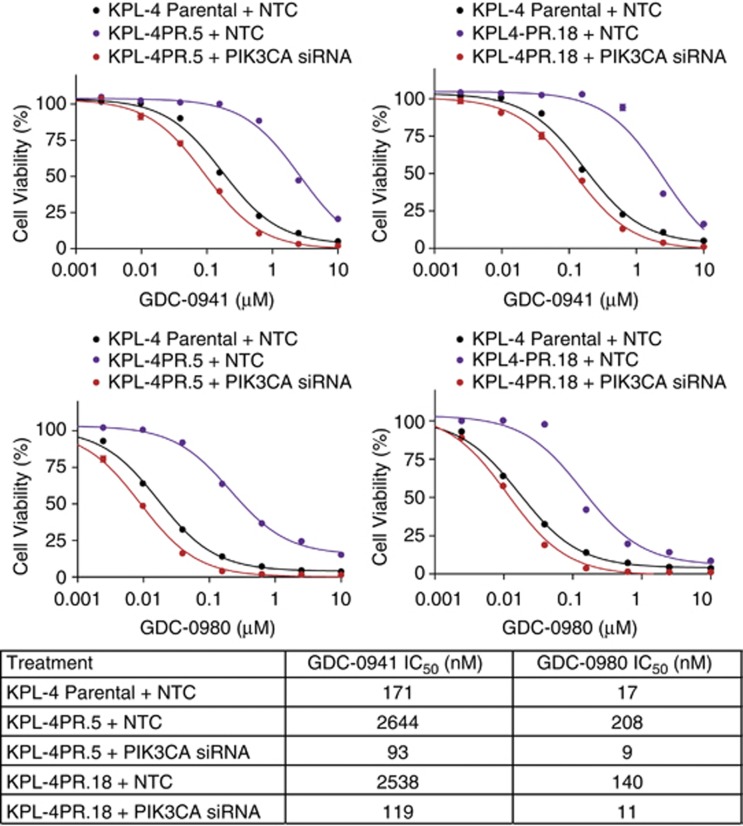
We next assessed whether PIK3CA knockdown restored sensitivity to PI3K inhibition in resistant cells. We found that treatment of KPL-4PR clones with PIK3CA siRNA duplexes restored sensitivity to both GDC-0941 and GDC-0980 to levels equivalent to parental cells treated with a control siRNA as measured by IC50 values (Figure 5). No effects were seen on sensitivity to MEK inhibition (data not shown), suggesting specific restoration of sensitivity to PI3K pathway inhibition.
Figure 5.
Cell viability experiments conducted on KPL-4 parental cells and resistant clones KPL-4PR.5 and KPL-4PR.18 transfected with PIK3CA siRNA or control (non-targeting scrambled) siRNA. Cells were treated with a concentration range of GDC-0941 or GDC-0980 after knockdown of PIK3CA. Table at the bottom shows cellular IC50 values for each of the various experimental conditions.
Discussion
We sought to identify and delineate mechanisms of resistance to a selective PI3K inhibitor, GDC-0941, in HER2-positive KPL-4 breast cancer cells that harbor a PIK3CA mutation and are dependent on signaling through the PI3K pathway. Comprehensive genomic analysis identified amplification of the mutant PIK3CA (H1047R) allele in resistant but not sensitive parental cells. Functional studies showed that the resistance was specifically due to the amplification event, as evidenced by decreased pathway activation and restored sensitivity to pathway inhibitors in cells with knockdown of mutant PIK3CA. To our knowledge, this is the first description of resistance to a PI3K inhibitor linked to activation of the PI3K oncogene itself. This contrasts strikingly to multiple reports of resistance mediated through parallel or downstream events.
This observation is unexpected for several reasons. First, one might reasonably expect that amplification of PIK3CA would predict for sensitivity, rather than resistance to PI3K inhibitors, as a number of preclinical studies have identified PIK3CA amplification as a candidate predictive marker for response to PI3K inhibition.15, 16 Rather, we found that amplification causes hyperactive pathway signaling and an apparent shift in drug sensitivity, likely due to increased expression of the activated target. This is consistent with previous reports of amplification of an oncogene driving resistance to therapeutics targeting another kinase in the same pathway. For example, amplification of both the BRAF and KRAS oncogenes has been reported to confer resistance to downstream inhibition of MEK.17, 18 Our findings suggest that amplification of a mutant PIK3CA allele causes an increase in the basal phosphorylation levels of AKT, leading to a concomitant increase in the IC50 for inhibition of AKT phosphorylation. Second, the role of PIK3CA amplification as a ‘driving oncogene' has been challenged. Several studies have suggested that the lineage-specific oncogene, SOX2 may be the oncogenic driver within the 3q26 amplicon.19 Similarly, the role of PIK3CA mutation in driving an increased pathway output has also been questioned based on proteomic and gene expression data suggesting that cell lines and tumors with PIK3CA mutations have reduced pathway output.20, 21 Our results suggest that amplification, at least of the mutant allele, can potently activate PI3K signaling.
Studies of resistance to EGFR inhibition have demonstrated that selective pressure results in clonal selection and expansion of rare cells with c-MET amplification present in the original tumor cell population at a frequency of 0.14%.22 To investigate whether a similar phenomenon occurred in KPL-4PR cells, we performed fluorescence in situ hybridization analysis on over 3000 nuclei derived from KPL-4 parental cells and did not find a single cell with high-level amplification that we observed in the resistant cells. Though our results cannot rule out the presence of very rare cells with amplification in the starting population, they do suggest such the cells must be present at a frequency below 0.03%.
An important question that arises from these studies is whether the doses at which preclinical resistance are observed are clinically relevant. KPL-4PR cells are able to grow efficiently in the presence of 1 μM GDC-0941, and pharmacokinetic/pharmacodynamic modeling suggests that the GDC-0941 plasma concentration required for tumor stasis is approximately 0.3 μM.23 Thus, concentrations at which we observed resistance are clinically relevant and are achievable based on phase I dose escalation studies.24
Several mechanisms of preclinical resistance to PI3K inhibition have now been described.8, 9, 10 Some are due to activation of parallel pathways (that is, Myc, Notch); another report points to activation of downstream signaling (that is, eIF4E amplification), and here, for the first time, we show the potential for activation of the target itself. The clinical relevance of any of these mechanisms is yet to be established, but the mechanisms described thus far provide hypotheses that can be clinically tested through rigorous analysis of biopsies collected at disease progression after administration of PI3K inhibitors.
Materials and methods
Cell lines and compounds
KPL-4 cells were obtained from Kurebayashi et al.25 and the identity of the isolate used here was verified by genotyping with a Multiplex STR assay (Genetica, Burlington, NC, USA). Parental and resistant clones were cultured in RPMI 1640 medium containing 10% fetal bovine serum, non-essential amino acids, 2 mmol/l L-glutamine and penicillin/streptomycin. Inhibitors for pan PI3K (GDC-0941), dual PI3K/mTOR (GDC-0980), β-sparing PI3K inhibitor GDC-0032, lapatinib and MEK1/2 (PD0325901) have been described previously and were produced and supplied by Genentech Medicinal Chemistry group.13, 26, 27, 28, 29 Other compounds were obtained from Calbiochem (Billerica, MA, USA) and TopoGEN (Port Orange, FL, USA).
PI3K inhibitor resistant line creation and doubling time analysis
KPL-4 resistant pool was established by gradual dose escalation of PI3K inhibitor, GDC-0941, for a period of 6 months to a final drug concentration of 1 μM. The KPL-4PR pool was cultured in the presence of the drug for 3 months before being subjected to fluorescence-activated cell sorting at one cell per well in a 96-well plate. Clones from the resistant pool were then expanded in the absence of the PI3K inhibitor, GDC-0941. Clones were stably resistant for up to 20 passages in the absence of GDC-0941. For doubling time determination, cells were plated at 4000 cells per well in 96-well plates 24 h before the addition of GDC-0941 at various concentrations. Images of live cell cultures were recorded with the IncuCyte TM Zoom Image system (Essen BioScience, Inc., Ann Arbor, MI, USA). Doubling times were calculated by Prism (GraphPad software Inc., La Jolla, CA, USA).
Small RNA interference assay and cell viability assay
PIK3CA SMARTpool, PIK3CA individual siRNA duplexes (S12, S15) and non-targeting scrambled control siRNAs were purchased from Dharmacon (Thermo Scientific, Pittsburgh, PA, USA). All PIK3CA siRNA transfections were performed using the SMARTpool unless specifically stated otherwise. Reverse transfections were conducted by first mixing 30 μl of Lipofectamine RNAiMAX (Life Technologies, Carlsbad, CA, USA) with 2–50 nM of siRNA in 1.5 ml Opti-MEM medium (Life Technologies) to allow complex formation. After 30 minutes, cells in an antibiotic-free growth medium at a density of 1.5 × 105 per flask were added into the culture flasks. Forty-eight hours after transfection, cells were trypsinized and counted. Cells were seeded at 3000 cells per well in 384-well plates for compound screening and also in 6-well plates for protein and RNA lysate collection the following day. Cells in 384-well plates were dosed the next day with inhibitors at threefold serial dilutions, starting at 10 μM, in quadruplicate. Three days after the addition of compounds, cell viability was assessed using the CellTiter Glo ATP Luminescence assay (Promega, Madison, WI, USA) as described previously.13
Receptor tyrosine kinase array
PathScan RTK Signaling Antibody Array kits (Fluorescent Readout; Cell Signaling Technology, Danvers, MA, USA) were used to measure 28 receptor tyrosine kinases and 11 intracellular signaling nodes. Cells were lysed in T-PER cell extraction reagent (Thermo Scientific) with additional 300 mM NaCl and protease and phosphatase inhibitor cocktails (Sigma, St Louis, MO, USA). Lysates were then diluted and hybridized to the slides following the manufacturer's protocol. Fluorescence intensities were quantitated on a Li-COR Odyssey scanner (Lincoln, NE, USA).
Western blot analyses
Cell lysates for western blots were collected in T-PER cell extraction reagent supplemented with protease and phosphatase inhibitor cocktails (Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer's protocol. Protein concentrations were determined by the BCA Protein Assay (Thermo Scientific) with a standard curve of bovine serum albumin provided in the kit. Lysates were resolved by electrophoresis on Novex NuPAGE 4–20% Bis-Tris gels (Life Technologies). For proteins with high molecular weight, Tris-acetate gels were used to achieve better separation. Proteins on gels were transferred to nitrocellulose membranes using the iBlot system (Life Technologies). Tris buffered saline & Tween 20 (TBST) containing 5% bovine serum albumin was used as a blocking and antibody dilution buffer. Immunodetection of proteins was carried out with Super Signal West Dura Chemiluminescence substrate (Thermo Scientific). Antibodies to pAKT (Ser473), pAKT (T308), pERK1/2 (Thr202/Tyr204), p4EB-P1 (Ser65), pS6 Ribosomal (Ser235/236), FOXO1 (Thr24), GSK3b (Ser9), pPRAS40 (Thr46) and PI3Kα were from Cell Signaling Technology. β-actin antibody from Santa Cruz Biotechnology (Santa Cruz, CA, USA) was used as a loading control. For inhibitor dose response western analysis, cells were plated at 400 000 cells per T-25 flask and cultured until they reached approximately 80% confluence. Cells were then treated with compounds at various concentrations for 24 h, then washed once with cold phosphate-buffered saline and processed as described above.
Gene copy number and mutation analyses
Relative gene copy numbers were determined using quantitative PCR. Threshold cycles (Ct) were measured using cell line genomic DNAs and gene-specific TaqMan Copy Number assays (Life Technologies) according to the manufacturer's instruction. Two assays for each gene were used. Relative copy numbers were calculated by the Delta Delta Ct method as previously described.15 The RNaseP was used as a reference gene (RNaseP Copy Number assay, Life Technologies) and pooled human blood genomic DNA (Roche Applied Science; 11691112001) was used as a calibrator sample with two copies for each gene. Molecular Inversion Probe (MIP) arrays were run and analyzed using OncoScan FFPE Express 2.0 Services (Affymetrix, Santa Clara, CA, USA). The nucleotide change underlying the PIK3CA (H1047R) substitution was detected using quantitative PCR with wild-type and mutation-specific probes, as described previously.15, 30
Deep sequencing of the PIK3CA gene
Sixty-four pairs of PCR primers were designed to generate tiling interleaved amplicons (70 bp average overlap) that cover DNA sequences of all exons and splicing junctions of the PIK3CA gene. PIK3CA amplicon libraries were generated from 50 ng of genomic DNA from each sample using Access Arrays (Fluidigm, South San Francisco, CA, USA) following the Multiplex Amplicon Tagging Protocol from the manufacturer. The resulting sequencing-ready amplicon libraries were sequenced (2 × 150 bp) on an Illumina (San Diego, CA, USA) MiSeq sequencer. The average sequencing depth for the PIK3CA amplicon library was 1595x (range 252–3918x). Protein altering variants (excluding known dbSNP variants) were reported using a variant frequency cutoff of 4%. No other variants in addition to PIK3CA H1047R were detected.
PIK3CA fluorescence in situ hybridization analysis
Fluorescence in situ hybridization analysis was performed on cell line samples as described previously.15, 31 Briefly, two bacterial artificial chromosome clones, RP11-245C23 and RP11-355N16, covering the entire PIK3CA locus and adjoining areas, were used as probes in these experiments. A commercially available probe for CEP3 (PathVysion; Vysis/Abbott Laboratories, Des Plaines, IL, USA) was also used to determine chromosome 3 copy number. The cytogenetic preparations for the KPL-4 parental cell line and the two resistant cell line clones were obtained using standard cytogenetic protocols.31
Acknowledgments
We would like to thank Scott Sproul for assistance with cell culture and Jeff Settleman for comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Oncogenesis website (http://www.nature.com/oncsis).
Supplementary Material
References
- Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther. 2009;8:1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–456. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- Baselga J, Campone M, Piccart M, Burris HA, 3rd, Rugo HS, Sahmoud T, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–529. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janku F, Tsimberidou AM, Garrido-Laguna I, Wang X, Luthra R, Hong DS, et al. PIK3CA mutations in patients with advanced cancers treated with PI3K/AKT/mTOR axis inhibitors. Mol Cancer Ther. 2011;10:558–565. doi: 10.1158/1535-7163.MCT-10-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner MR, Wilson TR, Settleman J. Mechanisms of acquired resistance to targeted cancer therapies. Future Oncol. 2012;8:999–1014. doi: 10.2217/fon.12.86. [DOI] [PubMed] [Google Scholar]
- Allison M. MEK inhibitor nears approval. Nat Biotechnol. 2013;31:4. [Google Scholar]
- Flaherty KT, Infante JR, Daud A, Gonzalez R, Kefford RF, Sosman J, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694–1703. doi: 10.1056/NEJMoa1210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, et al. Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med. 2011;17:1116–1120. doi: 10.1038/nm.2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellner MK, Uras IZ, Gapp BV, Kerzendorfer C, Smida M, Lechtermann H, et al. A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat Chem Biol. 2011;7:787–793. doi: 10.1038/nchembio.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic N, Utermark T, Widlund HR, Roberts TM. PI3K-targeted therapy can be evaded by gene amplification along the MYC-eukaryotic translation initiation factor 4E (eIF4E) axis. Proc Natl Acad Sci USA. 2011;108:E699–E708. doi: 10.1073/pnas.1108237108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra V, Eichhorn PJ, Garcia-Garcia C, Ibrahim YH, Prudkin L, Sanchez G, et al. RSK3/4 mediate resistance to PI3K pathway inhibitors in breast cancer. J Clin Invest. 2013;123:2551–2563. doi: 10.1172/JCI66343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunder ER, Knight ZA, Houseman BT, Apsel B, Shokat KM. Discovery of drug-resistant and drug-sensitizing mutations in the oncogenic PI3K isoform p110 alpha. Cancer Cell. 2008;14:180–192. doi: 10.1016/j.ccr.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C, Wallin JJ, Sampath D, GuhaThakurta D, Savage H, Punnoose EA, et al. Predictive biomarkers of sensitivity to the phosphatidylinositol 3' kinase inhibitor GDC-0941 in breast cancer preclinical models. Clin Cancer Res. 2010;16:3670–3683. doi: 10.1158/1078-0432.CCR-09-2828. [DOI] [PubMed] [Google Scholar]
- Ndubaku CO, Heffron TP, Staben ST, Baumgardner M, Blaquiere N, Bradley E, et al. Discovery of 2-{3-[2-(1-isopropyl-3-methyl-1H-1,2-4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): a beta-sparing phosphoinositide 3-kinase inhibitor with high unbound exposure and robust in vivo antitumor activity. J Med Chem. 2013;56:4597–4610. doi: 10.1021/jm4003632. [DOI] [PubMed] [Google Scholar]
- Spoerke JM, O'Brien C, Huw L, Koeppen H, Fridlyand J, Brachmann RK, et al. Phosphoinositide 3-kinase (PI3K) pathway alterations are associated with histologic subtypes and are predictive of sensitivity to PI3K inhibitors in lung cancer preclinical models. Clin Cancer Res. 2012;18:6771–6783. doi: 10.1158/1078-0432.CCR-12-2347. [DOI] [PubMed] [Google Scholar]
- Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, et al. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- Corcoran RB, Dias-Santagata D, Bergethon K, Iafrate AJ, Settleman J, Engelman JA. BRAF gene amplification can promote acquired resistance to MEK inhibitors in cancer cells harboring the BRAF V600E mutation. Sci Signal. 2010;3:ra84. doi: 10.1126/scisignal.2001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AS, Balmanno K, Sale MJ, Newman S, Dry JR, Hampson M, et al. Amplification of the driving oncogene, KRAS or BRAF, underpins acquired resistance to MEK1/2 inhibitors in colorectal cancer cells. Sci Signal. 2011;4:ra17. doi: 10.1126/scisignal.2001752. [DOI] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi S, Haibe-Kains B, Majjaj S, Lallemand F, Durbecq V, Larsimont D, et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc Natl Acad Sci USA. 2010;107:10208–10213. doi: 10.1073/pnas.0907011107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salphati L, Wong H, Belvin M, Bradford D, Edgar KA, Prior WW, et al. Pharmacokinetic-pharmacodynamic modeling of tumor growth inhibition and biomarker modulation by the novel phosphatidylinositol 3-kinase inhibitor GDC-0941. Drug Metab Dispos. 2010;38:1436–1442. doi: 10.1124/dmd.110.032912. [DOI] [PubMed] [Google Scholar]
- Sarker D, Kristeleit R, Mazina K, Ware JA, Yan Y, Dresser M, et al. A phase I study evaluating the pharmacokinetics (PK) and pharmacodynamics (PD) of the oral pan-phosphoinositide-3 kinase (PI3K) inhibitor GDC-0941. J Clin Oncol. 2009;27:3538. [Google Scholar]
- Kurebayashi J, Otsuki T, Tang CK, Kurosumi M, Yamamoto S, Tanaka K, et al. Isolation and characterization of a new human breast cancer cell line, KPL-4, expressing the Erb B family receptors and interleukin-6. Br J Cancer. 1999;79:707–717. doi: 10.1038/sj.bjc.6690114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkes AJ, Ahmadi K, Alderton WK, Alix S, Baker SJ, Box G, et al. The identification of 2-(1H-indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-t hieno[3,2-d]pyrimidine (GDC-0941) as a potent, selective, orally bioavailable inhibitor of class I PI3 kinase for the treatment of cancer. J Med Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- Rusnak DW, Lackey K, Affleck K, Wood ER, Alligood KJ, Rhodes N, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- Sutherlin DP, Sampath D, Berry M, Castanedo G, Chang Z, Chuckowree I, et al. Discovery of (thienopyrimidin-2-yl)aminopyrimidines as potent, selective, and orally available pan-PI3-kinase and dual pan-PI3-kinase/mTOR inhibitors for the treatment of cancer. J Med Chem. 2010;53:1086–1097. doi: 10.1021/jm901284w. [DOI] [PubMed] [Google Scholar]
- Wallin JJ, Edgar KA, Guan J, Berry M, Prior WW, Lee L, et al. GDC-0980 is a novel class I PI3K/mTOR kinase inhibitor with robust activity in cancer models driven by the PI3K pathway. Mol Cancer Ther. 2011;10:2426–2436. doi: 10.1158/1535-7163.MCT-11-0446. [DOI] [PubMed] [Google Scholar]
- Patel R, Tsan A, Tam R, Desai R, Schoenbrunner N, Myers TW, et al. Mutation scanning using MUT-MAP, a high-throughput, microfluidic chip-based, multi-analyte panel. PLoS One. 2012;7:e51153. doi: 10.1371/journal.pone.0051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C, Cavet G, Pandita A, Hu X, Haydu L, Mohan S, et al. Functional genomics identifies ABCC3 as a mediator of taxane resistance in HER2-amplified breast cancer. Cancer Res. 2008;68:5380–5389. doi: 10.1158/0008-5472.CAN-08-0234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.